Wetlands are among the most threatened ecosystems of the world (Zedler & Kercher 2005, Cui et al. 2012). Estimates over the last decades put wetland loss as high as 60 % worldwide (Davidson 2013), and 62 % for Mexico (Landgrave & Moreno-Casasola 2012). Mexico is a megadiverse country (Declaración de Cancún 2002) and a biodiversity conservation hotspot (Myers et al. 2000) that harbors 21,841 flowering plant species (Villaseñor & Ortiz 2014). According to the National Wetland Inventory (Dumac 2017), almost 6 % (128,000 km) of the Mexican territory is occupied by wetlands. There are 139 Ramsar sites in Mexico, which makes it the neotropical country with the highest increase in internationally protected wetlands in recent decades (Mauerhofer et al. 2015). Mexico has 1,283 aquatic and subaquatic angiosperms, of which 157 are endemic to the country (Villaseñor & Ortiz 2014). As to strictly aquatic plants, there are 240 species (Mora-Olivo et al. 2013). According to Lot et al. (1993) Mexico has 747 of vascular aquatic plants. Aquatic plants may belong to the groups Charophyta, Briophyta, Pteridophyra, Gymnosperms and Angiosperms (Lot 2012), and are a major component of aquatic ecosystems (Dar et al. 2014).
Temporary wetlands span over approximately 0.81 million km2 of the Earth’s surface (Pekel et al. 2016). They undergo severe changes in water saturation levels, and at times can dry completely (Martínez & García 2001). Temporary wetlands are dynamic and can change in shape and size (Frohn et al. 2009). They function as a connection among different ecosystems, either terrestrial or aquatic (Aavik et al. 2013, Ishiyama et al. 2014, Uden et al. 2014), provide ecosystem services (Marton et al. 2015), and substantially contribute in maintaining biodiversity (Balian et al. 2008). Temporary wetlands harbor almost 73 % of the aquatic plants and 31 % of the strictly aquatic plants in Mexico (Mora-Olivo et al. 2013). In particular, temporary wetlands in central Mexico are highly diverse ecosystems (Rico-Romero 2015). The largest number of aquatic plants is concentrated at lower altitudes (Rzedowski 1978), but at least 147 of the Mexican strictly aquatic plants populate wetlands located above 1,000 m a.s.l. (Mora-Olivo et al. 2013). Scientific studies of temporary wetlands are scarce, which contributes to habitat loss. In such a context, floristic inventories of temporary wetlands contribute to the knowledge and conservation of a rapidly disappearing ecosystem (Calhoun et al. 2016). The objectives of our paper are to determine the floristic composition and analyze the level of similarity among temporary highland wetlands in central Mexico.
Materials and methods
The 39 studied temporary wetlands range in elevation from 1,900 to 2,700 m a.s.l. in the states of Aguascalientes (localities 1-12), Guanajuato (13-18), Jalisco (19-22), Michoacán (23), Querétaro (24-32), San Luis Potosí (33, 34), and Zacatecas (35-39, Figure 1, Appendix 1, Figure 2). Wetlands were located using bibliographical references, by field trips, and through the support of local researchers. The areas lie between the latitudes of 20º and 24º North, and longitudes 100º and 103º West (Appendix 1), in the Mexican Transvolcanic Belt and the Mexican Plateau. Weather is semiarid temperate, and temperate subhumid, with the following Koeppen classifications: ‘BS1kw’, ‘C(wo)’, and ‘C(w1)’. Mean annual temperature ranges from 12 ºC to 18 ºC. Mean annual precipitation is 600 to 800 mm with the highest precipitations in June or July. Natural vegetation surrounding the wetlands is composed of oak forests and grasslands with agricultural activities present (INEGI 2017, CONABIO 2017). Water parameters were as follows (averages): pH 5.98, dissolved oxygen 4.87 mg/L, conductivity 126 µSTm, resistivity 0.027 mΩ, total dissolved solids 62 ppm, and salinity 0.06 PSU (Appendix 1).
