One of the most exciting examples of Neotropical myrmecophytic plants is one that occurs in a small group of 15 species of ant-acacias, which belong to the Acacia genus (Rico-Arce 2007). At present, the Acacia genus has been reclassified and subdivided into several genera, among which is Vachellia (Murphy 2008, Miller & Seigler 2012, Kyalangalilwa et al. 2013), to which the myrmecophytic acacias belong. However, because this particular group is globally recognized as “swollen-thorn acacias” or “ant-acacias” and the circumscription of Acacia genus is under debate, in the present work the nomenclature of Acacia sensu lato will be conserved.
The mutualism between ant-acacias and their mutualist ants originated in Mesoamerica, approximately 5.44 million years ago, in the late Miocene (Gómez-Acevedo et al. 2010). As a consequence of ant interactions, the ant-acacias show some morphological variations compared to their relatives non-myrmecophilous species. These include: 1) domatia, which are swollen hollow stipular thorns, where the ants live; 2) beltian bodies located in the leaflet tips, which are a source of food for the ant larvae; 3) extrafloral nectaries (one or more) in the leaf rachis; and 4) a prominent involucel (Janzen 1974, Rico-Arce 2007). The ants protect plants against herbivores and florivores, but their patrolling activities on flower structures may have a negative effect on the fitness of host plants, since they have a limited potential as pollinators and interfere with the effectiveness of pollinators. In response, plants have developed strategies to control the access of the ants to the flowers, restricting them basically to foliage (Nicklen & Wagner 2006, Willmer et al. 2009).
Ecological studies have shown that flowers in general exclude ants prior to pollination by means of the secretion of ant-repellents, probably through volatile chemicals (e.g., Federle et al. 1997, Willmer & Stone 1997, Ghazoul 2001, Raine et al. 2002, Nicklen & Wagner 2006). Some species of Acacia have been studied in this regard; in these cases, the opening of the inflorescences is carried out when ants are less active, as in A. constricta, A. hindsii (myrmecophytic) and A. zanzibarica (Willmer & Stone 1997, Raine et al. 2002, Nicklen & Wagner 2006), though in A. collinsii (myrmecophytic) this temporal separation is not present, and the activity of the ants and pollinators concurs (Ghazoul 2001). Nevertheless, only the temporal coincidence between the absence of ants and the anthesis period is considered in such studies, such that most evidence is circumstantial (Willmer et al. 2009).
Until now, no morphological study has been carried out on the floral organs involved in the probable exclusion of ants during the flowering period. In this sense, floral development studies are ideal for clarifying this type of characters, showing the time and manner in which certain adaptations and peculiarities are presented in each floral organ, taking into account all the events from inception to maturation, and enabling a better understanding of the relationship between morphology and pollination biology (Endress 2006, Scut & Vandenbussche 2014, Iwamoto & Bull-Hereñu 2018, Ronse de Craene 2018).
In this study, a floral development examination was performed in Acacia cornigera (L.) Willd., which is the most representative Neotropical ant-acacia. It has a very broad geographical and ecological range, from Mexico to Colombia. Acacia cornigera is a shrub or tree up to 10 m tall. It inhabits seasonally flooded and disturbed wet forests, although it is also common in savannas, grasslands and tends to be invasive in secondary vegetation. It shows great morphological variation along its geographical range (Janzen 1974, Rico-Arce 2007). This species is also particularly interesting because it has unusual floral characteristics such as tapered cylindrical inflorescences and peltate floral bracts, although the latter are also present in other ant-acacias including A. globulifera and A. sphaerocephala (Janzen 1974, Seigler & Ebinger 1995).
The aim of this study was to analyze if the floral development of the myrmecophytic A. cornigera is different from a non-myrmecophytic species and determine if there is a morphological variation probably associated with avoiding the ants during the flowering period. The questions considered in this study were: 1) are the patterns of floral development affected by the relationship with ants? 2) is there any floral organ or structure involved in avoiding the presence of ants during the flowering period? and 3) at what stage of development do these modifications arise, if at all?
Materials and methods
Inflorescences of Mexican Acacia cornigera in some degree of maturation were collected during March 2015 in the locality of Guadalupe Victoria, Santiago Pinotepa Nacional (16o 20´ N, 98o 03´ W), Oaxaca and in May 2015 in “Los Tuxtlas”, Tropical Biology Station (18o 34´-18o 36´ N, 95o 04´- 95o 09´ W), Veracruz. The characteristic vegetation of Santiago Pinotepa Nacional is rainforest and sand dunes, savannas and cattail wetlands, to a lesser extent, whereas in “Los Tuxtlas” there is mainly evergreen lowland rainforest. In both regions, there are also open sites (disturbed vegetation), as a consequence of agriculture and urban sprawl and A. cornigera is capable of growing in these disturbed sites. The two populations were examined in order to consider the morphological variation due to geographic range.
