Plant-pollinator interactions are reciprocal, as the pollinator has a direct effect in the plant and vice-versa. The floral features, such as color, size and shape are closely related to the pollinator attraction, due to the rewards offered by the plant (pollen, oils, fragrances, resins and nectar), in the process, the plant receives or donates pollen with every visit (Grajales-Conesa et al. 2011, Domínguez & Pérez 2013). The nectar is produced in nectaries located inside and/or outside the flowers. The nectaries located in any part of the flower are called floral nectaries, while those developed on vegetative structures of the plant re called extrafloral nectaries (Bernardello 2007). Floral nectaries are directly associated with pollination and reproductive efficiency (Richards 1986, Pacini et al. 2003, Irwin et al. 2004, Díaz-Castelazo et al. 2005, Villamil et al. 2013). On the other hand, extrafloral nectaries are generally associated to mutualist relationships among plants and animals, mainly insects providing protection against herbivores and receiving sugars as a reward (Heil & McKey 2003, Leins & Erbar 2010).
The nectaries produce sugar-rich fluids (mainly saccharose, glucose and fructose), also contain proteins (mainly enzymes like transglucosidases and transfructosidases), amino acids, lipids, organic acids, ascorbic acid, minerals, phosphates, alkaloids and vitamins (Fahn 1979, 1988, Leins & Erbar 2010). The nectaries can be composed of structural tissues (nectariferous parenchyma cells and glandular tissue associated with vascular bundles) or a group of glands (formed by secretory trichomes on the epidermis). Glandular tissues are composed of secretory parenchyma, epidermis (which can also be secretory) and a nearby vascular bundle (Pacini et al. 2003, Pacini & Nicolson 2007). If the epidermis is not one of the secretory tissues, then the nectar comes out of gaps in the cellular wall and cuticle, or through stomata that have lost the capacity of closing the pore because the guard cells are no longer contracting and lack chlorophyll (Leins & Erbar 2010). The cuticle in the nectary can be permeable through various structures, like pores or microchannels secreting nectar, or it can burst due to the pressure of nectar itself (Fahn 1979, Nepi 2007).
Cactaceae usually present floral nectaries, although some species have also extrafloral nectaries, mainly associated to areoles in stems (Buxbaum 1953), like in Opuntia stricta (Oliveira et al. 1999), Cylindropuntia acanthocarpa (Pickett & Clark 1979), Sclerocactus scheeri (Mauseth 1982), Ferocactus histrix (Del Castillo 1994) and F. cylindraceus subsp. lecontei (Ruffner & Clark 1986). Although the floral nectaries are present in the studied species of Cactaceae, the presence of nectar and characterization of nectaries have not been thoroughly proved and described. Most studies carried out on Cactaceae regarding floral nectaries provide information on their location and morphology, even though some of them deepen in their structure, anatomy, way of secreting nectar and chemical composition of sugars and other substances related to the process of pollination and their pollinator types, e.g. Hylocereeae and Rhipsalideae (Almeida et al. 2013), Polaskia chende, P. chichipe and Stenocereus quevedonis (Gudiño et al. 2015). Three different types of nectaries have been proposed for Cactaceae: a) Chamber; b) Annular, and c) Furrow nectaries (Buxbaum 1953), although this classification does not cover all the types of nectaries of the species within the family. For example, Fuentes-Pérez et al. (2009) studied five species of the genus Opuntia and they propose that the term “furrow” not apply to the type of nectaries they have. Therefore, the objective of the herein presented work is to study the location, morphology, anatomy and secretion of nectar in the floral nectaries of Strombocactus, an endemic genus of Mexico in risk of extinction.
The genus Strombocactus belong to the tribe Cacteae in the Cactoideae subfamily (Anderson 2001). It was described originally as Mammillaria by De Candolle (1828) and was later proposed as a monotypic genus by Britton & Rose (1922), Strombocactus disciformis (DC.) Britton & Rose. Currently, two species are recognized, S. disciformis (DC.) Britton & Rose (with the subspecies S. disciformis subsp. disciformis and S. disciformis subsp. esperanzae Glass & S. Arias), and S. corregidorae S. Arias & E. Sánchez (Arias & Sánchez-Martínez 2010). These species are distributed in a canyon system belonging to the states of Querétaro, Guanajuato, and Hidalgo (Hernández et al. 2004, Sánchez-Martínez et al. 2006). The species of Strombocactus have similar habitat: small rock crevices filled with fine soil, on steep slopes and vertical walls, and emerge in places with calcareous shales, preferably in areas devoid of vegetation or sparsely vegetated (Álvarez et al. 2004, Arias & Sánchez-Martínez 2010).