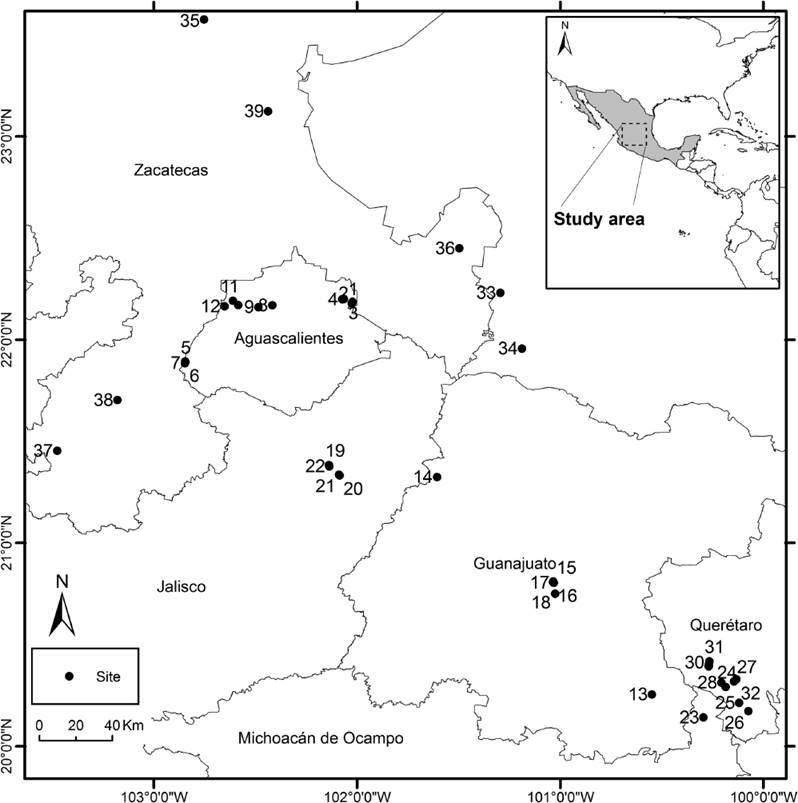
Figure 1 Map of studied temporary wetlands located in the highlands of Aguascalientes, Guanajuato, Jalisco, Michoacán, San Luis Potosí, Querétaro and Zacatecas States, central Mexico.

Figure 2 A) Site 15 in Guanajuato, October 2015. B) Site 15 starting to became dry in Guanajuato, December 2016. C) Site 7 in Aguascalientes, October 2016. D) Site 34 in San Luis Potosí, October 2016. E) Site 35 in Zacatecas, October 2015. F) Site 32 in Querétaro, October 2016. G) Site 29 surrounded by houses in Querétaro, October 2016. H) Site 26 it was covered with soil and asphalt during 2016 in Querétaro
Plant specimens were collected in the 39 wetlands from August 2015 to November 2016. Collection and herborization followed Lot (1986). Vouchers were deposited at Autonomous University of Queretaro Herbarium “Jerzy Rzedowski”, QMEX with duplicates to be distributed in Mexico (CIIDIR, IBUG, MEXU, SLPM, and XAL) and Brazil (LUSC), acronyms according to Thiers (continuously updated). Family classification for ferns followed Smith et al. (2006), and Angiosperm Phylogeny Group IV (APG 2016) for angiosperms. Nomenclature followed the International Plant Name Index (IPNI 2017). We used the concept of Lot et al. (1986, 1993) to define life form (rooted emergent, rooted submersed, rooted floating, free floating, free submersed), and affinity as aquatic plant, which includes three categories (strictly aquatic, subaquatic, and tolerant). Strictly aquatic plants definition followed Mora-Olivo et al. (2013), subaquatic are according to Lot et al. (2013) for monocots and Lot et al. (2015).
We elaborated a presence/absence list per site, and calculated the floristic distance (Jaccard 1908, Krebs 1999) and the floristic correlation among wetlands (Friendly 2002). Graphs and maps were elaborated with ArcGIS® version 9.3 and R version 3.31 (R Development Core Team 2017), through corrplot, vegan packages (Wei y Simko 2017)
We included geographical and altitudinal distribution (Mora-Olivo et al. 2013, Lot et al. 2013, Lot et al. 2015, GBIF 2017, Tropicos 2017), conservation status: either national or international (SEMARNAT 2010, IUCN 2017), use: biofilters, medicinal (Medline 2017), and finally, weeds were defined as such if they are included in Villaseñor & Espinosa-García (1998). Records for the Mexican states were considered as new if the species was not included in Mickel & Smith (2004) for ferns, Lot et al. (2013) for monocotyledons, Flora del Bajío (Rzedowski & Rzedowski 2017) for several families, and Martínez et al. 2017 for Solanaceae.