Floral material was fixed in 70 % ethanol. The inflorescences were dissected under a Luxeo 4Z (LABOMED, Fremont, CA, U.S.A.) stereoscopic microscope. The dissected material was dehydrated in 100 % ethanol for at least 24 hours, and critical-point dried using liquid CO2 in an Emitech K550 (Quorum Technologies Ltd, Lewes, United Kingdom) apparatus. Subsequently, the material was mounted on aluminium stubs, sputter-coated with gold in a Q150R ES Quorum (Quorum Technologies Ltd, Lewes, United Kingdom) and observed using a Hitachi SU1510 (Hitachi, High-Technologies Corporation, Tokyo, Japan) scanning electron microscope.
Results
The inflorescences of Acacia cornigera were peduncle-elongated and tapered-cylindrical. Patterns of bract and floral organogenesis were similar in the two populations. The floral bracts were peltate and measured ~ 1.37 mm long. The flowers (~ 2.02 mm long) were sessile and consisted of more than five lobes of sepals and petals (~ 1.32 and ~ 1.54 mm long respectively); more than 50 stamens (~ 1.94 mm long), and a single carpel (~ 1.97 mm long).
Organ inception. The first bracts to emerge were located in the apex of the inflorescence. Bract maturation was basipetal along the inflorescence. There was no inflorescence petiole and the inflorescence parenchyma were evident, measuring about 0.62 × 0.85 mm (Figure 1A). Each bract then enlarged and began to develop a petiole. There were small unicellular trichomes on the margin of the bract (Figure 1B). The adaxial and abaxial surfaces of each bract are glabrous. Floral meristem below each bract was not yet seen at this stage (Figure 1C, D). Shortly after bract development, the inflorescence parenchyma became elongated and a short peduncle could be observed (Figure 1E). Each floral bract was large, and their characteristic peltate form was evident, but the petiole was still short. All sides of the bract were imbricated with each other (Figure 1F).
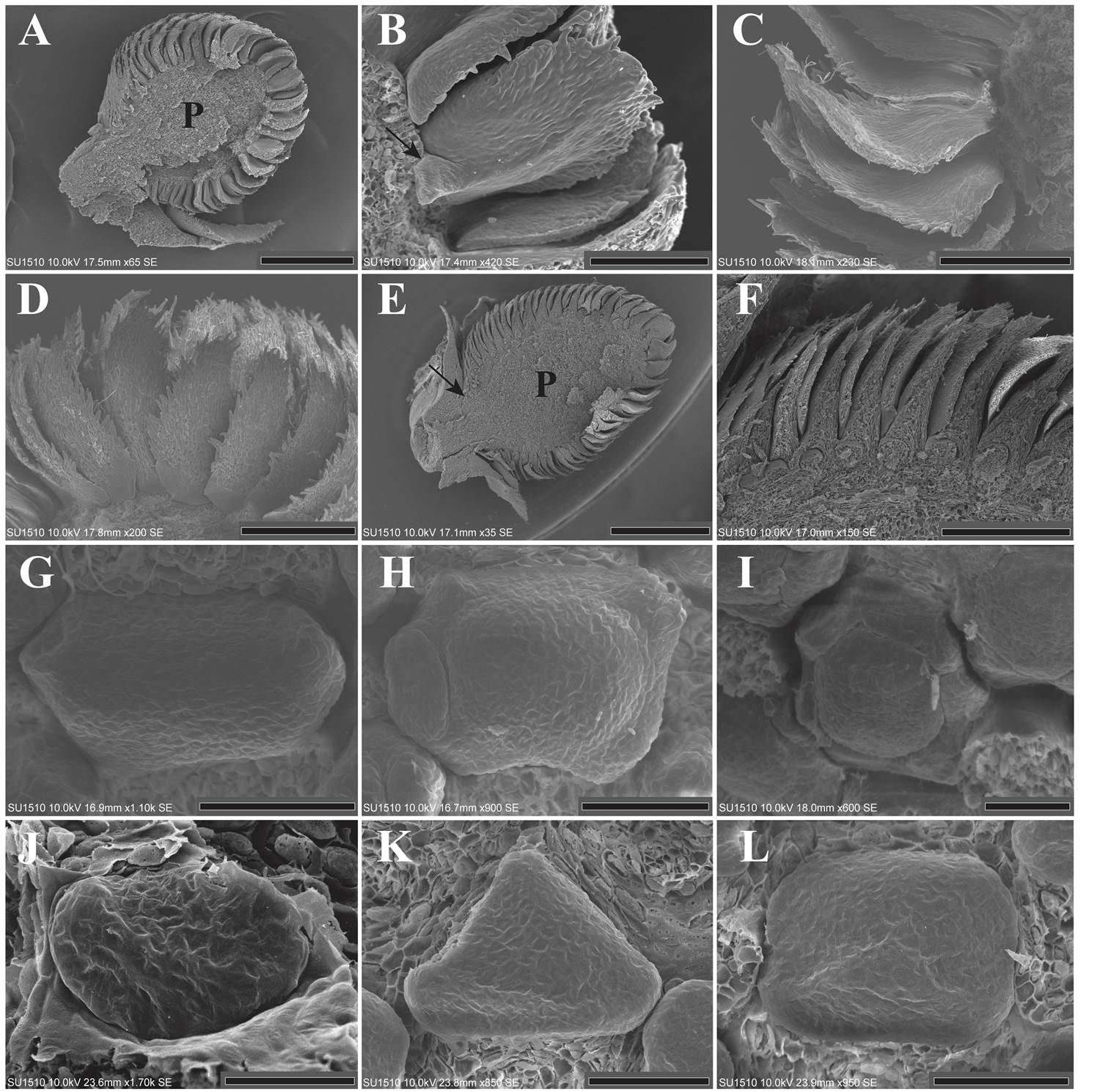
