The study of the reproductive biology of flowering plants explores diverse aspects that impact the success of sexual reproduction (i.e. fruit set and seed set) and therefore fitness, including: phenology and flowering patterns, reproductive behaviors of congeners that can lead to synchronic flowering, duration of anthesis, temporal function of the sexes, intra-floral spatial overlap of stigma and anthers, and the success of female and male functions, as well as the behavior and efficiency of interacting floral visitors (Dafni 1992, Mandujano et al. 2010, Martínez-Peralta & Mandujano 2011).
Flowering events can be triggered by a diversity of environmental changes (Parmesan & Yohe 2003, Rosenzweig et al. 2008, among others). Exposure to low temperatures (Evans 1971) and rainfall events (Alvim 1960) can trigger flowering in certain environments. However even though there is an environmental cue, non-overlapping flowering periods are selectively favoured by species within a community as separating flowering phases leads to a more efficient use of pollinators as resources over the course of the year (Waser 1978, Waser 1986, Dafni 1992).
In terms of phenologies and flowering patterns, Gentry (1974) grouped species into five types: 1) species that flower year - round (Bat flowers); 2) species with a low abundance of floral resources that are present during a short time (Steady state); 3) species with abundant floral resources over a short time (Cornucopia); 4) species with highly abundant floral resources for a very short time or in pulses (Big bang); and 5) species whose floral resources are present during several distinct events over the course of the year or in several pulses (Multiple big bang). These patterns can have an impact on the reproductive success both of individuals and of flowers that appear over time, since temporal overlap with other flowers can lead to facilitation or competition for pollinators, which can be essential for plants that require intercrossing. In the Cactaceae flowering usually occurs in a specific period that can encompass several months with one to several peak flowering periods (Multiple big bang, i.e. Cereus hexagonus, Echinocereus coccineus, Neobuxbaumia mezcalaensis, Neobuxbaumia macrocephala, Stenocereus griseus, Stenocereus queretaroensis, Pilosocereus sp., among others) (Valiente-Banuet 1997, Ruíz et al. 2000, Scobell & Scott 2002, Mandujano et al. 2010), or can be very short lived of only a few days (Big bang, i.e. Ariocarpus fissuratus, Mammillaria pectinifera, Echinomastus erectocentrus, Echinocereus engelmannii var. acicularis) (Martínez-Peralta & Mandujano 2011, Valverde et al. 2015). Of the three studies that deal with flowering in Astrophytum (Martínez-Ávalos 2007, Strong & Williamson 2007, López-Flores 2012) none have information on the length or pattern of flowering.
Sexual reproduction in flowering plants can be analyzed at two levels. The first one corresponds to the breeding system which describes the reproductive attributes (functional and morphological) of individuals flowers and populations (Wyatt 1983, Richards 1997, Neal & Anderson 2005). The second level is the mating system that is the pattern in gene transmission between generations through seeds, and these can be by selfing, outcrossing or different proportions of these two extremes, treated as a measure of genetic relatedness (Brown 1990, Neal & Anderson 2005). Floral characteristics such as corolla diameter, distance between the anthers and stigma in perfect flowers, temporal separation between the maturation of male and female functions (i.e. protandry and protogyny), and the proportion of pollen grains to ovules have been used as indicators of a species breeding systems -cleistogamy, autogamy, facultative autogamy, facultative xenogamy, or obligate xenogamy (Barret & Eckert 1990, Dafni 1992, Cruden 1977). This classification eases the generation of hypotheses on the possible breeding systems and selective pressures that result in possible adaptations in morphology or behavior in flowers and among individuals in a population (Dafni 1992, Waser 1993).
Both mating and breeding systems affect the genetic makeup and thus genetic structure of populations as well as the success of the subsequent processes of seed germination and seedling establishment, events which are particularly important in isolated, low density populations, as well as in populations of threatened species as there is an increase in the effects of genetic drift and potentially lower fitness (Esparza-Olguín et al. 2005, Camargo-Smidt et al. 2006, Saunders & Sipes 2006, Strong & Williamson 2007, Martínez-Peralta & Mandujano 2011). In the Cactaceae the effects of small population size is particularly important as many species have small isolated populations (Martorell & Peters 2005, Valverde & Zavala 2006, Hernández & Gómez-Hinostrosa 2011, Martorell et al. 2015).