Results
We found 126 species of 80 genera and 39 families (Appendix 2). Three were Charophyta, four Pteridophyta, and 119 Angiosperms. The richest family was Cyperaceae (27 species), followed by Asteraceae (17), Poaceae (16), Plantaginaceae (5), Fabaceae (5), and Juncaceae (4, Figure 3-A). The genera with the highest number of species were Eleocharis (16 species, Figure 4-A), Cyperus (8), and Juncus (4). The rest of the genera had three species or less. Persicaria mexicana had the widest distribution (32 localities, Figure 3-B, Figure 4-B), followed by Marsilea mollis (28 localities), Luziola fluitans (26 localities, Figure 4-C), Heteranthera peduncularis (25 localities, Figure 4-D), Nymphoides fallax (25 localities, Figure 4-E), Eleocharis macrostachya (24 localities), and Paspalum distichum (23 localities). Thirty-six species (28 %) were found in only one locality. Life forms were emergent (93 species), submersed (14 species), rooted floating (13 species), free floating (five species) and free submersed (one species). We found 49 strictly aquatic, 21 subaquatic, 38 tolerant, and 18 with no previous record as aquatic plant. All plants were herbaceous.
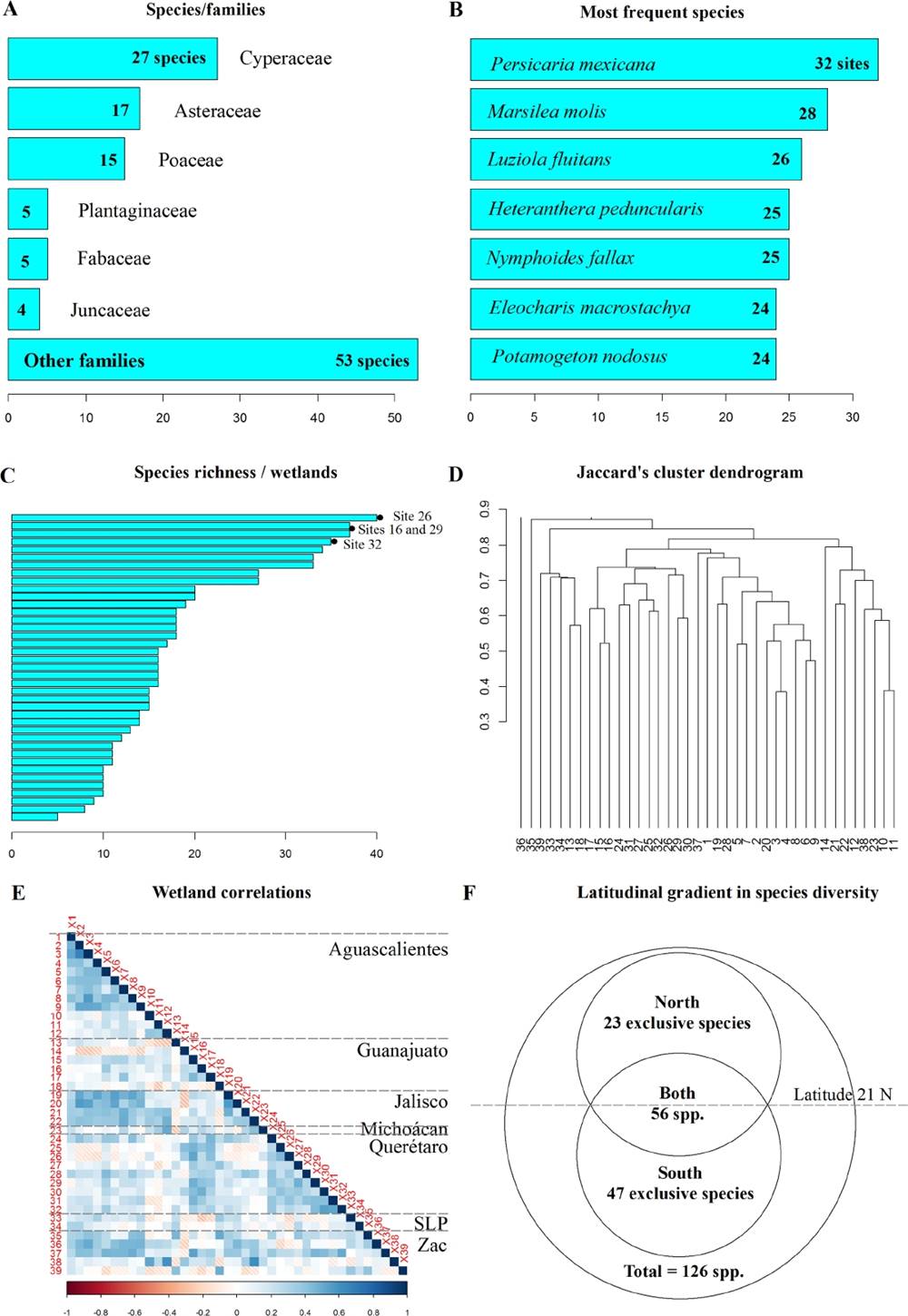
Figure 3 A) Specie richest families; B) More frequent species; C) Number of species per locality; D) Dendrogram cluster by Jaccard; E) Floristic correlation between localities; F) Specie distribution in higher and lower latitudes from 21º N.