Figure 1 A: Longitudinal section of an inflorescence in the first stages of maturation, only bracts are present and floral meristem is absent. The parenchyma is evident (LT). B: Bract showing a short petiole (arrow) and trichomes on the border (LT). C-D: Bract enlargement, showing trichomes on the margin of each bract. The abaxial and adaxial surfaces are glabrous (LT). E-F: Longitudinal section corresponding to the time of sepal inception. The bracts are imbricated with each other. The arrow shows the short petiole of the inflorescence. The parenchyma has increased in size (LT). G-L: Sepal inception showing two, three or more sepal primordia in the localities of LT (G-I) and SPN (J-L). The sepal primordia emerge either sequentially or simultaneously. The bracts were dissected. P = Parenchyma; LT = Los Tuxtlas; SPN = Santiago Pinotepa Nacional. Scale bar: A = 500 µm; B = 100 µm; C, D = 200 µm; E = 1 mm; F = 300 µm; G, H, I, K, L = 50 µm; J = 30 µm.
The pattern of organ inception in the sepals is irregular. Some floral meristems presented two laterally opposite primordia, while others three, four, or more than five sepal primordia, which emerge either sequentially or simultaneously (Figure 1G-L). After inception, the sepal primordia are basally connate by precocious congenital fusion (Figure 2A). Contrary to the calyx, the petal primordia emerged simultaneously. The emergence of more than five sepal and petal lobes is evident (Figure 2A-F). The petal primordia were glabrous as well as congenitally and basally fused (Figure 2G, H).
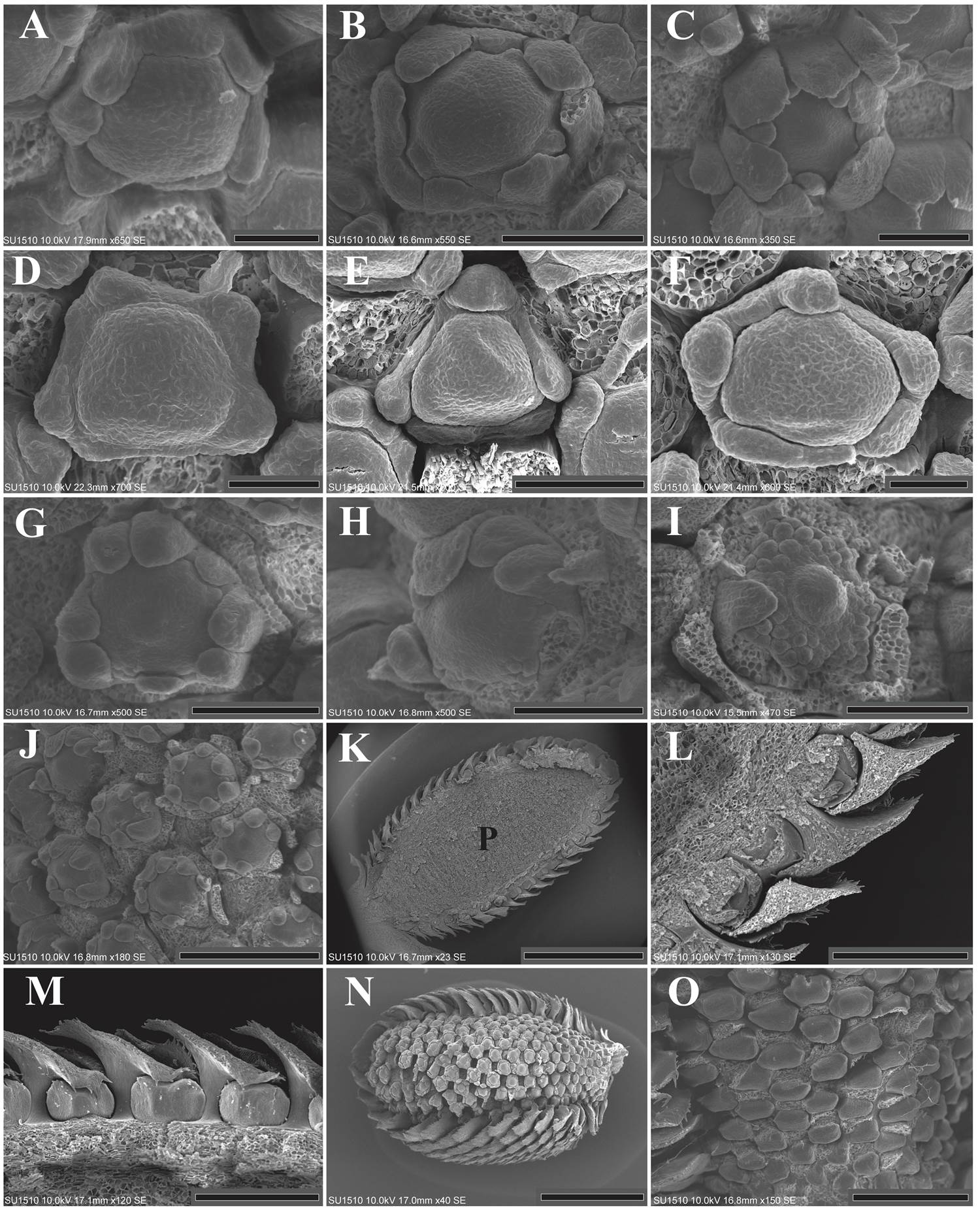
Figure 2 A-F: Floral meristems from LT (A-C) and SPN (D-F), showing the presence of more than five sepal lobes and the congenital fusion of the calyx. The simultaneous inception of the petal primordia is also appreciated. The bracts were dissected. G: Enlargement of the petal lobes toward the floral center. The stamens and gynoecium primordia can also be observed. The sepals were dissected (LT). H: Flower showing the inception of the stamen primordia. Five stamen primordia emerged in alternate position with respect to the petals. The inception of the remaining stamens is in lateral and acropetal mode. Some sepals and petals were dissected. The carpel is also appreciated (LT). I: Inception of the remaining stamen primordia. The carpel begins to enlarge but its ventral slit is not formed yet. Sepals and petals were dissected (SPN). J: Lateral view of an inflorescence showing the precocious inception of the carpel primordia, before androecium inception is completed. Bracts and sepals were dissected (LT). K: