Reproductive phenology studies the temporal frequency of flowering and fructification stages of plant life cycle and their relationship with environmental factors (Rathcke & Lacey 1985, Borchert 1994). These reproductive structures are involved in the formation and maturation of seeds, as well as in their dispersal, and they determine the regeneration of individuals in a community. Therefore the reproductive phenology represents the first approximation to understand the dynamics of the reproductive success of plants (Rathcke & Lacey 1985, Donohue 2009).
Abiotic factors are signals that trigger the development of the reproductive structures (Rathcke & Lacey 1985, Borchert 1994, Valdez-Hernández et al. 2010). For example in temperate forests of Mexico it has been found that the rainy season matches with the beginning of flower and fruit production in tree species (Cornejo-Tenorio & Ibarra-Manríquez 2007, Cortés-Flores et al. 2013). However environmental factors such as temperature, humidity, light amount and its quality as well as soil chemical properties change in a single area. These variations generate micro-environments, which can be defined as local modifications of weather and they are the result of the spatial differences in altitude, slope and orientation in a site (Chen et al. 1995).
In a similar way these environmental variations give result to different flowering and fructification patterns of individuals of the same species growing in the same area (Donohue 2009, Anderson et al. 2012). This plasticity is an attribute of plants that allows them to ensure survival and reproduction, mainly in heterogeneous and changing ecosystems (Sultan 2000, Hernández-Verdugo et al. 2015).
The study of reproductive phenology at a micro-environmental scale, allows knowing which are the abiotic factors that favor reproductive success in plants. In this sense, current studies on reproductive phenology in temperate forests of Mexico are scarce and none of them considers the space and time heterogeneity generated by micro-environments (Cortés-Flores et al. 2013).
For example in Mexico, an important species that lacks ecological studies is Sambucus nigra L. subsp. canadensis, although it is considered a characteristic species of Abies religiosa temperate forests located between 2,500 and 2,800 m a.s.l. (Arreguín-Sánchez 2001, Applequist 2013). Besides its biological importance, the study of this species is interesting because of the ecological differences that sets it apart from its sister subspecies S. nigra subsp. nigra, which has been cataloged as one of the main invasive weed species of Europe (Atkinson & Atkinson, 2002; Kabuce & Priede 2006). In contrast, S. nigra subsp. canadensis is considered as a natural element of temperate forests in America (Arreguín-Sánchez 2001, Applequist 2013, Hanan-Alipi et al. 2009).
In the Abies religiosa forest, also named fir forest, in the Magdalena river basin (MRB), Mexico City, individuals of Sambucus nigra subsp. canadensis are distributed in an aggregate pattern in open canopy areas with signs of anthropogenic disturbance (Santibáñez-Andrade 2009). This study evaluates the relation of its reproductive phenology with the environmental factors of precipitation, temperature, amount of light and chemical characteristics of the soil, with the objective to know how each one of them influences its reproductive dynamics.
Materials and methods
Study site. The Abies religiosa forest in the Magdalena river basin (MRB) is in the SW of the valley of Mexico between 2,900 and 3,650 m a.s.l. located at 19° 13’ 53’’, 19° 18’ 12’’ N y 99° 14’ 50’’, 99° 20’ 30’ O, covering an extension of 3,100 ha (Figure 1A and B). It is a highly heterogeneous and species rich site, that belongs to the remaining vegetation and soil conservation area of Mexico City (Santibáñez-Andrade et al. 2015).