Materials and methods
Studied species. Twenty floral buds and 20 flowers in anthesis were analyzed from a minimum of 10 different individuals of every species of Strombocactus, during February and March of 2016 and 2017. Buds and flowers of S. disciformis subsp. disciformis and S. corregidorae were collected in the municipality of Cadereyta de Montes, Querétaro. We did not find S. disciformis subsp. esperanzae in their natural population in the municipality of Xichú, Guanajuato, for that buds and flowers were collected from the living collections of the Botanical Garden in the National Autonomous University of Mexico (UNAM) and the Regional Botanical Garden of Cadereyta, Querétaro. The samples were fixed in FAA (formaldehyde, ethylic alcohol 96 %, glacial acetic acid, distilled water; ratios 10:50:5:35, respectively) for 48 hours.
Floral morphology. The color and shape flower to each species was based on botanical descriptions. The anthesis type (diurnal/nocturnal) was described based on personal observations in the field and in the greenhouse in at least 30 flowers of each taxon.
Light microscopy and scanning electron microscopy. To know the anatomy of the floral nectaries and their micromorphology, the fixed material in FAA was washed with water to eliminate the excess of fixative, dissected and dehydrated in a gradual ethanol series (from 30 to 100 %) and embedded in Paraplast. The embedded material was cut in sections of 6 - 10 μm thick with an American Optical 820 rotatory microtome and stained with safranin and fast green. The obtained preparations were observed with an Olympus Provis AX70 microscope and microphotographs were taken. For nectary micromorphology, several flowers were dissected and dehydrated in a graded ethanol series (from 30 to 100 %). Afterwards, they were critically-point dried with CO2 in a critical-point dryer CPD-030 Bal-Tec, and then mounted on metallic sample holders with a carbon conducting tape and later covered in gold with a Denton Vacuum Desk-II ionizer so that they could be observed and photographed with the scanning electron microscope JSM-5310LV (Tokyo, Japan). Some of the photographs were artificially colored with the Adobe Photoshop CS6 software.
Histochemical tests. Four tests were carried out on the obtained sections of the embedded material in Paraplast: (1) Oil red “O” for lipidic reserves, (2) Periodic acid-Schiff (PAS) for insoluble polysaccharides, (3) Naphthol Blue Black - PAS for proteins and insoluble polysaccharides, respectively, and (4) Lugol for starch (López-Curto et al. 2005, Márquez-Guzmán et al. 2016). The obtained preparations in every histochemical sample were observed in an Olympus Provis AX70 microscope and microphotographies were taken.
Nectar concentration. The collection of nectar was carried out in February 2018 in plants of the living collection of the Botanical Garden (UNAM), for the three studied taxa. The samples of nectar were obtained at 3 pm from 15 flowers of different individuals of each taxon of Strombocactus. The extraction of nectar was done with an insulin syringe (commercial brand) and the concentration was measured using a refractometer Brix30 (Spectrum Technologies, Inc.). Degrees Brix are used as the measuring unit of dissolved solids, which correspond to the percentage of saccharose in a solution. One degree Brix is 1 gram of saccharose dissolved in 100 grams of solution. Therefore, a solution with 50 °Brix has 50 grams of saccharose (Suárez & Diana 2003).
Results
Floral morphology. The flowers of the three taxa of Strombocactus are infundibuliform and diurnal. The units of the perianth (tepals) of S. disciformis subsp. disciformis are white to yellowish white, abaxially magenta at the midvein (Figure 1A, B). The tepals in S. disciformis subsp. esperanzae are intense magenta, slightly paler towards the margins (Figure 1C, D). The units of the perianth in S. corregidorae can be intense or pale yellow, with reddish tones on the underside of the tepals (Figure 1E, F).