Unfortunately, the reproductive characteristics of the majority of the Cactaceae are unknown (i.e. descriptions of their flowers, fruits and phenology), and only a limited number of studies integrate population-level reproductive dynamics. Mandujano et al. (2010) systematized the current knowledge on the reproductive biology in the Cactaceae and showed that most species have bisexual flowers although some may be functionally or morphologically unisexual with relatively short floral life spans (1-2 days) with some opening for longer periods of up to six days. Most species are outcrossers but some species have mixed mating systems with no clear pattern regarding self incompatibility systems. Most of the studied species are bee pollinated however some are bat (mainly columnar cacti), bird and even ant pollinated. For the ornamental species of Cactaceae reproductive biology provides information on the best times of year to allow or restrict seed collection, and how to implement pollination practices that increase fruit and seed production, which could be used for intensive management for commercial interests of selected species (Reyes & Arias 1995, Bárcenas 2006, Jiménez & Jiménez-Sierra 2007, Jiménez-Sierra 2011, Matias-Palafox & Rocha 2013). In the case of most Cactaceae species, these activities increase commercial interest and are an ideal alternative for the conservation of natural populations, and at the same time reduce poaching and increase economic benefits for local communities.
Here, we present the results of the first study of the reproductive biology of the “star cactus”, Astrophytum ornatum (DC.) Britton & Rose, which is based on observations over a full year in the Barranca de Metztitlán Biosphere Reserve (RBBM) in Hidalgo, Mexico, where we answered the following questions: What is the reproductive phenology of the species? What are its mating and breeding systems? and What is its reproductive success (fruit set and seed set) during the reproductive events throughout the year? The expectation was that A. ornatum had a flowering period in either a fixed period (A. asterias and A. capricorne; Bravo-Hollis & Sánchez-Mejorada 1991, Mandujano et al. 2015) or over the year (A. myriostigma; López-Flores 2012), and have an obligate xenogamous breeding system as has been found for its congener A. asterias (Strong & Williamson 2007).
Materials and Methods
Study species. Astrophytum ornatum is a short, cylindrical-stalk cactus that can reach up to 160 cm in height and 30 cm in diameter. It is characterized by its stalk, which has 5-8 acute ribs in a spiral growth pattern and light gray-green color, with stigma composed of groups of small white trichomes which are especially dense in juveniles. The areoles present one or two central and 10 radial spines. Its flowers are diurnal, solitary, and actinomorph, 5-7 cm long and canary yellow. The fruits are globose, dehiscent, and 2.5-3 cm long (Vázquez-Lobo et al. 2016), and according to Zepeda-Martínez et al. (2013) contain 113 ± 4.6 (mean ± SD) black seeds. This cactus grows in the highlands of the southern part of the Chihuahuan Desert (Guanajuato, Hidalgo, Querétaro and San Luis Potosí), mainly on steep slopes with calcareous soils (Bravo-Hollis & Sánchez-Mejorada 1991, Anderson 2001). The species is designated as threatened by Mexican law (“amenazada,” status “A” in NOM-059-SEMARNAT-2010), and is found in the Appendix II of the CITES (Guzmán et al. 2003, Arias et al. 2005, SEMARNAT 2010). Zepeda-Martínez et al. (2013) reported an average population growth rate (lambda, λ) in equilibrium with a seed bank and a low rate of transition to the seedling stage in one population. Despite being widely cultivated and commercialized in Mexico, all germplasm is acquired from wild populations (Matias-Palafox & Rocha 2013).
Study Area. The study was carried out in the Cactus Sanctuary Garden (Jardín Santuario de Cactáceas) of the RBBM (Hidalgo, 20°35’ N; 99°41’ W; 1,294 m a.s.l.). The RBBM includes the Metztitlán Canyon, which belongs to the Trans-Mexican Volcanic Belt and Sierra Madre Oriental provinces and is made up of canyons carved by the affluents of the Pánuco River. These canyons have high biological richness and are considered one of the most important zones in Mexico for cacti, with more than 70 species, of which 11.42 % are endemic and 15 % are threatened (Sánchez-Mejorada 1978, Jiménez-Sierra & Reyes 2000, CONABIO 2003, SEMARNAT 2010). The climate is dry semi-warm, with summer rains (BS0hw), a mean annual temperature between 18 and 22 °C and mean annual precipitation below 500 mm. Xerophitic scrub dominates the area, which is considered a Pleistocene refuge of Mexican desert biota with affinity to the Chihuahuan Desert, and which acts as a biological corridor among the arid zones throughout the Mexican Central Plateau (CONABIO 2003).
Phenology. During the study period (June 2010 through June 2011) monthly censuses were taken of 35 reproductive individuals found in a 250 m2 strip, contained within the total area occupied by the species, which does not exceed 450 m2. In each census the number of reproductive structures was recorded (buds, flowers, and fruits per individual) to describe the reproductive phenology and estimate probabilities of transition between reproductive structures. When flowers were found, the individuals were followed until anthesis ended.
In order to determine whether there was a relationship between individual size (height, cover or volume) and the production of reproductive structures per year, as well as to explore the relationship between productivity and climate variables during the year of the study (mean monthly temperature, maximum temperature, relative humidity, and global radiation) non-parametric correlations were carried out (Spearman, p).