Figure 4 A) Eleocharis densa site 27, richest genus. B) Persicaria mexicana site 13, most frequent specie. C) Luziola fluitans site 16, recurrent specie. D) Heteranthera peduncularis site 8, recurrent specie. E) Nymphoides fallax site 38, recurrent specie. F) Potamogeton nodosus site 2, endangered. G) Nymphaea gracilis site 21, endangered. H) Azolla microphylla site 25, multiple economic use.
The richest wetland had 40 species (site 26 in Querétaro, Figure 3-C), followed by 37 (sites 16 in Guanajuato and 29 in Querétaro). However, one of the wetlands had only five species (site 36 in Zacatecas). The surveyed wetlands had a level of similarity among themselves (Figure 3-D). Wetlands with the highest correlations among them were those from Querétaro (sites 24, 25, 26, 27, 29, 30, 31, and 32, Figure 3-E), followed by those of Aguascalientes (sites 1, 2, 3, 4, 5, 6, 7, 8 and 9, Figure 3-E). Aguascalientes localities also showed a high correlation with Jalisco’s Highlands (sites 19, 20, 21, and 22, Figure 3-E) and southern Zacatecas (site 36 and 37, Figure 3-E). In addition, Querétaro localities showed a correlation with Guanajuato (Figure 3-E). We observed the existence of a latitudinal gradient and found 47 species growing only at the lowest latitude wetlands (Figure 3-F). We only sampled 15 of the 39 wetlands at latitudes lower than 21°, but we found 103 of the 126 species (82 %) on these wetlands, 47 of which were exclusive. Wetlands higher than 21° latitude presented 79 species (63 %), and only 23 were exclusive. Forty-eight species are endemic to the MegaMéxico region: Callitriche heterophylla, Eleocharis densa, E. ignota, E. reznicekii, E. tenarum, E. yecorensis, Eragrostis plumbea, Eriocaulon bilobatum, Galium proliferum, Glandularia teucriifolia, Helenium mexicanum, Heteranthera peduncularis, Heterosperma pinnatum, Isoetes mexicana, Jaegeria glabra, J. purpurascens, Karinia mexicana, Lobelia fenestralis, L. irasuensis var.fucata, Luziola fluitans, Marsilea mollis, Nierembergia angustifolia, Nymphaea gracilis, Nymphoides fallax, Plantago linearis, Polygala alba, P. subalata, Potamogeton diversifolius, Rorippa mexicana, Sagittaria demersa, Schkuhria schkuhrioides, Sisyrinchium convolutum, Sporobolus atrovirens, Stevia eupatoria, Tagetes lucida, T. pringlei, Trifolium wormskioldii, Tripogandra purpurascens, Utricularia perversa, and Verbena carolina. Nineteen species are cosmopolitan. Two species are introduced to Mexico: Egeria densa, and Glyceria fluitans.