Figure 1 Flowers of Strombocactus. A, B. S. disciformis subsp. disciformis. C, D. S. disciformis subsp. esperanzae. E, F. S.corregidorae. A. Flower in anthesis. B. Flowers at early anthesis, the outer tepals with slight magenta color in the underside of the limb (arrows). C. Flower in anthesis. D. Flowers at the beginning of anthesis. E. Flower in anthesis. F. Flower at the end of anthesis, outer tepals slightly reddish on the underside (arrows).
Location and morphoanatomy of the floral nectary. The three taxa present a small and open chamber nectary, which is limited to the proximal end of the inner side of the receptacular tube (Figures 2A, D, G; 3A, D, G). The nectary is located underneath the insertion region of the innermost filaments of the perianth, specifically in the hypanthium. It presents a ring shape and in the upper region its ends are free and exerted showing widened and undulated protuberances, which longitudinally appear corniculate (Figure 3A, D, G), and more conspicuous in S. disciformis subsp. esperanzae (Figure 3D). The subnectariferous parenchyma is directly associated with vascular bundles that are parallel in the hypanthium, in the upper region of the ovary, and perpendicular to the nectary (Figure 3A, D, G). Underneath the epidermis there are approximately 7-12 layers of parenchymatic secretory cells, and several layers of associated parenchymatic, non-secretory cells, which are connected to the adjacent secretory cells; on the other end are located the vascular bundles (Figure 3A, D, G).
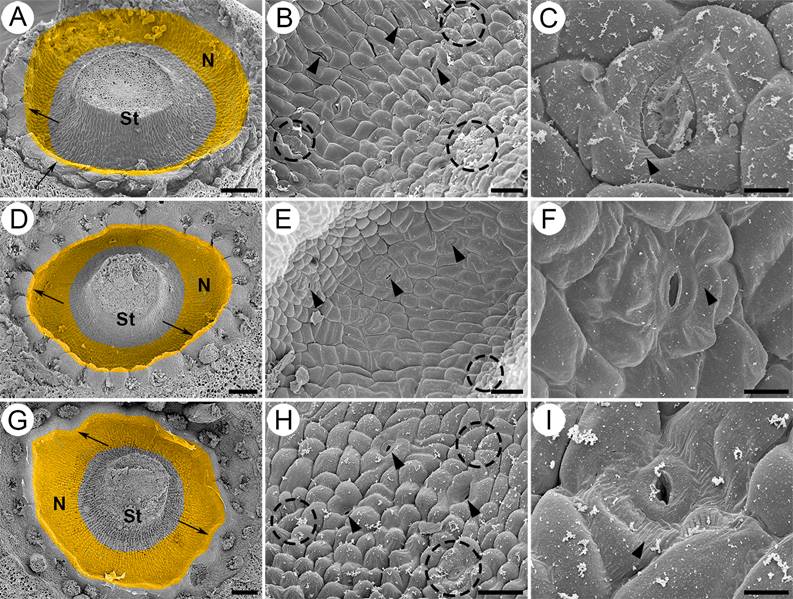
Figure 2 Micromorphology of the nectary in Strombocactus. A-C. S. disciformis subsp. disciformis. D-F. S. disciformis subsp. esperanzae. G-I. S. corregidorae. A, D, G. Front view of the nectary chamber (close-up). The secretory tissue is a ring underneath the insertion of the innermost filaments. The ring has an apical protuberance (arrows). B, E, H. Close-up of the epidermis of the nectary chamber; nectarostomata are observed (arrowheads); the epidermal cells are swollen in S. corregidorae (H) and less swollen in S. disciformis subsp. esperanzae (E); remains of the secretions are observed on the epidermal cells (dotted circles). C, F, I. Close-up of nectarostomata, which are open before anthesis; striated ornamentation is observed in the cuticle (arrowhead). N, nectary; S, style. Scale bars: A, D, G = 200 µm; B, E, H = 40 µm; C, F, I = 10 µm.