Longitudinal section showing the parenchyma of an inflorescence in stages corresponding to the androecium inception (LT). L: Close-up of figure 2K, the bract petiole is evident. M: Protective role of the bracts. The bottom of the bract covered the floral meristem. The top of the bract is free (LT). N: Lateral view showing the slightly asynchronous maturation of the inflorescence. The flowers located near the petiole of the inflorescence delay their maturation. Some bracts were dissected (LT). O: Close-up of the basal flowers in which maturation has been delayed (LT). P = Parenchyma; LT = Los Tuxtlas; SPN = Santiago Pinotepa Nacional. Scale bar: A, D, F = 50 µm; B, C, E, G, H, I = 100 µm; J, O = 300 µm; K = 2 mm; L, M = 400 µm; N = 1 mm.
Five stamen primordia emerged acropetally, each one in alternate position with respect to the petals. After first stamen primordia emerged, other stamens were initiated in lateral and acropetal mode on either side of the first five primordia (Figure 2H). The order of initiation of the subsequent stamen primordia was also acropetal (Figure 2I). The carpel primordium emerged at the center of the flower by the time of the initiation of the first sets of stamens (Figure 2G, H, J).
The parenchyma reached a size of 2.01 × 4.45 mm, the inflorescence peduncle remained short (Figure 2K) and the bract petiole was elongated (Figure 2L). The protective role of the bract and their imbricate pattern was more evident, the bottom of the bract covered the floral meristem, while the top was free (Figure 2M). It is important to note that the development of flowers within the inflorescence was slightly asynchronous, with flowers at the base of the inflorescence developing later in both populations (Figure 2N, O).
Organ elongation. In mid stages of flower maturation, the sepals remained fused only basally, but the distal part of each sepal had enlarged and remained free (Figure 3A). Slightly after, the trichomes began to emerge, the sepals enlarged toward the center of the flower, covering and protecting the internal organs (Figure 3B-C). Regarding the corolla, each petal grew and curved toward the center of the flower. Then, the distal part of each petal enlarged, becomes covered by trichomes, and the corolla enclosed the reproductive organs (Figure 3D).