We found 19 species that can occur in low and high elevations: Diplachne fusca, Echinochloa crus-galli, Egeria densa, E. minima, E. montana, E. parishii, E. schaffneri, E. yecorensis, Heteranthera limosa, H. peduncularis, Juncus arcticus, Lemna minuta, Ludwigia octovalvis, L. peploides, Najas guadalupensis, Paspalum distichum, Potamogeton nodosus, and Schoenoplectus californicus, and Triglochin scilloides; 24 species occur only above > 1,000 m a.s.l.: Callitriche heterophylla, Echinochloa crus-pavonis, E. oplismenoides, Eleocharis aciculares, E. densa, E. ignota, E. macrostachya, Eriocaulon bilobatum, Glyceria fluitans, Isoetes Mexicana, Jaegeria glabra, Juncus dichotomus, J. ebracteatus, J. microcephalus, Karinia mexicana, Lemna gibba, L. obscura, Limosella aquatica. Luziola fluitans, Nymphoides fallax, Potamogeton diversifolius, Sagittaria demersa, Sisyrinchium convolutum, and Tripogandra purpurascens. We did not find information on altitudinal distribution for the rest of the species collected in this study.
Twenty seven species were listed as threatened, 25 of which are on the international list (IUCN 2017) in the “least concern” category: Azolla microphylla, Callitriche heterophylla, Cyperus esculentus, Diplachne fusca, Distichlis spicata, Echinochloa crus-galli, Elatine brachysperma, Eleocharis aciculares, E. atropurpurea, E. densa, E. macrostachya, Glyceria fluitans, Hippuris vulgaris, Lemna gibba, L. minuta, Limosella aquatica, Ludwigia octovalvis, Najas guadalupensis, Paspalum distichum, Poa annua, Polygonum punctatum, Potamogeton nodosus (Figure 4-F), Setaria parviflora, Trifolium amabile, and Triglochin scilloides. Two species, Nymphaea gracilis (Figure 4-G), and Trifolium wormskioldii, are listed by SEMARNAT (2010) as threatened.
As to the actual or potential uses of the species, 21 have economic importance, 12 as medicinal, and eight as biofilter/ biofuel, and one with both purposes (Azolla microphylla, Appendix 2, Figure 4-H). Medicinal: Azolla spp. have antibacterial activity (Abraham et al. 2015), also Cosmos bipinnatus has the same medicinal property (Olajuyigbe & Ashafa 2014, Sohn et al. 2013). The oil of Baccharis salicifolia is a natural repellent (García et al. 2005). Bidens aurea acts like omeprazole (De la Lastra et al. 1994). Ludwigia octovalvis is used against cancer (Chang et al. 2004). Polygonum punctatum has antibiotic, anti-inflammatory and anti-hyperalgesic properties (Alves et al. 2001). Rumex crispus has sun protection properties (Demirezer & Uzun 2016). Schkuhria schkuhrioides is antimicrobial (Delgado et al. 1998). Stevia eupatoria has anti-mutagenic and anti-oxidant properties (Cariño-Cortés et al. 2007). Symphyotrichum subulatum has anti-inflammatory properties (Lee et al. 2012). Tagetes lucida has medicinal properties as anti-depressive (Bonilla-Jaime et al. 2015) and T. micrantha has diverse medicinal properties (Linares & Bye 1987). Biofilter/ biofuel: Azolla filiculoides and A. microphylla are attractive species for the production of renewable biofuels (Miranda et al. 2016). Egeria densa can remove heavy metals from the environment (Tsuji et al. 2017), as can Eleocharis aciculares, E. macrostachya, and, E. montana (Ha et al. 2011, Olmos-Márquez et al. 2012). Lemna gibba, L. obscura, L. minuta are indicated for phytoremediation of contaminated water (Gallardo-Williams et al. 2002, Gür et al. 2016, Di-Baccio et al. 2017).
Forty four species were recorded as Mexican weeds: Allium glandulosum, Baccharis salicifolia, Bahia absinthifolia, Bidens aurea, Cosmos bipinnatus, Cuphea wrightii, Cyperus esculentus, C. flavescens, C. virens, Dalea foliolosa, Echinochloa crus-galli, E. crus-pavonis, Egeria densa, Eleocharis aciculares, E. montana, Glandularia teucriifolia, Glyceria fluitans, Helenium mexicanum, Heteranthera limosa, Lemna gibba, L. minuta, L. obscura, Ludwigia octovalvis, L. peploides, Najas guadalupensis, Nothoscordum bivalve, Paspalum distichum, Plantago linearis, Poa annua, Polygonum punctatum, Potamogeton diversifolius, P. nodosus, Pycreus niger, Rorippa mexicana, Rumex crispus, Schkuhria schkuhrioides, Setaria parviflora, Sisyrinchium convolutum, Sporobolus indicus, Tagetes lucida, Tagetes micranta, Trifolium amabile, Tripogandra purpurascens, and Verbena carolina.