Floral cycle and visitors. During the second peak of spring flowering (April), 20 flowers in anthesis from different individuals of Astrophytum ornatum were followed such that, perianth diameter and opening of the stigma lobes (using a 6” digital caliper) were recording every 2 hours. We also attempted unsuccessfully to quantify the nectar produced using 2 µl microcapillary tubes. Temperature was recorded using a Hobo data logger (Onset, U12-012).
During the April flowering event, the frequency and behavior of visiting insects was also recorded. At midday, each of two observers recorded insect visits to 15 flowers from 12:40 h to 14:10 h. The activity of the visitors was classified as: flight above the perianth (FP), landing on the perianth (LP), flight above the stigma (FS), landing on the stamen (LST), immersion of the visitor into the flower (I), deposition of pollen on the stigma (POS), visible transport of pollen (TP), and predation of the perianth (PP). Specimens were collected preserved in 70 % alcohol and identified in the laboratory.
Flower morphometry. From fresh flowers (N = 20 from individuals outside the 250 m2 strip, collected in November 2010), the following floral characters were measured using a digital caliper: 1) flower height; 2) width of flower tube; 3) perianth diameter; 4) length of the tube covered by stamen; 5) style length; 6) length of stigma lobes; 7) number of stigma lobes; 8) distance between stigma lobes and anthers; 9) nectar chamber; 10) polar diameter of the ovary; 11) equatorial diameter of the ovary; 12) number of ovules (Figure 1). The number of ovules and number of pollen grains per anther per flower were counted using a stereoscopic microscope (Carl Zeiss; DV4; 0.8x). To estimate the total pollen grains per flower, the average number of grains on ten anthers per flower was multiplied by the number of stamens per flower (Cruden 1977).
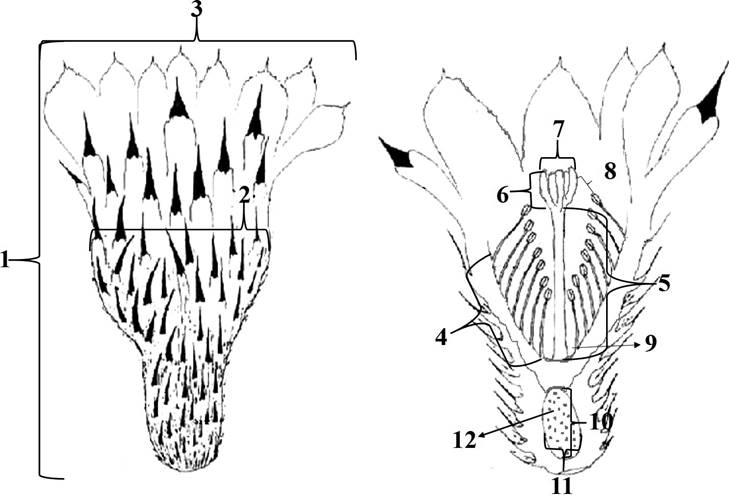
Figure 1 Flower of Astrophytum ornatum: 1) flower height; 2) width of flower tube; 3) perianth diameter; 4) length of the tube covered by stamen; 5) style length; 6) length of stigma lobes; 7) number of stigma lobes; 8) distance between stigma lobes and anthers; 9) nectar chamber, 10) polar diameter of the ovary; 11) equatorial diameter of the ovary; 12) number of ovules (modified from Bravo-Hollis and Sánchez-Mejorada 1991).
Breeding system. The outcrossing index (OCI) proposed by Cruden (1977) was used to determine the breeding system. For this, 20 flowers (each from distinct individuals) were examined to determine herkogamy (spatial separation of anthers and stigma) and dichogamy (temporal separation of the male and female functions), and pollen/ovule ratio (P/O). The corolla diameter was quantified as the average maximum perianth opening recorded during anthesis. Herkogamy was estimated using the minimum distance between the anthers and stigma lobes, considered herkogamy if this value was statistically different than zero (Nassar & Ramírez 2004). To determine whether male and female functions overlapped in time (homogamy) or dichogamy was presented, the number of flowers with dehiscent anthers and with receptive stigmas was recorded. The stigma was considered receptive when it presented a sticky surface (Martínez-Peralta & Mandujano 2011, Flores-Martínez et al. 2013). P/O ratio was estimated by dividing the total number of pollen grains by the number of ovules from the same flower (Cruden 1977).