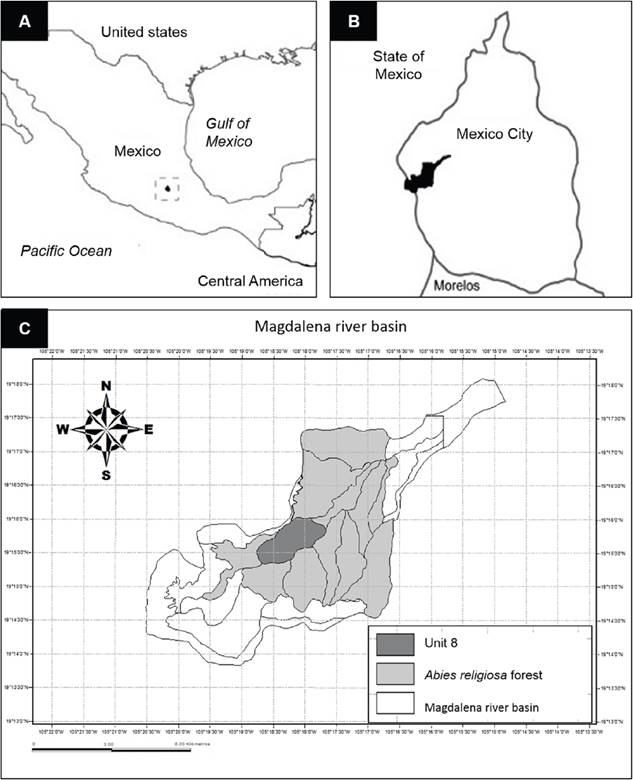
Figure 1 Study area A) Mexico City location, B) Magdalena river basin location (MRB) in Mexico City and C) Landscape unit number 8 in the Abies religiosa forest of the MRB (Map of Mexico and Mexico City modified from Galeana-Pizaña et al. 2013).
According to Köppen´s classification, it has a temperate subhumid weather C(w2)(w)b(i’), it has a minimum temperature of 14 ºC, a maximum of 20 ºC and a mean one of 18 ºC, an annual precipitation of 950-1,300 mm (Dobler-Morales 2010) (Figure 2). The forest canopy is dominated by Abies religiosa, Sambucus nigra subsp. canadensis and Salix paradoxa, while other genders such as Senecio, Salvia and Ageratina are found in the understorey (Santibáñez-Andrade et al. 2015).
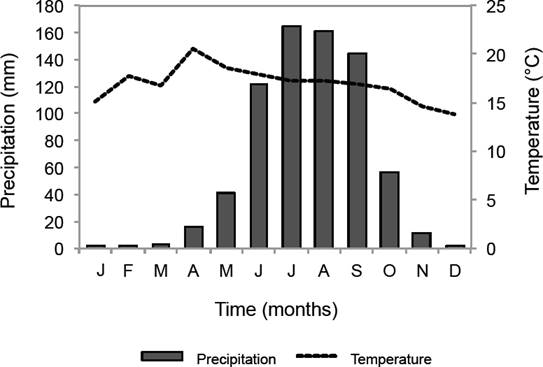
Figure 2 Climograph from the weather stations of Desviación Alta al Pedregal (1967-2000) and Monte Alegre (1967-1987) (modified from Delgadillo-Durán 2011).
Species description. Sambucus nigra subsp. canadensis (Adoxaceae), known as the elderberry, is a shrub or tree that grows up to 15 m height. It has corymbs inflorescences, with small, less than1 cm diameter bisexual, white flowers, with five petals. The fruit when mature is a glossy dark purple to black round drupe, of 3-8 mm diameter that contains 3-5 seeds (Figure 3) (Arreguín-Sánchez 2001, Atkinson & Atkinson 2002). Flowers are polinized by bees, beetles and flies, while fruits are mainly bird dispersed (Debussche & Isenmann 1994).
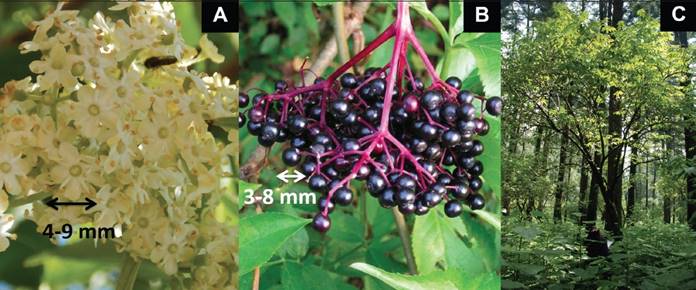
Figure 3 Reproductive structures of Sambucus nigra subsp. canadensis A) White inflorescences, B) Drupes (fruits) and C) Adult. (Drupes modified from Kabuce and Priede, 2006).