Two species probably yet undescribed of the genus Eleocharis were found at site 7, Aguascalientes, and site 35, Zacatecas (S. González pers. comm.). New records of 20 species were found for the following Mexican states: Aguascalientes: Eleocharis parishii, E. reznicekii, Eriocaulon bilobatum, Isoetes mexicana, Lemna oscura, Potamogeton nodosus, and Schoenoplectus californicus. Guanajuato: Eleocharis tenarum, E. yecorensis, Echinocloa oplismenosides, Eriocaulon bilobatum, Juncus ebracteatus, and Lemna minuta. Michoacán: Jaegeria purpurascens. Querétaro: Azolla microphylla, Eleocharis ignota, and Lemna oscura. San Luis Potosí: Lemna oscura. Zacatecas: Elatine brachysperma, Eriocaulon bilobatum, Heteranthera limosa, Isoetes mexicana, Juncus arcticus, Marsilea mollis, and Nierenbergia angustifolia.
Discussion
In several wetland studies, Asteraceae, Cyperaceae and Poaceae arise as the most important families and emergent species stand out as the most abundant life form in wetlands. (Pott & Pott 2000, Rolon et al. 2010, Magalhães et al. 2016). Cyperaceae and Poaceae are among the richest aquatic monocotyledons plant families in Mexico (Lot et al. 2013). Aquatic Cyperaceae have morphological adaptations that enables them to survive drought spells (Rocha & Martins 2011). As to distributions, aquatics plants frequently are cosmopolitan, but a few only prosper in specific environments and are endemic (Rzedowski 1978). Cyperaceae and Poaceae also have a high endemism among Mexican aquatic plants (Lot et al. 2013). Allium glandulosum, Azolla filliculoides, Eleocharis ignota, Hippuris vulgaris, Jaegeria glabra and Sagittaria demersa are some of the 47 species registered only below 21° N. S. demersa is endemic to MegaMexico and is considered rare, or even threatened (Lot et al. 2002). The Cyperaceae Eleocharis parashii, E. atropurpurea, E. coloradoensis, E. minima, E. reznicekii and Schonoplectus californicus are among the 47 species registered above 21° N latitude. Some of the 56 species are present at both north and south of latitude 21° N were Callitriche deflexa, C. heterophylla, Eleocharis aciculares, E. densa, E. dombeyana, E. macrostachya, E. montana, E. schafenerri, E. tenarum, E. yecorensis, Eriocaulon bilobatum, Heteranthera limosa, H. peduncularis, Najas guadalupensis, Nymphoides fallax, Triglochin scilloides, and Utricularia perversa. Species with restricted distribution to Mexico or Central America are Eleocharis reznicekii, Eriocaulon bilobatum, Sisyrinchium convolutum (Lot et al. 2013), Utricularia perversa, and Nymphoides fallax (GBIF 2017, Tropicos 2017). Altitudinal distribution presented species which strictly occur at higher elevations, and others that are able to develop at both low and high elevations. Rzedowski (1978) and Mora-Olivo et al. (2013) suggest that there is a pattern of lower species diversity at higher elevation wetlands. However, there are two possible explanations for such a perception: 1) highland wetlands are under-detected and under-collected because many have a temporary water regime, and 2) at higher elevations terrain slopes hinder large water areas and many of the wetlands occupy small areas.