Mating system. This was determined using five pollination and one control treatment under natural conditions (N = 20 flowers, per treatment, one flower/individual): 1) Control, flowers were marked with no other manipulation and were accessible to pollinator activity; 2) Artificial cross-pollination, flowers were isolated with organdy (to avoid contact by floral visitors) and manually pollinated using pollen from other plants (pollen obtained from stamens from 6 distinct flowers, placed in a test-tube, and applied to each isolated flower using a paint brush); 3) Artificial self-pollination, flowers were pollinated with their own pollen using a paintbrush and subsequently isolated with organdy; 4) Natural self-pollination, flowers were isolated with organdy and were otherwise unmanipulated; 5) Geitonogamy, flowers were manually pollinated with pollen from different flowers from the same plant, then isolated, and 6) Apomixis, the flower’s stigma was mechanically isolated using a small plastic tube to avoid contact with pollen (Arroyo-Pérez 2014, Matias-Palafox 2007, Jiménez et al. 2007). All treated flowers were labeled, and approximately four weeks after anthesis the mature fruits were collected. Fruit and seed number were counted, and fruit set and seed set for all treatments were compared using a Chi squared (χ2) test.
Reproductive success over time. To compare the reproductive success among the four flowering events observed over the course of the year (November 2010, March 2011, April 2011, and May 2011), 20 flowers (from distinct individuals) per event were marked during anthesis and their mature fruits were collected. For each fruit, we determined the dry mass, number of seeds, and total seed mass. The difference in fruit set (transition from flower to fruit) was analyzed using a Chi squared (χ2) test, dry mass, seed number and seed mass were analyzed with a one-way analysis of variance (ANOVA) and Tukey-Kramer tests. Correlations among the variables of fruit per event were estimated using Spearman’s Correlation Coefficient (p). All statistical analyses in this study were carried out using JMP® statistical software v 10.0.0 (Cary: SAS Institute Inc., 1989-2012).
Results
Phenology. Even though flower buds were found year-round, the presence of two or more flowers was only observed on four occasions, which we called “flowering events”. All four events occurred during the dry season: November 2010, March 2011, April 2011, and May 2011 (Figure 2). Most individuals (89 %), always had at least one reproductive structure. In November, 15 % of individuals had flowers, in March and April, this number increased to 88 % and 91 %, respectively, and in May a decrease to 33 % was observed.
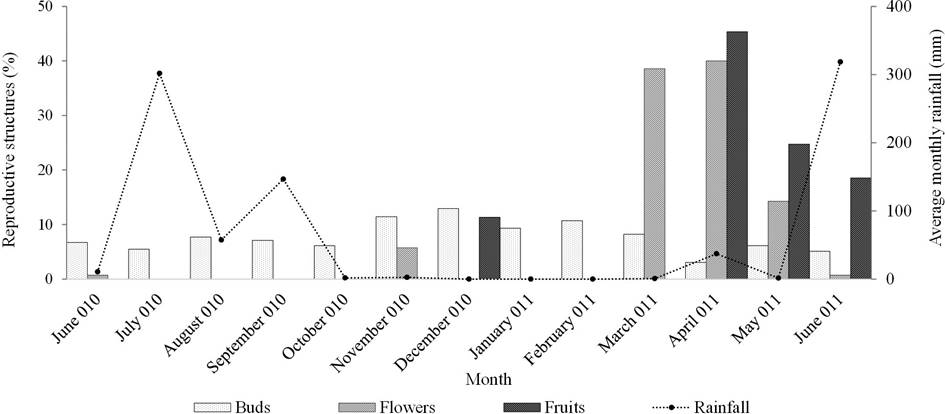
Figure 2 Reproductive phenology of Astrophytum ornatum in the Cactus Sanctuary Garden of the RBBM. Precipitation data taken from the Tlatepexe station (INIFAP 2012).
In June 2010 and 2011, only one flower was observed on each occasion, and neither produced fruits; as such we did not consider these to be population-level flowering events. Of the four population-level flowering events, three lasted a single day (28 March, 14 April, and 28 May), while the November flowering lasted 2 days (November 12-13). The largest number of flowers was found in the spring. The production of buds per population was 1,612, with an average of 46 (± 25.7 SD) per individual per year. The annual probability of transition from bud to flower was 0.11. The average flowers per individual was 4 (± 2.6) and the annual probability of transition from flower to fruit was 0.86, with an average of 3.5 (± 2.7) fruits per individual per year.
The individual contribution of flowering was not evenly distributed, since 8.57 % (N = 3) of individuals did not produce flowers despite having buds, and 30 % of individuals produced 40 % of the flowers observed (Figure 3).