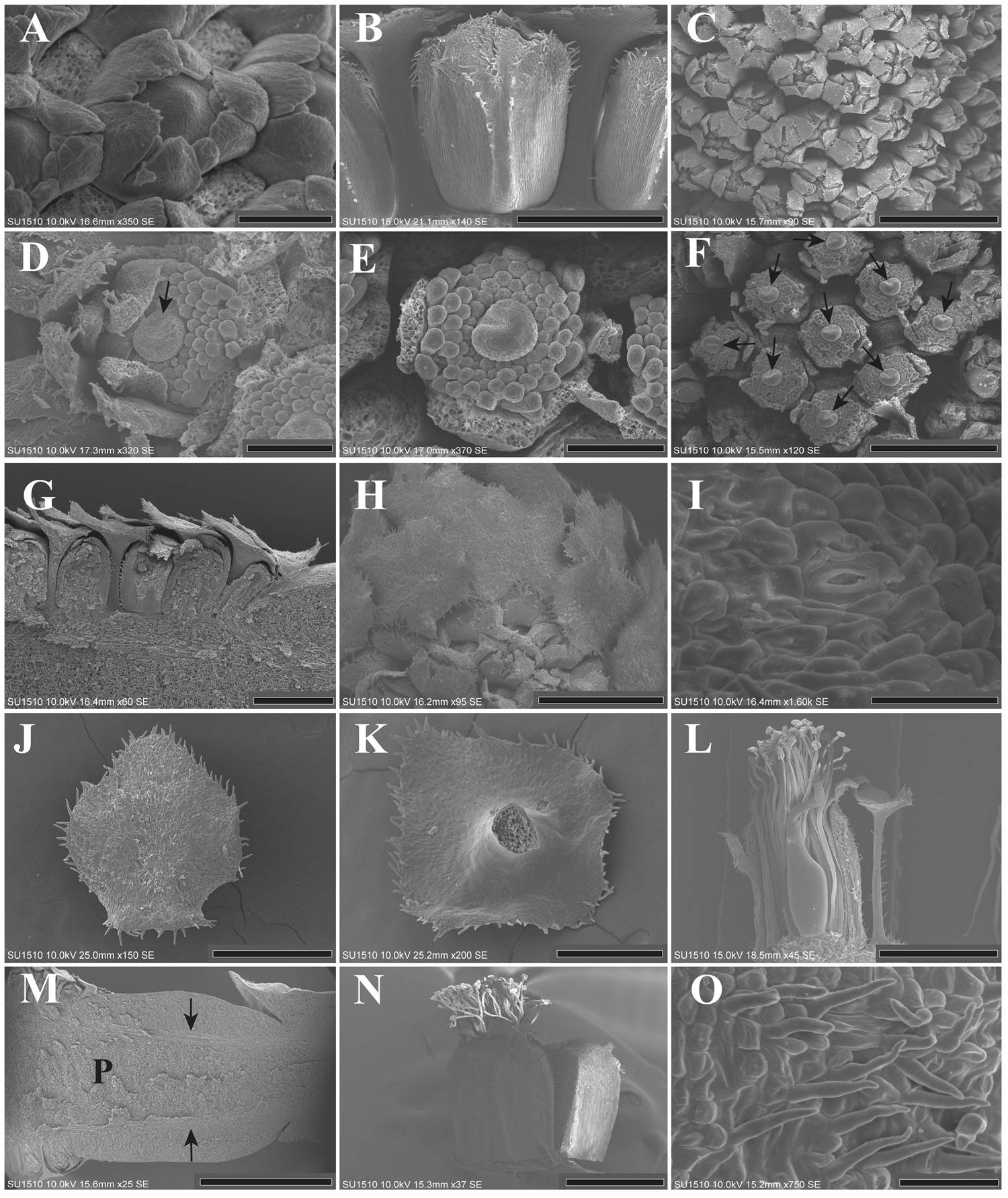
Figure 3 A: Flower in mid stages of maturation showing the congenital fusion of the sepals. The bracts were dissected (LT). B: Lateral view of a flower showing the calyx tube. The presence of trichomes on the distal side is conspicuous (SPN). C: Lateral view of an inflorescence. The distal side of the calyx is not fused, but it protects the inner organs. The bracts were dissected (SPN). D-E: Flower in mid stages of maturation. Some sepals and petals were dissected. Trichomes are observed on the apex of both sepals and petals, as well as globose cells within the apex of the corolla elements. The stamen primordia begin to elongate. In the gynoecium meristem, ventral slit formation can be observed (SPN). F: Lateral view of the inflorescence, the orientation of the ventral slit (arrows) in each flower is variable. Bracts, sepals and petals were dissected (SPN). G: Longitudinal section of an inflorescence showing the imbricate pattern and protective role of the bracts in stages corresponding to the stamen enlargement (LT). H: Lateral view of the inflorescence showing the protective role of the bracts. The bracts covering the flowers. Some bracts were dissected (SPN). I: Close-up of the stoma (arrow) located on the abaxial side of the mature bract (SPN). J: Abaxial view of a mature bract, with globose cells and some trichomes on the borders (SPN). K: Adaxial side of a mature bract, slightly rough (SPN). L: Lateral view of a mature flower showing bract size compared to flower size. The trichomes on the bract petiole are conspicuous (LT). M: Longitudinal section of the inflorescence rachis, showing two vascular bundles, pointed out by arrows. The parenchyma had reached their maximum size (SPN). N: Post-anthetic flower showing the gamosepalous calyx. The stamen size is one third longer than the calyx. There is an unopened flower beside it. The trichomes on the apex and on the borders of the calyx are evident (LT). O: Close-up of the calyx trichomes (LT). P = Parenchyma; LT = Los Tuxtlas; SPN = Santiago Pinotepa Nacional. Scale bar: A, D, E = 100 µm; B, F = 400 µm; C, G, H = 500 µm; I = 30 µm; J = 300 µm; K = 200 µm; L, N = 1 mm; M = 2 mm; O = 50 µm.