Figure 3 Longitudinal sections of flowers in anthesis of Strombocactus. A-C. S. disciformis subsp. disciformis. D-F. S. disciformis subsp. esperanzae. G-I. S. corregidorae. A, D, G. The nectary below the insertion region of the innermost filaments, the arrows indicate the location of the nectary, the arrowheads indicate the vascular bundles. B, E, H. Both the nectariferous and subnectariferous parenchyma, the arrowheads indicate vascular bundles. C, I. Sections of nectarostomata, showing the subnectarostomic cavity (arrow), intercellular space (arrowheads). F. Enhancement of E; secretory cells with intercellular spaces (arrowheads). E, epidermis; F, filament; Np, nectariferous parenchyma; O, ovary; Sc, secretory cells; Sp, subnectariferous parenchyma; St, style. Scale bars: A, D, G = 200 µm; B, E = 100 µm; C, I = 12 µm; F, H = 40 µm.
The nectary of the three taxa of Strombocactus is composed of an epidermis of isodiametric cells, slightly bulked towards the exterior (Figure 2B, E, H), being more bulked in S. corregidorae (Figure 2H) and less in S. disciformis subsp. esperanzae (Figure 2E). Throughout the epidermis, there are several open stomata prior and during the anthesis, which is the secretory period of nectar, showing sugar remains even after the process of fixation, washing and dehydration of the material during the SEM process (Figure 2B, C, E, F, H, I). The cuticle of the epidermal cells exhibits slightly striated ornamentation (Figure 2C, F, I). The epidermis is unistratified, with vacuolated cells and thin walls (Figure 3B, E, F, H), under the stomata, there are large substomatal chambers (Figures 3C, I; 4F, G; 6B).

Figure 4 Histochemical tests of the nectary of S. disciformis subsp. disciformis. Longitudinal (A-C, H-J, L, O) and transversal (D-G, K, M, N) sections. A-C. Oil red “O” test. Only the cuticle presented lipids (arrows). D-I. The test of Periodic acid-Schiff (PAS) was positive for insoluble polysaccharides in nectary, style and superior wall of the ovary. The arrowheads indicate vascular bundles. G. A nectarostome. J, K. Lugol test was positive for starch in the style and non-nectariferous parenchyma of the hypanthium. The arrowheads indicate vascular bundles. L. The Maltese cross was observed with polarized light, which indicates the presence of starch in the same sites as the lugol test. The arrowheads indicate vascular bundles. M-O. Naphtol Blue Black-PAS test positive for proteins and insoluble polysaccharides in nectary. E, epidermis; N, nectary; Ne, nectarostome; Sc, secretory cells; Sp, subectariferous parenchyma; St, style. Scale bars: A, D, E, N = 50 µm; B, F, K = 25 µm; C, G = 12 µm; H, I, J, L, M, O = 100 µm.
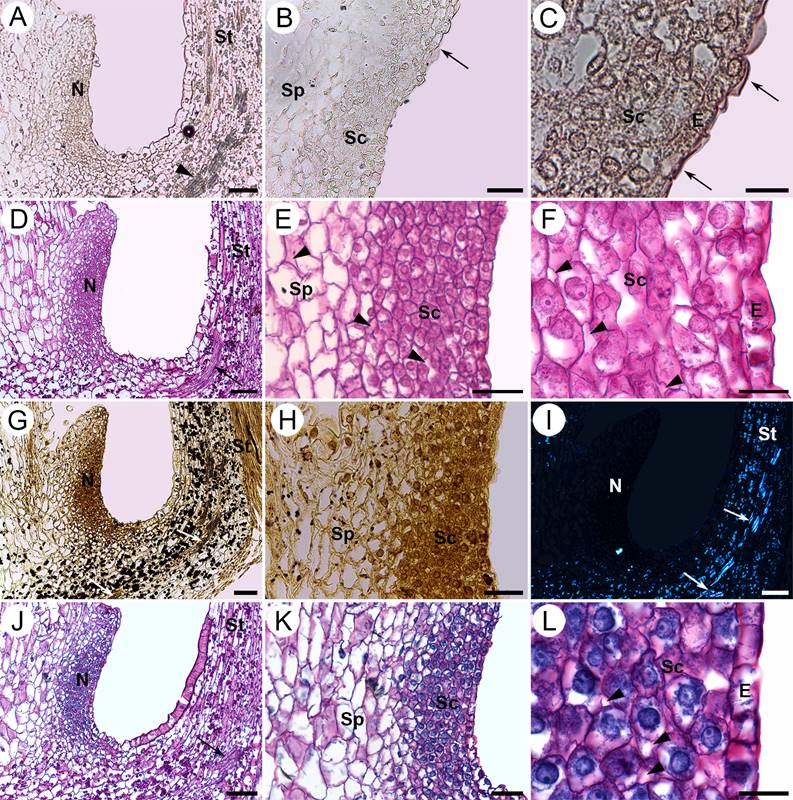