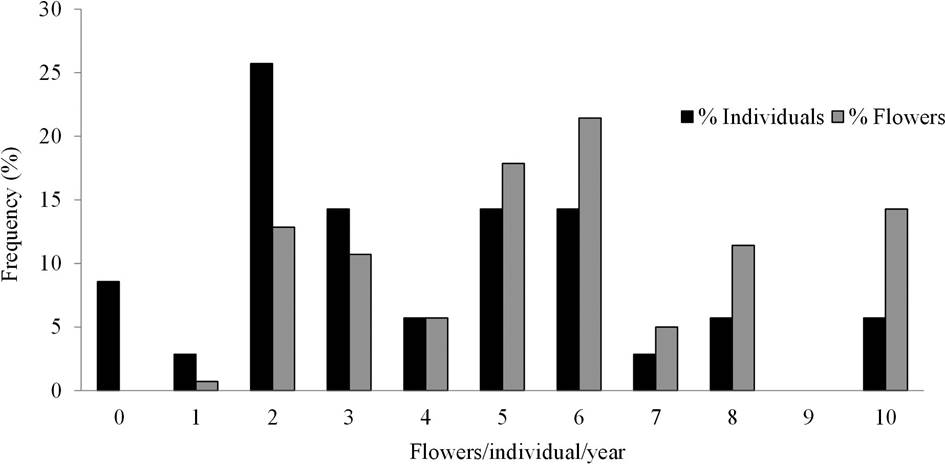
Figure 3 Relative contribution of individual plants to the flowering. It can be seen that 30 % of individuals contribute to the 40 % of the flowers, while 8 % did not produce flowers.
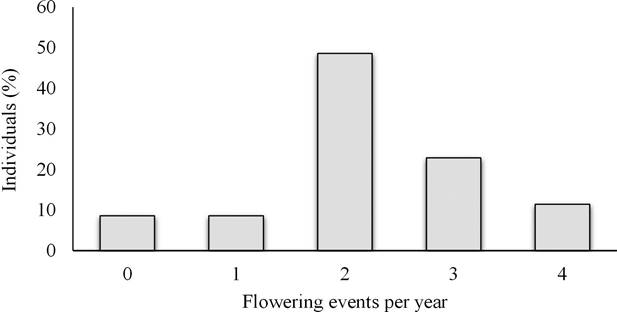
The maximum number of events in which an individual presented flowers was four (N = 4), and the majority of individuals presented only two flowering events (48 %; Figure 4). The relative production of flowers per individual and per event is presented in Figure 5.
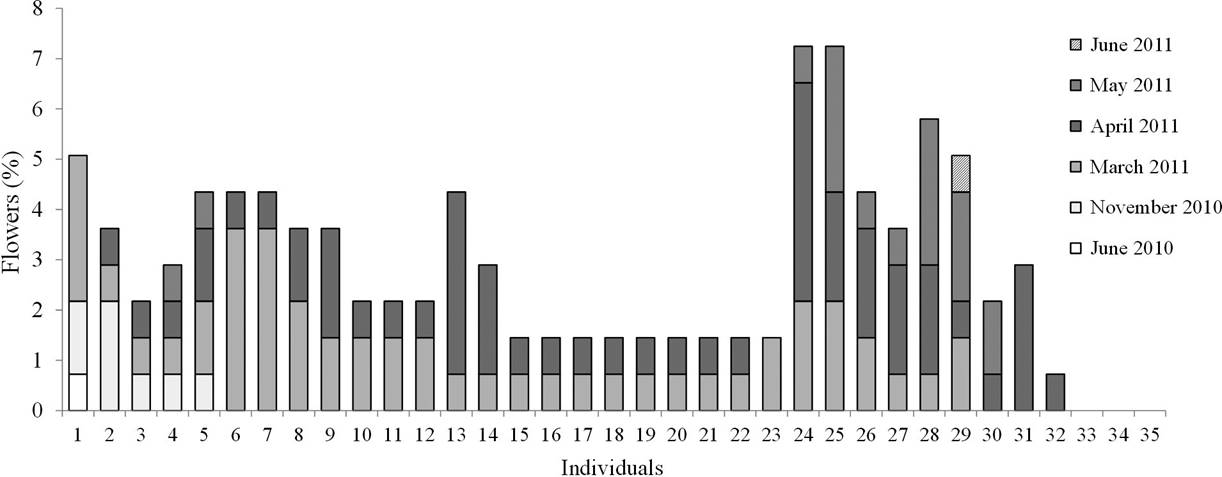
Figure 5 Annual individual contribution to flowering. The height of the bars represents the annual and temporal relative contribution of each individual.
There was a significant relationship between individual height and annual production of flower buds (p = 0.5461; P < 0.001), and the number of buds was inversely related to minimum temperature recorded during the previous month (p = -0.61; P < 0.025).
Floral cycle and visitors. Anthesis began between 9:30 and 10:00 h and ended around 17:00 h. Anthesis took 7 to 14 h (for those that happened to have 2 day anthesis).
The maximum perianth opening was recorded at 13:30 h, shortly before the maximum daily atmospheric temperature at 15:30 h (43 °C). The maximum opening of the stigma lobes coincided with the presence of surface exudation as well as with the dehiscence of the anthers; therefore, flowers were considered homogamous (Figure 6). Though nectar amount could not be quantified, the behavior of pollinators suggested that nectar was produced in very small quantities.