The Abies religiosa forest of the MRB is integrated by 11 landscape units based on their topographic and vegetation features (Santibáñez-Andrade et al. 2015). This study was carried out in the landscape unit number eight (Figure 1C), since it has an important abundance of Sambucus nigra subsp. canadensis in areas of anthropogenic disturbance (Santibáñez-Andrade 2009). In this unit seven plots of 25 × 25 m were selected, following a simple-random method, they were established through their location on (x, y) coordinates, leaving at least 50 m of distance between them, having at least 15 individuals per plot. In each plot we marked all the elderberry individuals that were higher than 3 m height and with reproductive structures, having a total of 103 individuals.
We registered the percentages of young and mature flowers and fruits monthly and during a year (April 2012 to May 2013). We considered the open flowers as mature ones and flower buds as the young ones. Purple fruits were considered as mature ones and those green as young ones. To determine the amounts of young and mature flowers and fruits per individual we used the percentage scale proposed by Fournier and Charpantier (1975) and modified by Carabias-Lillo and Guevara-Sada (1985), in which, in a scale of one to five the percentage of flowers and fruits is estimated with respect to the total of branches (Table 1).
Table 1 Scale proposed by Fournier and Charpantier (1974) and modified by Carabias-Lillo and Guevara-Sada (1995).
| Rank | Percentages of reproductive structures |
|---|---|
| 0 | Absence of the structure |
| 1 | Presence of the structure, in an interval of 1-10% |
| 2 | Presence of the structure in an interval of 11-25% |
| 3 | Presence of the structure in an interval of 26-50% |
| 4 | Presence of the structure in an interval of 51-75% |
| 5 | Presence of the structure in an interval of 76-100% |
With the aim of knowing the synchrony in time of flowering and fructification between the individuals of Sambucus nigra subsp. canadensis, we calculated the index proposed by Augspurger (1983) through the following equation:
Where: Xi is the individual synchrony index, n is the number of individuals in the population, fi is the number of days in which the individual i has a phenological event and e.g. is the number of days in which both individuals i and j have a phenological event in common.
With the individual synchrony index, we obtained the population synchrony index (Z) with the equation proposed by Augspurger (1983):
Where: Z represents the population synchrony index, n is the number of individuals in the population and Xi is the synchrony index per individual obtained with equation [1]. When Z equals one, individuals in the population show complete synchrony.
From April 2012 to May 2013 the means of monthly precipitation were consulted in the weather station of Monte Alegre D.F (SMN 2013). In order to register the environmental temperatures during the study year continuously, in each plot a HOBO data logger (HOBO Onset Corporation USA) was installed at the soil surface. With the aim to characterize the light conditions in the plots, every two months at 8 o´clock in the morning, we took three hemispheric photographs (Nikon digital camera Nikon D80 with a “fisheye” lens EX SIGMA 4.5 2:28 DCHSM), for all of them, the superior part of the lens facing north. They were analyzed with the Gap Light Analyzer GLA, 2.0 (Frazer et al. 2000) software, to determine the canopy gap fraction (CGF) and the global site factor (GSF), that is defined as the percentage of light transmitted (in moles), and it was calculated according to Canham et al. (1990) with the following equation:
Where: GSF is the global site factor, FSD is the percentage of direct light and FSI is the percentage of diffuse light.
In both seasons of the year, dry and rainy, in each plot five soil samples of 250 g of the first 10 cm and without litter were taken. Four samples corresponded to the corners of the plot and one more to the center of it. These were combined per plot and were sent to the soil fertility laboratory in the Colegio de Postgraduados campus Montecillo, for their chemical analysis. Analyzed variables were pH, through the relation of soil and water 1:2, electric conductivity in water (E.C.), with the relation 1:5 and an electric conductivity bonding, percentage of organic matter (O.M.), through a humid digestion with Walkley-Black determination, and the availability of inorganic phosphorus (P) concentration was obtained through the extraction of NaHCO3 0.5 M (pH 8.5) and with colorimetric determination. The percentage of nitrogen (N) was determined as well, with a moist digestion with a mixture of sulfuric acid with Kjeldahl distillation through a steam stripping and a sulfuric acid 0.05 N tritation. Potassium (K) concentration was determined as well, with a pH 7 NH4OAc extraction through flame photometry. In order to know the moisture content of the soil in the study plots, every two months soil samples were weighed and oven dried at 100 °C, moisture content percentage was calculated through the Reynold’s (1970) equation:
Where: H% is the soil moisture, Wemo is the weight of the moist soil, Wedr is the weight of the dry soil.