In the androecium, the stamens begin to elongate prior to their differentiation into filaments and anthers. The elongation of the filaments was asynchronous and began with the stamen located near the petals (Figure 3D, E). Likewise, the carpel grew, and the ventral slit became evident when the stamen primordia began to differentiate (Figure 3D, E). The orientation of the ventral slit in each flower of the inflorescence was variable (Figure 3F).
Organ maturation. In later stages, all bracts exhibited a long petiole, and their imbricate pattern remained (Figure 3G, H). The abaxial side of each mature bract had some stomata (Figure 3I), globose cells and unicellular trichomes on their borders (Figure 3J). The adaxial side was glabrous (Figure 3K). The bract reached practically the same height as the corolla. The petiole was long with some trichomes (Figure 3L). The parenchyma (~ 30.55 mm long) and peduncle (~ 9.73 mm long) of the inflorescence had reached their maximum size (Figure 3M).
The sepals were almost completely fused (Figure 3N). The abaxial side had globose cells and abundant trichomes (Figure 3O) and the adaxial side was grooved (Figure 4A). The petals were fused forming almost a complete tube slightly larger than the sepals (Figure 4B). The abaxial side of each petal was covered by trichomes (Figure 4C), the adaxial side was glabrous except at the tip, where petals had globose cells and some stomata (Figure 4D, E). The anthers were bithecal, dorsifixed and with a longitudinal dehiscence. Filaments and anthers harboured globose cells (Figure 4F). The filaments of the external set of stamens (i.e. close to the petals) were longer than the filaments of the internal set, which were next to the carpel (Figure 4G). The filaments were free and exerted, one third longer than perianth and bracts (Figures 3L, N, 4G).
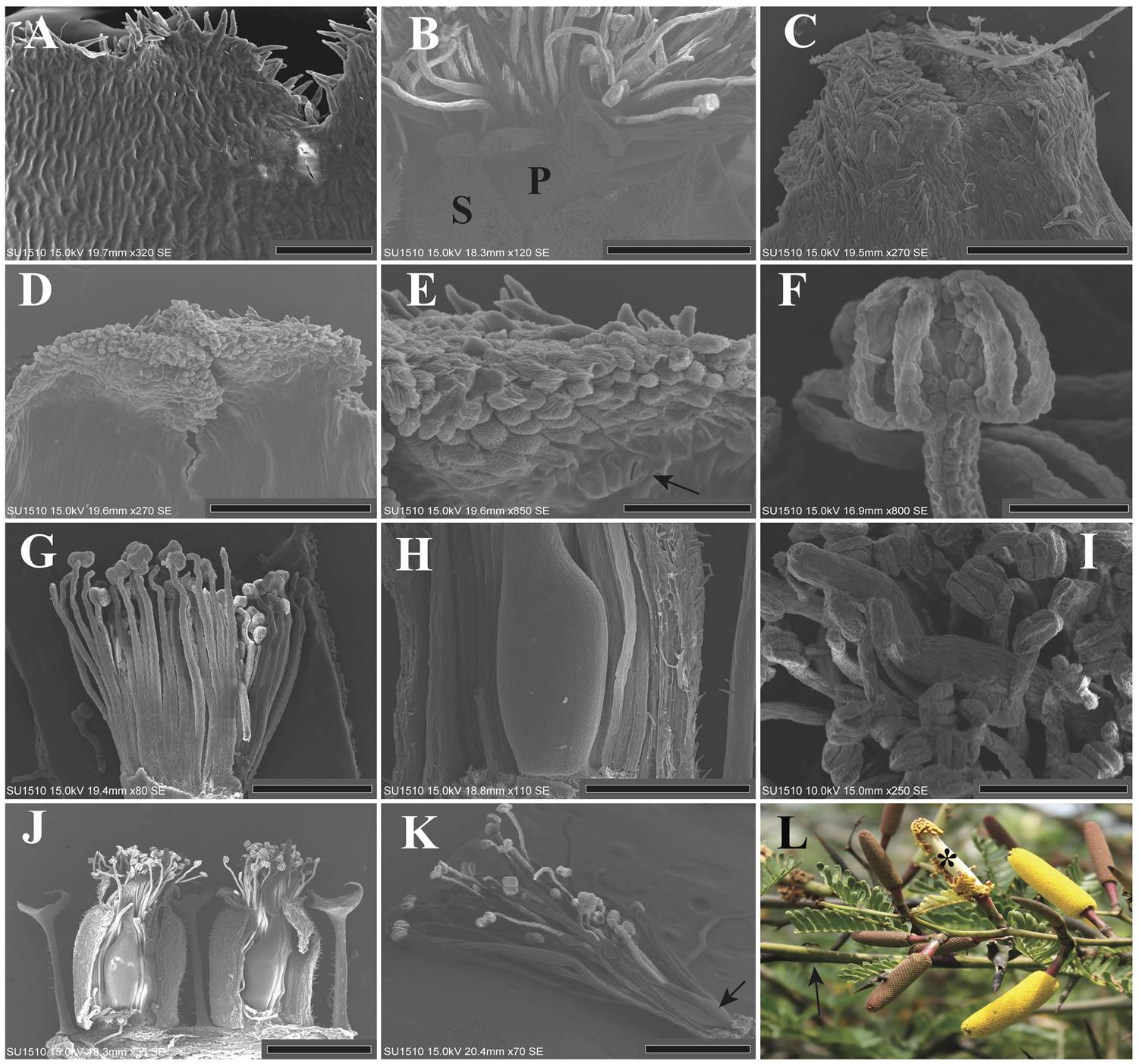