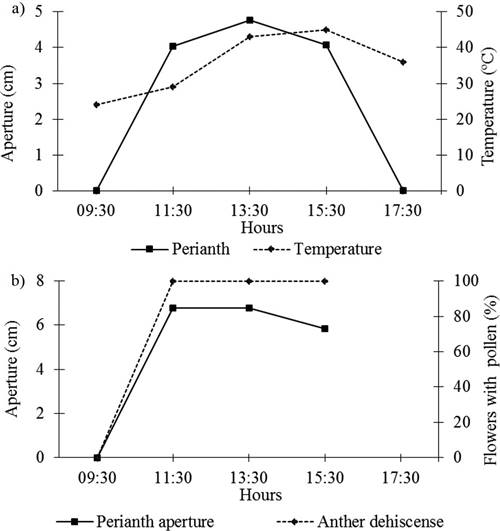
Figure 6 Floral behavior of Astrophytum ornatum in the Cactus Sanctuary Garden (April 14, 2011; N= 20 flowers): a) Perianth aperture and temperature duration of anthesis; b) Behavior of the stigma lobes and anther dehiscence.
During the hour and a half of observation, the following 32 floral visitors, were recorded: the social bee Apis mellifera (47 %), and the solitary bees Augochlorella sp. (3 %), Lasioglossum sp. (28 %) and Perdita sp. (13 %). Beetles of the Nitidulidae family (6 %) and grasshoppers (Orthoptera, 3 %) were also observed, feeding on pollen and perianth, respectively. Bees (A. mellifera; Lasioglossum sp. Perdita sp. y Augochlorella sp.) had all the behavioral patterns of a typical pollinator (Figure 7).
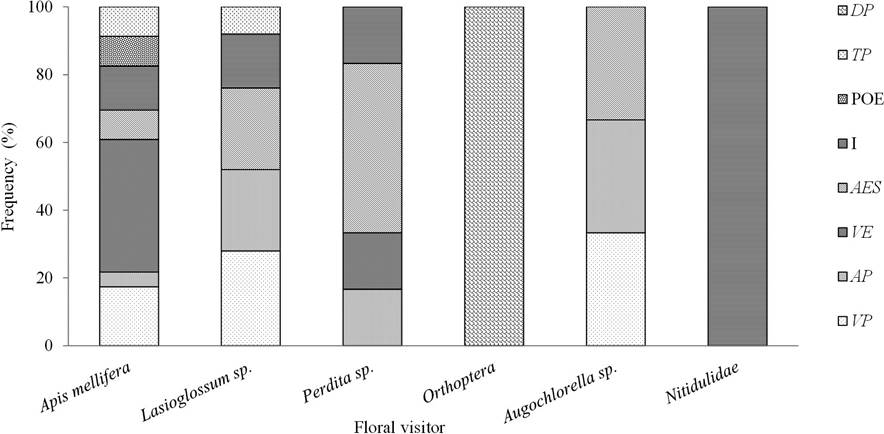
Figure 7 Activity of floral visitors Astrophytum ornatum: Flight above the perianth (FP); landing on the perianth (LP); flight above the stigma (FS); landing on the stamen (LST); immersion of the visitor into the flower (I); deposition of pollen on the stigma (POS); visible transport of pollen (TP), and predation of the perianth (PP).
Floral morphometry. The flowers of Astrophytum ornatum originate form areoles close to the apex of the stalk. Their height is 66 (± 7) mm, and they have 662 (± 94) stamen with sulphur yellow filaments and chrome yellow anthers. The pistil has a stigma with 7 (± 2) sulphur yellow lobes. The floral morphometry suggests a melitophilous pollination syndrome (Table 1; Figure 1).
Table 1 Floral characters of Astrophytum ornatum (N = 20 flowers).
| Floral attributes | Mean (mm) | S. D. | |
|---|---|---|---|
| 1. | Flower height | 66 | 7 |
| 2. | Width of flower tube | 25 | 3 |
| 3. | Perianth diameter | 33 | 11 |
| 4. | Length of the tube covered by stamen | 26 | 4 |
| 5. | Style length | 132 | 4 |
| 6. | Length of stigma lobes | 8 | 1 |
| 7. | Number of stigma lobes | 7 | 2 |
| 8. | Distance between stigma lobes and anthers* | -0.01 | 0.37 |
| 9. | Nectar chamber | 3 | 1 |
| 10. | Polar diameter of the ovary | 8 | 2 |
| 11. | Equatorial diameter of the ovary | 18 | 66 |
| 12. | Number of ovules * | 115 | 31 |
| Number of stamens | 662 | 94 | |
| Number of pollen grains per anther * | 306 | 44 | |
| Pollen-ovule ratios* | 1893:1 | 601 | |
* Values used for the determination of the Cruden’s outcrossing index (OCI) and the pollen- ovule ratios (P/O).
Breeding system. The flowers are hermaphrodite. The maximum aperture of the perianth was 33 ± 11 mm. Homogamy was observed, with no herkogamy, since the lobes of the stigma were slightly covered by the anthers (-0.01 ± 0.37 mm; paired t = 0.103; P > 0.05), such that Cruden’s OCI = 3, and the ratio of pollen per ovule per flower was 1,893 (± 601).