Figure 5 Histochemical tests of the nectary of S. disciformis subsp. esperanzae. Longitudinal sections. A-C. Oil red “O” test, in which the arrows indicate the cuticle with positive result for waxes. The arrowheads indicate a vascular bundle. D-F. Periodic acid-Schiff (PAS) test was positive for insoluble polysaccharides in the nectary, style and superior wall of the ovary. The arrow indicates a vascular bundle. The arrowheads indicate intercellular spaces. G, H. Lugol test, was positive for starch in the style and non-nectariferous parenchyma located in the hypanthium. The arrows indicate vascular bundles. I. Under polarized light, starch grains appear bright showing the Maltese cross in the same sites in which the lugol test was positive. The arrows indicate vascular bundles. J-L. Naphthol Blue Black - PAS test was positive for proteins and insoluble polysaccharides in the nectary. The arrow indicates a vascular bundle. The arrowheads indicate intercellular spaces. E, epidermis; N, nectary; Sc, secretory cells; Sp, subnectariferous parenchyma; St, style. Scale bars: A, D, G, I, J = 80 µm; B, E, H, K = 40 µm; C, F, L = 16 µm.

Figure 6 Histochemical tests of the nectary of S. corregidorae. Longitudinal (A, B, D, E, H-M) and transversal (C, F, G, N, O) sections. A-C. Oil red “O” test, in which the arrows indicate the cuticle which was positive for the presence of waxes. B. Enhancement of a nectarostome. D-G. Periodic acid-Schiff (PAS) test was positive for insoluble polysaccharides in the nectary, style and upper region of the ovary. The arrows indicate vascular bundles. The arrowheads indicate intercellular spaces. H-I. Lugol test was positive for starch in style and non-nectariferous parenchyma located in the hypanthium. The arrow indicates a vascular bundle. J. The Maltese cross was observed under polarized light, indicating the presence of starch in the same places as in the lugol test. The arrow indicates a vascular bundle. K-O. Naphthol Blue Black - PAS test was positive for proteins and insoluble polysaccharides in the nectary. The arrows indicate vascular bundles, the arrowheads, intercellular spaces. E, epidermis; N, nectary; Ne, nectarostome; Sc, secretory cells; Sp, subnectariferous parenchyma; St, style. Scale bars: A, C, E, I, L = 40 µm; B, G, M, O = 16 µm; D, H, J, K = 100 µm; F, N = 20 µm.
Under the epidermis there is the nectariferous secretory parenchyma (Figures 3B, E, F, H; 4C, E, N; 5E, H, K; 6F, N), which comprise isodiametric cells, small and very compact, with thin walls, large and central or eccentric nucleus, patent vacuoles and dense cytoplasm; there are also some intercellular spaces (Figures 3C, F; 5F, L; 6G, O). The secretory tissue of the nectary is 10-12 layers thick in S. disciformis subsp. disciformis (Figures 3B; 4E, F); 8-9 in S. disciformis subsp. esperanzae (Figures 3E, F; 5E H) and 7-8 in S. corregidorae (3H; 6F, N). The subnectariferous parenchyma is subjacent to the nectariferous secretory parenchyma, whose cells are distinguished for being larger than the ones of the nectariferous parenchyma (Figures 3B, E, H; 4E, N; 5E, H, K; 6F, I, N). These cells present thin walls, conspicuous vacuoles, poorly dense cytoplasm and eccentric nucleus (Figures 3F; 4E, N; 6N). This parenchyma presents intercellular spaces and abundant nearby vascular bundles, with both xylem and phloem (Figures 4D, E, M, N; 5E; 6F, N).