Figure 4 A: Adaxial view of the calyx, showing a grooved ultrastructure (LT). B: Close-up of the corolla tips. The petals (P) barely stand out in size with respect to the sepals (S) (LT). C: Top view of the corolla apex abaxial side, showing the trichomes (LT). D: Top view of the adaxial side of the corolla. The apex is ornamented, while the rest is glabrous and slightly grooved (LT). E: Close-up of the corolla tip. Globular and rough cells can be appreciated, as well as an open stoma, pointed by an arrow (LT). F: Frontal view of a mature bithecal anther, globose cells are noticeable (LT). G: Lateral view of a pre-anthetic dissected flower. Calyx and corolla were removed. The anthers are dorsifixed (LT). H: Close-up of a mature ovary. The ovary is sessile and glabrous. Some sepals, petals and stamens were dissected (LT). I: Top view of a mature flower, showing anthers, style and stigma with globular cells (LT). J: Lateral view of two mature flowers. Some sepals and petals were dissected. The size and ornamentation of all floral parts and peltate bracts is evident (LT). K: Mature male flower. Calyx and corolla were dissected. The arrow shows the atrophied gynoecium (LT). L: Inflorescences in some stages of maturation. The white parenchyma (indicated by an asterisk) of one inflorescence is exposed. The immature inflorescences are reddish-brown in color, while the inflorescences in anthesis are yellow. The arrow indicates an ant patrolling on the nearby branch (LT). LT = Los Tuxtlas; SPN = Santiago Pinotepa Nacional. Scale bar: A = 100 µm; B = 400 µm; C, D, I = 200 µm; E, F = 50 µm; G, H, K = 500 µm; J = 1 mm.
Each mature gynoecium consisted of one carpel with a glabrous and sessile ovary (Figure 4H). The style had globose cells and the undifferentiated stigmatic surface presented the same characteristics, the style is folded, so the stigma was only slightly longer than the stamens (Figure 4I, J). In some samples male flowers were found near the peduncle and/or at the apex of the inflorescences. These male flowers had an atrophied carpel, which consists of one moderately developed ovary, and a poorly elongated style (Figure 4K). The parenchyma of the inflorescence is white, the perianth and peltate bract are of a reddish-brown color, while the stamens and gynoecium are yellow, which gives the characteristic color to the inflorescences in anthesis (Figure 4L).