Mating system. No fruits or seeds were obtained from the self-pollination, geitonogamy or apomixis treatments. The fruit set and seed set values for the control treatment and artificial cross-pollination treatments were significantly different, with higher values for artificial cross-pollination for both parameters (Table 2) suggesting pollinator limitations.
Table 2 Reproductive success of Astrophytum ornatum in pollination experiments (N = 20 flowers/treatment; April 2011).
| Treatments | Fruit set | Seed set |
|---|---|---|
| Treatment control | 0.65 ± 0.1 | 0.47 ± 0.2 |
| Artificial cross-pollination treatments | 0.95 ± 0.05 | 0.70 ± 0.2 |
| Statistical test | χ2 = 6.19; P < 0.05; df =1 | F1,30 = 12.04; P = 0.001 |
Reproductive success over time. The fruit set of marked flowers did not differ significantly among flowering events (χ2 = 4.36; P > 0.05; df = 3). Fruits produces in May had a higher average mass than those produced in the other events (F3,56 = 25.02; P < 0.05); however, the total mass of the seed batch from each fruit did not differ among events (F3,48 = 2.258; P > 0.05). The number of seeds of the flowering events in May and November (98.50 ± 25 and 92.50 ± 7.7 respectively) were significantly larger than those in March and April (45.94 ± 5.7 and 53.69 ± 7.0 respectively; F3,57 = 18.0; P < 0.05; q = 2.64; P = 0.05; Table 3).
Table 3 Reproductive success of Astrophytum ornatum in various flowering events.
| Flowering Event | Fruit set | Seed set | Dry weight of fruits ± SD | Total number of seeds ± SD | Total weight of seeds ± SD |
|---|---|---|---|---|---|
| November | 0.7 | 0.80a | 0.380 ± 0.03 | 92.50 ± 7.7a | 0.1899 ± 0.02 |
| March | 0.8 | 0.40b | 0.308 ± 0.04 | 45.94 ± 5.7b | 0.1387 ± 0.03 |
| April | 0.65 | 0.47b | 0.345 ± 0.02 | 53.69 ± 7.0b | 0.1267 ± 0.01 |
| May | 0.9 | 0.86a | 0.696* ± 1.67 | 98.50 ± 25a | 0.234 ± 0.070 |
Different letters indicate significant differences.
Discussion
The flowers of Astrophytum ornatum have a melitophilous pollination syndrome -flowers actinomorphic, diurnal anthesis, yellow and abundant pollen content that has been suggested as a compensatory attractant for pollinators given the low production of nectar (Dafni 1992). Floral characteristics attract the native solitary bees Augochlorella sp., Lasioglossum sp., and Perdita sp. and the exotic social bee Apis mellifera. The flowers are similar to those of other cacti of the Subfamily Cactoideae, and particularly to those of Astrophytum asterias (Zucc.) Lem., which is found in Northern Mexico (Martínez-Ávalos 2007, Martínez-Ávalos et al. 2007; Strong & Williamson 2007, Mandujano et al. 2010). Mean anthesis duration is brief, lasting from one to two days as is reported for other small cacti including: Ariocarpus fissuratus (Engelm.) K.Schum. Astrophytum asterias, Melocactus curvispinus Pfeiff. and Echinomastus erectocentrus (J.M.Coult.) Britton & Rose (Mandujano et al. 2010).
Despite Astrophytum ornatum produced buds continuously with an average of 46 (± 25.6) buds/individual/year, it produced on average 4 (± 2.6) flowers/individual/year and only 3.5 (± 2.7) fruits/individual/year. This is due to the fact that 89.2 % of buds were aborted two or three weeks after their appearance. The constant production of flower buds implies a constant reproductive investment with little reproductive success. The continuous production of buds suggests that the species has the capacity to develop a flowering pattern that is continuous over time - i.e. “Steady state”, sensu Gentry (1974). However, the flowering pattern observed was episodic, consisting of four distinct peaks, which could corresponds with a “Multiple big bang” pattern, sensu Gentry (1974), in which the probability from bud to flower is so low, that it is a bottleneck for fruit production (a component of seed limitation). The continuous production of buds constitutes a high energetic cost, since these structures are non-photosynthetic but contain all of the components for the formation of functional flowers.