Differences in floristic composition found among sampled wetlands can also be explained by the surrounding vegetation cover and land use, as well as by physical and chemical water characteristics (Declerck et al. 2006, Lacoul & Fredman 2006, Ot’ahel’ová et al. 2007, Dar et al. 2014, Lu et al. 2014). Geographically isolated temporary wetlands can contribute to the landscape functions (Cohen et al. 2016). Localities 1 (Aguascalientes), 29 (Querétaro), 35 and 39 (Zacatecas) had higher conductivity, dissolved solids and salinity than the rest. Localities 1 and 29 are very close to a town, especially site 29 (Figure 2-G) which is delimited by houses. However, site 29 is among the richest localities, with 37 species. Wetlands 35 and 39 had higher salinity, and had a lower number of species (15 and 10, respectively). Both localities also had pH values above eight. Besides water contamination and nutrient deposition in the water, temporary wetlands are vulnerable to landscape conversion, drainage and obliteration. Site 26 (Querétaro) presented the highest number of species in 2015, however in 2016, it was covered with soil and asphalt, and was completely surrounded by houses (Figure 2-H). We found some aquatic species in 2016, as Triglochin scilloides and Jaegeria purpurascens, even when the site was already dry. Vegetation restoration of a 100 m belt surrounding a temporary wetland can significantly improve water quality (Bird & Day 2014). Submersed species as Najas guadalupensis and Chara spp. are important in maintaining ecological processes in wetlands exposed to high level of nutrients (Dierberg et al. 2002). Aquatic plants are also economically important as biofilters to remove excess of nutrients (Kostel 2016), as well as to control eutrophication (Fisher & Acreman 2004). Lemna spp. acts as a filter and inhibits submersed plants growth by blocking the light (Arroyave 2004, Rai 2008, Cuasquer et al. 2016).
A large proportion of the plants listed as weeds (Villaseñor & Espiosa-Garcia 1998), are also aquatic, either strict or subaquatic (Lot et al. 2013, Mora-Olivo et al. 2013, Lot et al. 2015), for example: Baccharis salicifolia, Cyperus esculentus, C. flavescens, C. virens, Echinochloa crus-galli, E. crus-pavonis, Eleocharis aciculares, E. montana, Heteranthera limosa, Ludwigia octovalvis, L. peploides, Najas guadalupensis, Paspalum distichum, Plantago linearis, Poa annua, Polygonum punctatum, Potamogeton diversifolius, P. nodosus, Pycreus niger, Rorippa mexicana, Rumex crispus, Schkuhria schkuhrioides, Sisyrinchium convolutum, Sporobolus indicus, and Tripogandra purpurascens. We could not consider them so, since they are in their typical habitat, the temporary wetlands. The concept of weed depends of the moment, place, and conditions where the plant is developing (Lorenzi 1991). In addition, 15 of the 44 species cited as weed are also cited as economically important, such as potentially medicinal (nine species): Baccharis salicifolia, Bidens aurea, Cosmos bipinnatus, Ludwigia octovalvis, Polygonum punctatum, Rumex crispus, Schkuhria schkuhrioides, Tagetes lucida, and Tagetes micrantha, or as biofilters (six species): Egeria densa, Eleocharis aciculares, E. montana, Lemna gibba, L. minuta, and L. obscura. On the other hand, we did not find previous record as aquatic plant for 17 species of Asteraceae (Acmella repens, Aster moranensis, Bahia absinthifolia, Bidens aurea, Cosmos bipinnatus, Gnaphalium luteo-album, Heterosperma pinnatum, Tagetes lucida, and T. micrantha), Fabaceae (Dalea foliolosa, Macroptilium sp., Mimosa aculeaticarpa, Trifolium amabile, and T. wormskioldii), Rubiaceae (Galium cf. proliferum), and Verbenaceae (Glandularia teucriifolia, Verbena carolina). We could consider the above species as weeds for the temporary wetlands where recorded.
In spite of recent compilation studies for Mexican aquatic plants (such as Lot et al. 2013, Lot et al. 2015), several states (especially Aguascalientes and Zacatecas) need a higher collecting effort. We found seven new records and probably one undescribed species for each state.
Given that temporary wetlands present a high anthropic degradation, but still have a high biodiversity (Pollock et al. 1998, Balian et al. 2008, Murray-Hudson et al. 2012), with economically species (Pott & Pott 2000, Magalhães et al. 2016), they should be a conservation priority. Hence, studies of landscape influence on species occurrence are a new challenge to create strategies for conservation of temporary freshwater wetlands. Knowledge and awareness of the distribution, biodiversity, and economic potential of botanical species in temporary wetlands is the first step to establish conservation policies (Calhoun et al. 2016), as they are a very peculiar and highly threatened environment of central Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)