Histochemical tests. The test of oil red “O” for cuticle of the epidermal cells in the nectary of the three species of Strombocactus was positive (Figures 4A-C; 5A-C; 6A-C), due to the waxy compounds it presents, although no other structure that presented lipidic compounds (Table 1).
Table 1 Histochemical tests in the nectary of the genus Strombocactus. NBB-PAS, Naphthol Blue Black-Periodic acid-Schiff; Np, nectariferous parenchyma; PAS, Periodic acid-Schiff; Sp, subnectariferous parenchyma. Test was positive (+) or negative (-).
| Taxon | S. disciformis subsp. disciformis | S. disciformis subsp. esperanzae | S. corregidorae | |||
|---|---|---|---|---|---|---|
| Histochemical tests / Tissue type | Np | Sp | Np | Sp | Np | Sp |
| Oil red “O” | - | - | - | - | - | - |
| PAS | + | + | + | + | + | + |
| Lugol | - | - | - | - | - | - |
| NBB-PAS | + | + | + | + | + | + |
With the PAS test applied on flowers prior and during anthesis of the three taxa, there were abundant insoluble polysaccharides in the cellular walls and the cytoplasm of the nectariferous parenchyma, indicating its secretory features, and they were scarce in the subnectariferous parenchyma (Figures 4D-G, I; 5D-F; 6D-G; Table 1). These compounds were also abundant in the style parenchyma and the upper region of the ovary (Figures 4H; 5D; 6D). The presence of starch in the style and non-nectariferous parenchyma of the hypanthium was corroborated with the Lugol test (Figures 4J, K; 5G, H; 6H, I), which could suggest a storage function of this tissue. A distinctive Maltese cross was observed under polarized light, which corroborates the presence of starch in these tissues (Figures 4L; 5I; 6J).
After the Naphthol Blue Black - PAS test for proteins and insoluble polysaccharides, all three taxa presented high amounts of proteins in the cytoplasm of the secretory cells compared to those of the subnectariferous parenchyma (Figures 4M-O; 5J-L; 6K-O; Table 1), which indicate greater activity in the secretory tissue.
Concentration of nectar. The secretion of nectar in the species of Strombocactus is too little to be quantified, even though it can be observed under stereoscopy (Figure S1). The average concentration of solutes in the nectar of S. corregidorae was 16.12 ± 4.48 °Brix, in S. disciformis subsp. disciformis was 22.09 ± 7.42 °Brix and in S. disciformis subsp. esperanzae was 8.65 ± 3.98 °Brix.
Discussion
The nectaries of Strombocactus had not been thoroughly studied before. Only Buxbaum (1953) mentioned information regarding S. disciformis. In the genus Strombocactus, the nectary forms a ring which is located surrounding the base of the style, below the insertion region of the innermost series of filaments. Therefore, the nectary chamber is part of the hypanthium and collects the nectar, which together with the pollen itself are the greatest rewards for pollinators (Simpson & Neff 1983, Proctor et al. 1996, Domínguez & Pérez 2013).
According to the classification of Bernardello (2007), the nectary in the species of Strombocactus corresponds to a hypanthial nectary, due to its location underneath the insertion of the innermost filaments, surrounding the style. According the classification of Buxbaum (1953), three types of nectary (chamber, disc, and furrow) are recognized for different Cactaceae. Chamber type is found in some genera of Cactoideae (Buxbaum 1953, Fuentes-Pérez 2004, Urías 2009, Almeida et al. 2013, Torres-Sánchez 2013, Gudiño et al. 2015), while the nectary type disc has been reported for some species of Maihueniopsis, Opuntia and Maihuenia (Fuentes-Pérez 2008). The furrow nectary has a half-open or closed chamber due the curved base of the innermost stamens or by a protuberance of the receptacular hypanthial tissue (Buxbaum 1953), and it has been reported for some species of the tribes: Rhipsalideae (Almeida et al. 2013), Trichocereeae and Cacteae, including Strombocactus (Buxbaum 1953). However, based on our results the term “furrow” cannot be used to describe the nectary because the innermost stamens are neither curved nor forming a protuberance, as in Hatiora gaertneri, Lepismium cruciforme and L. warmingianum (Almeida et al. 2013). The three taxa of Strombocactus present a ring with bulky protuberances in the upper edge, which corroborates this structure as an annular nectary based on our results, similar to that of Rhipsalis (Almeida et al. 2013). We also consider essential to revise once again the type of nectary present in the genera related to Strombocactus (e.g., Turbinicarpus s.s., Ariocarpus, Epithelantha; Vázquez-Sánchez et al. 2013), as the flowers are equally small, with short receptacles, which could eventually be a feature of taxonomic importance.