Analysis. The evaluated variables did not show a normal distribution when tested with Shapiro-Wilk, therefore the correlations between the percentages of flowers and mature fruits with the environmental variables were calculated through the Spearman´s test with the STATISTICA 8 software (Stat Soft, Inc. 2007). With the objective to know the relation between the environmental factors of the dry and the rainy seasons in the seven plots and the young flowers and fruits of the individuals, we carried out a Canonical Correspondence Analysis (CCA) with the software PC-ORD version 5.10 (McCune & Mefford 2006). The evaluated micro-environmental characteristics in this study were analyzed with the ANOVA and Tukey (P < 0.05) multiple comparisons tests. The micro-environmental differences in electric conductivity, organic matter and potassium concentration that did not show a normal distribution, were analyzed with the Kruskal-Wallis and Student-Newman-Keuls (P < 0.05) multiple comparisons tests non parametric statistic test with STATISTICA 8 (Stat Soft, Inc. 2007) (P < 0.05) software.
Results
Relation between the reproductive phenology and the environmental factors. From March to October Sambucus nigra subsp. canadensis showed reproductive structures (Figure 4). The main flowering season was from March to June, and a second, less intense flowering season was detected in September (Figura 4). Most individuals (66 %) showed the highest percentage of flowers (26.67 %) in May. Fruits were observed from May to October and the maximum percentage of fruits was reached in July (30 %), however an important increase of individuals with fruits (47 %) was registered in August (Figure 4). In the population level, a low synchrony of the reproductive phases was observed: the synchrony index value for flowering was 0.47 and 0.24 for fructification.
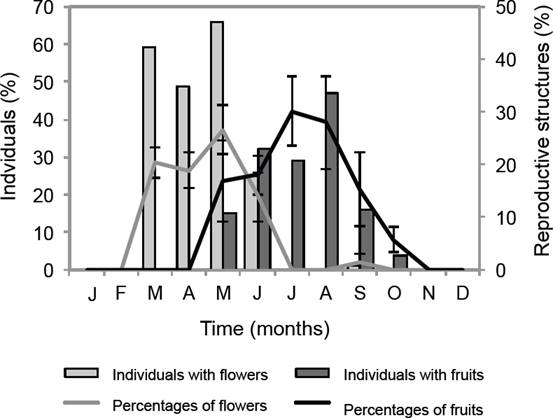
Figure 4 Reproductive phenology pattern of Sambucus nigra subsp. canadensis; monthly percentages of individuals with flowers and fruits (primary axis). Monthly percentages of flowers and fruits (secondary axis).
According to the relation between reproductive phenology and the environmental factors, the percentages of flowering were correlated in a significant and positive way with the canopy gap fraction (% CGF) and in a negative way with the soil moisture content (P < 0.05). Percentages of fruits were correlated in a significant and positive way with the precipitation and temperature (P < 0.05) (Table 2).
Table 2 Spearman’s rank correlation coefficient values (rs) between the percentages of flowers and fruits and the abiotic factors (P < 0.05) (*significant differences).
| Flowers (%) | Fruits (%) | |
|---|---|---|
| Precipitation (mm) | 0.07 | *0.89 |
| Temperature (º C) | 0.45 | *0.82 |
| Light (% GSF) | 0.43 | -0.16 |
| Canopy gap (% CGF) | 0.67* | 0.15 |
| Soil moisture content (%) | -0.63* | 0.06 |
Micro-environmental groups. The Canonical Correspondence Analysis (CCA) between reproductive phenology and the environmental factors, identified three micro-environmental groups, and their explained variance was 50.9 % for axis 1 and 28.3 % for the second variation axis (Table 3). The results of the Monte Carlo permutations test indicated that the ordination provides a significant representation of the percentages of flowers and fruits and the registered environmental variables (P < 0.05). In group one (plots five and two) the percentages of young fruits in the dry and rainy seasons, as well as the mature fruits percentages of the dry season, were related in a positive way with the amount of light (GSF) (Figure 5). In micro-environment two (plots one and three) the increase in mature flowers during the dry and rainy seasons, was related in a positive way with high values of organic matter (O.M.), nitrogen (N), soil moisture and phosphorus (P), this last was the one with the smallest relation (Figure 5). Micro-environment three (plots seven and four) did not show any direct association between reproductive phenology and the micro-environmental factors (Figure 5).