Discussion
Despite the usefulness of floral organogenesis studies, this type of studies remains scarce for the Acacia s.l. genus. The most representative include the perianth ontogeny of some species (Ramírez-Domenech & Tucker 1990), and complete floral organogenesis of A. pycnantha (Buttrose et al. 1981), A. celastrifolia (Prenner 2011), A. berlandieri, A. pennatula and A. saligna (Gómez-Acevedo et al. 2007). For A. pennatula, the authors included two populations and found no difference in any aspect of the floral development. Similarly, the patterns of floral development in the two populations of A. cornigera studied here were alike. This indicates that the inception patterns and maturation of floral organs are not affected by the climatic characteristics where the plants inhabit. Acacia cornigera is distinguished by its thick, tapered cylindrical inflorescences (Janzen 1974, Seigler & Ebinger 1995). This study demonstrates that this characteristic is a result of the parenchyma growing during the inflorescence development. This species has more than 1,000 flowers per inflorescence (Janzen 1974), and the thickening of the parenchyma could be a response for supporting that large number of flowers.
Regarding the floral development found here for A. cornigera, the inception patterns of the calyx (irregular), corolla (simultaneous), androecium (acropetally in alternate sectors) and gynoecium (precocious), agree with previous reports for typical species of the Acacia genus (Gómez-Acevedo et al. 2007). A characteristic of the perianth found in A. cornigera, is the inception of more than five organs both in the calyx and in the corolla. It is important to remark that this feature does not affect the inception pattern; nevertheless, in mid stages, equalization occurs, which is an ontogenetic process by means of which the mature perianth is pentamerous (Ramírez-Domenech & Tucker 1990). Deviations from the pentamerous merosity in the perianth have been reported in other legumes as Ceratonia siliqua (Tucker 1992), Inga congesta, I. grandis, I. hispida (Paulino et al. 2017), Lecointea hatschbachii (Mansano et al. 2002), Stryphnodendron adstringens (Pedersoli & Teixeira 2016) and Swartzia dipetala (Paulino et al. 2013).
The presence of some male flowers at the base of the inflorescence indicates that A. cornigera is an andromonoecious species. The andromonoecy is a plastic response only when the later-developing flowers are functionally male (Miller & Diggle 2007), like in the species studied. Also, andromonoecy is considered a form of phenotypic plasticity, through which a plant can adjust the energy allocation to male and female functions in response to changes in resource availability (Miller & Diggle 2003), and a response to the ecological conditions and resources available for reproduction, increasing floral display and pollinators attraction (Reuther & Claβen-Bockhoff 2013).
On the other hand, myrmecophytic plants present conflicts of interest between their resident ants and their pollinators during the flowering periods. The ants may have a negative effect on plant fitness by decreasing the pollinator visitation time (Nicklen & Wagner 2006 and literature cited therein). In this sense, the persistence of the ants depends on the plant’s mechanisms and success in preventing them from visiting flowers at particular times, and in this way promoting the temporal separation between the activities of ants and pollinators. This is accomplished through the presence of floral repellents, either by means of specific structures (pollen and anther glands) or by emission of fragrances (Raine et al. 2002, Stone et al. 2003). There is only one study in the Acacia genus where pollen was evaluated as a repellent factor for ants; the case of the non-myrmecophyte A. constricta, where the ants avoid contact with pollen, which repels not only the resident ants, but also those belonging to three more species of visiting ants (Nicklen & Wagner 2006). In this study, no pollen was found in any of the mature inflorescences, because all the anthers were already open. The anthers of A. cornigera are eglandular and lack any conspicuous modification, so it is unlikely that they act as ant repellents.
Floral fragrances are considered the attractants and repellents per excellence (Junker & Blüthgen 2010, Tölke et al. 2019), although the latter function is more expected among plants that form mutualistic interactions with ants and have been characterized as the chemical compounds that facilitate access to pollinators (Willmer & Stone 1997). Petals are considered the main fragrance-emitting organs, and the structures involved are the epidermis (osmophores), trichomes and stomata (Marinho et al. 2014, Huchelmann et al. 2017, Tölke et al. 2019). In A. cornigera, the presence of stomata on mature bracts and petals could be indicative of their participation in the emission of volatile compounds either for attracting pollinators like in A. berlandieri (Gómez-Acevedo et al. 2007) or even to repel resident ants, which is consistent with ecological studies that reveal that ants do not approach mature inflorescences, even though they do patrol them at immature stages (Willmer & Stone 1997, Ghazoul 2001), but this fact needs to be studied in greater detail with anatomical and histochemical studies in both petals and bracts.
In conclusion, we can argue, that contrary to the vegetative characteristics, the floral organs of Acacia cornigera do not seem to have been affected by its relationship with ants, given that floral development (inception, elongation and maturation of organs) coincides with previous reports in non-myrmecophyic species of the Acacia genus, suggesting that these patterns are evolutionary stable. Nevertheless, the characters that are expressed in mature stages, such as the stomata on bracts and petals may be indicative of the presence of secretory structures, and hence, these pieces could be directly involved in resolving the conflict of interest between ants and pollinators during the flowering period, favoring in this way pollination.











 nova página do texto(beta)
nova página do texto(beta)