As for the anatomy, the nectary of the species of Strombocactus is composed of epidermis with nectarostomata, a nectariferous parenchyma with numerous vascular bundles associated to the subnectariferous parenchyma. This arrangement and features of the subtending cells of the epidermis are typical of the floral nectaries described in some species of Cylindropuntia, Opuntia, Pterocactus, Pereskiopsis, Maihuenia, Maihueniopsis, Tephrocactus, Tunilla (Fuentes-Pérez 2008), Epiphyllum (Almeida et al. 2010, 2013), Neobuxbaumia (Torres-Sánchez 2013; currently knowkn as Cephalocereus, Tapia et al. 2017), and Stenocereus (Urías 2009).
It is noteworthy that the epidermal cells on nectarial tissue of the three taxa of Strombocactus does not show tannins, as in other species of columnar cactus like Escontria chiotilla, Cephalocereus mezcalaensis, Stenocereus pruinosus (Fuentes-Pérez 2004), C. tetetzo, C. columna-trajani (Torres-Sánchez 2013) and some species of Opuntia and Tephrocactus (Fuentes-Pérez 2008). The latter genera have large flowers, with more open floral tubes developing on stems with more magnificent exposition to solar radiation, in contrast with the taxa of Strombocactus, whose size barely surpasses the soil and is many times found in association with a nurse plant.
The nectar in the species of Strombocactus is secreted through modified stomata called nectarostomata, which cannot regulate its opening or closure (Nepi 2007). The discharge of nectar through nectarostomata is the most common way of secretion. According to Leins & Erbar (2010), these are stomata whose occlusive cells lack chloroplasts and are not functional in gas exchange, and they are present in many dicotyledons (Bernardello 2007). The nectar secreted by nectarostomata is frequent in Cactaceae (Fuentes-Pérez 2004, Almeida et al. 2013, Torres-Sánchez 2013, Gudiño et al. 2015). It has also been reported the secretion of nectar through secretory trichomes in Disocactus ackermannii, Epiphyllum guatemalense and Hylocereus undatus (Almeida et al. 2013), across pores in Polaskia chichipe, and through fissures in the cuticle in P. chende (Gudiño et al. 2015).
During the study of the species of Strombocactus and given the density of the cytoplasm, size of the vacuoles, large nucleus and numerous intercellular spaces in the nectariferous parenchyma (secretory cells), the tissue is considered to present merocrine secretion, meaning that the cells remain alive during this process without disintegrating tissue (Fahn 1979, Nepi 2007). The Naphtol Blue Black test indicates great metabolic activity of these cells, as is it proves the presence of abundant proteins. On the other hand, starch was not detected, probably because this is not a storage tissue and because carbohydrates break down rapidly to produce nectar or other components, which is the case of Platanthera chlorantha (Orchidaceae, Stpiczynska et al. 2005).
Regarding the characteristics of the nectar in Strombocactus species (low volume and percentage of saccharose < 33 %), it is necessary detailed analysis its chemical composition and its temporal production dynamics to generate a more concrete idea of how these characteristics are associated with pollinators as suggested by Baker & Baker (1983, 1990), Cruden et al. (1983) and Nicolson & Thornburg (2007) in other species. However, based on floral characteristics of the three Strombocactus taxa studied, as diurnal anthesis, infundibular shape flower, color flower, nectar and pollen as rewards, these attributes indicate that they can be pollinated by bees, as proposed by Faegri & Van Der Pijl (1979), Fenster et al. (2004) and Domínguez & Pérez (2013) based on the flower features associated with the pollination syndromes.











 nueva página del texto (beta)
nueva página del texto (beta)