Table 3 Statistical summary for Canonical Correspondence Analysis (CCA).
| Axis 1 | Axis 2 | |
|---|---|---|
| Eigenvalues | 0.071 | 0.039 |
| Variance explainded (%) | 50.9 | 28.3 |
| Variance comulative (%) | 50.9 | 79.2 |
| Pearson Correlatión | 1.00 | 1.00 |
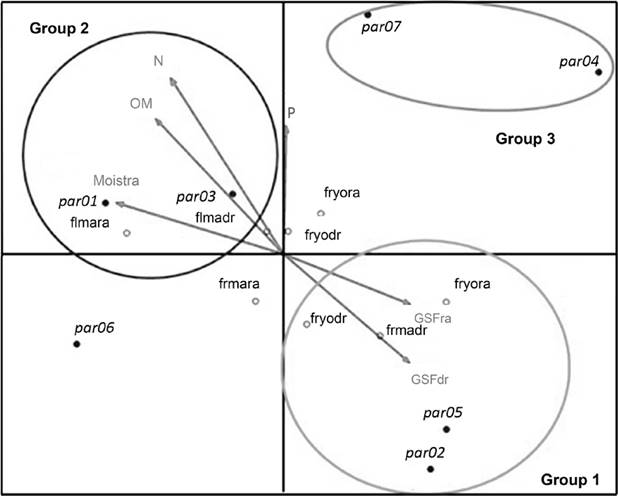
Figure 5 Canonical Correspondence Analysis (CCA) of the reproductive phenology of Sambucus nigra subsp. canadensis and the abiotic factors in the dry and rainy seasons; par = plot and number, fl = flowers, fr = fruits, yo = young, ma = mature, dr= dry season, ra = rainy season, Moistra = soil moisture content in the rainy season , MO = organic matter, N = nitrogen, P = phosphorus, GSFra = global site factor in the rainy season and GSFdr = global site factor in the dry season.
Reproductive phenology of the micro-environmental groups. The reproductive phenology pattern was different between the three micro-environments (Figure 6). However, no significant differences were found for the percentages of flower and fruit numbers during the year (F3,284 = 0.24, P = 0.76 and F3.284 = 2.00, P = 0.15 respectively).
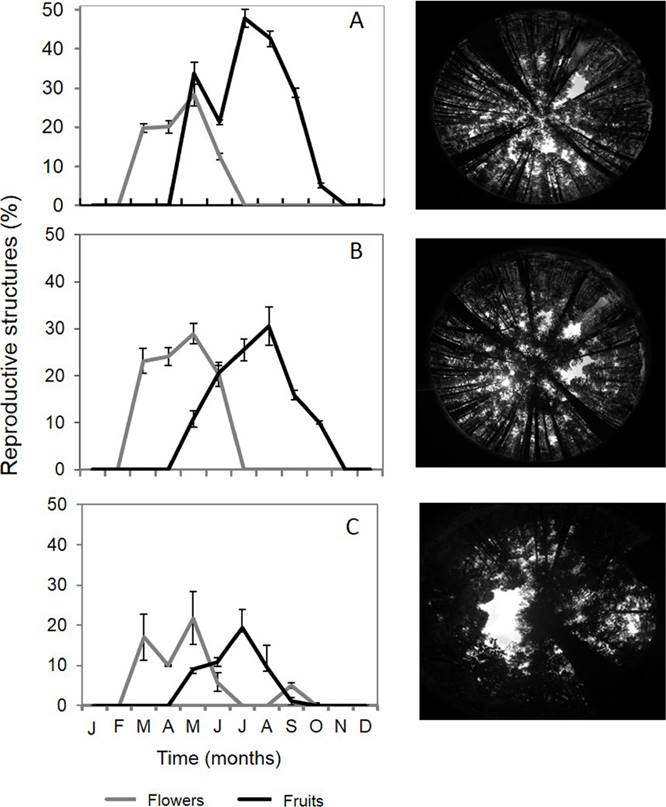
Figure 6 Monthly percentages of flowers and fruits in the three micro-environmental groups and hemispheric photographs of each micro-environment; A) Group one, B) Group two and C) Group three.