It has been suggested that the flowering patterns observed in a variety of species are the consequence of the tuning of individual physiology to a series of environmental signals that act as triggers for flowering; these include hours of light, cold or warm temperatures, rains or droughts, or the amount of heat accumulated by the plant over a period of time (Bernier et al. 1981, Diekmann 1996, Fenner 1998). However, for cacti, the specific stimuli involved have not been identified. Over the year of observation, we were unable to find a clear relationship between the environmental variables considered and the flowering patterns observed, although the four flowering events occurred during the dry season -spring and fall- as has been reported in other cactus species -Ariocarpus agavoides, A. bravoanus, A. fissuratus, A. kotchoubeyanus, A. scaphirostris, A. trigonus, A. asterias, Echinocactus platyacanthus, Echinocereus pulchellus var. pulchellus, Ferocactus histrix, Lophophora diffusa, Mammillaria crucigera, M. huitzilopochtli, M. oteroi, M. pectinifera, Turbinicarpus horripilus, T. pseudomacrochele- (Del Castillo 1988, Contreras & Valverde 2002, Navarro & Flores 2002, Martínez et al. 2004, Martínez-Ávalos 2007, Matias-Palafox 2007, Jiménez-Sierra 2008, Jiménez-Sierra & Eguiarte 2010, Martínez-Peralta & Mandujano 2011, Díaz-Segura 2013, Flores-Martínez et al. 2013, Arroyo-Pérez 2014, Valverde et al. 2015). However, we found an inverse relationship between buds and minimum temperatures in the preceding month which would indirectly suggest an environmental cue.
The continuous production of flower buds by Astrophytum ornatum is an unusual phenomenon, which had rarely been reported for any other species of cactus (Mandujano et al. 2010), making this the first report of this cactus with a permanent or constant production of buds. This strategy could be considered energetically costly, since it implies a significant energetic cost that could otherwise be used for other vital processes. Low temperatures (4 °C) that predate bud abortion could represent a lack of physiological response to environmental conditions such that a relatively recent environmental change is uncoupled with previous regulated floral cycles. The constant presence of buds could be seen as a pre-adaptive characteristic that would favor individuals when they are found in more benign environments (i.e. wetter environments), which would allow the species to stretch its flowering and fruiting periods as has been observed when individuals are raised in the greenhouse (pers. obs.). Under constant environments, a species with a single large flowering period would be expected to maximize reproductive success and thus would pose a better strategy than a steady state strategy that would have a lower reproductive success. However, arid environments pose a challenge such that conditions vary strongly and therefore a strategy that spreads the risk over time would be favorable as reproductive success is maximized.
The ephemeral population-level flowering events, lasting one to two days - constitute one of the shortest intra-population flowering records for cactus populations (Mandujano et al. 2010), and leads to a high degree of intra-population floral synchrony. This phenomenon deserves special attention because at the same time as synchronized exposure of flowers is an important resource for both pollinators and predators, the optimal movement of pollen must be assured to guarantee cross-pollination, since pollination experiments suggest this species is self-incompatible or presents strong inbreeding depression due to its small population size and high degree of isolation. As such, flowers produced outside the short flowering events, as occurred in June of both years, have little or no chance of reproductive success. In addition, the fruit and seed set values from the control treatment were low compared to the artificial cross-pollination treatment, which suggests pollinator scarcity, as has been reported in A. asterias (Strong & Williamson 2007). Even though our data set for floral visitors only included those who arrived at midday, the high percentage of the exotic bee Apis mellifera may be contributing factor to the low success (Dupont et al. 2004).
Astrophytum ornatum can be considered a rare species, since its populations have low density (< 2 individuals/m2), which are generally present in areas smaller than 500 m2 (Esparza-Olguín et al. 2002, Valverde & Zavala 2006, Jiménez-Sierra & Matias-Palafox 2015). In addition, local populations are frequently separated by several kilometers; as such, the evolution of high floral synchrony could result as advantageous to facilitate pollination among conspecifics and achieve reproductive success (Martínez-Peralta & Mandujano 2011). Isolated small populations, coupled with its narrow niche breadth, removal of individuals from wild populations, and projected climate change, leaves this species at a critical level of extinction risk (Carrillo-Angeles et al. 2016, Martorell et al. 2015).
The reproductive outlook becomes more complicated when other biotic factors are considered. For example, during flowering peak of Astrophytum ornatum in April, another cacti species that cohabits the area, Turbinicarpus horripilus (Lem.) John & Říha, also present a flowering peak. The synchronous flowering of these two species, both with melitophilous flowers, and common visitors (L. Matias-Palafox Unpub. Data) could lead to interspecific competition or promote facilitation of the already scarce pollinators, which would affect reproductive efficiency (Rathcke 1983). This could explain the low fruit set values of A. ornatum during April (0.65), as well as the reduced number of seeds per fruit during this period.
The results of this study emphasize the importance of maintaining current numbers of adult individuals in the population, since reproductive success depends on the cross-pollination by bees. Implementing artificial cross-pollination is also a priority, as well as the seed sowing in greenhouses. Juvenils obtained could be reintroduced to natural environments or could be used by local residents to cover the commercial demand for this plant and reduce poaching from wild populations.











 text new page (beta)
text new page (beta)