The individuals of the micro-environmental group one showed the highest percentage of fruits in July (47 %) (Figure 6A) and the highest synchrony index value (Z = 0.23, Table 4). In the case of the individuals in group two, both the percentages of flowers and fruits reached values close to 30 % (Figure 6B), in this micro-environment the highest synchrony index value for flowering was observed (Z = 0.22), as well as the highest percentage of soil moisture content (60 ± 34.26), M.O. amount (20 ± 0.41) and of N (0.66 ± 0.01). Analysis of variance for the three micro-environmental groups showed that the amounts of O.M. and N are significantly different between them (K13.91, P < 0.001 and F1,215 = 15.4, P < 0.001 respectively) (Table 4). Finally, the individuals of micro-environment three stand out since they showed the lowest percentages of reproductive structures (< 21 %) (Figure 6C).
Table 4 Comparison of the micro-environmental characteristics and the synchrony index values for flowers and fruits in the three micro-environmental groups (*, ** significant differences P < 0.05).
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Canopy gap (% CGF) | 17.39 ± 5.92 | 15.72 ± 5.55 | 15.91 ± 5.31 |
| Light (% GSF) | 23.34 ± 7.09 | 21.85 ± 8.33 | 24.82 ± 6.53 |
| Organic matter (O.M) (% W-B) | *16.20 ± 1.76 | *20.50 ± 0.41 | **19.60 ± 0.97 |
| Nitrogen (N) ( % Kjeldahl) | *0.59 ± 0.01 | **0.66 ± 0.01 | **0.66 ± 0.042 |
| Phosphorus (P) (mg/Kg) | 4 ± 1.60 | 6 ± 2.47 | 6 ± 2.91 |
| Potassium (K)(meq/100g) | 0.8 ± 0.19 | 0.7 ± 0.06 | 0.8 ± 0.24 |
| pH (1:2 H2O) | 5.7 ± 0.16 | 5.5 ± 0.19 | 5.7 ± 0.33 |
| Electric conductivity (E.C) (dS/m 1:5 H2O) | 0.05 ± 0.003 | 0.05 ± 0.004 | 0.05 ± 0.003 |
| Moisture content (%) | 46.76 ± 19.21 | 60.42 ± 34.26 | 55.35 ± 25.98 |
| Flowers synchrony index (Z) | 0.13 | 0.22 | 0.12 |
| Fruits synchrony index (Z) | 0.23 | 0.08 | 0.07 |
Discussion
Reproductive phenology of tree species in temperate forests of Mexico is directly related to the precipitation season (Cornejo-Tenorio & Ibarra-Manríquez 2007; Cortés-Flores et al. 2013). This is also the case of the studied species: the general pattern of the reproductive phenology of Sambucus nigra subsp. canadensis showed a relation with the rainy season, in particular in terms of the fructification pattern. Ramírez-García & Nepamuceno-Martínez (1986) and Cornejo-Tenorio & Ibarra-Manríquez (2007) suggest that most of fleshy fruits are produced during the highest precipitation season, since big quantities of water are needed for their maturation. This coincides with the findings of our study, since the percentages of S. nigra subsp. canadensis fruits were positively correlated to precipitation.
Water availability for plants does not only depend on the changes in precipitation, but also on soil moisture content. This is why phenology studies besides taking into account the precipitation values, they should also search about the role of soil moisture content (Borchert 1994). In the case of Sambucus nigra subsp. canadensis the negative relation between the soil moisture and flower percentages, suggests that low contents of soil moisture favor the development of flowers, result that has also been reported for other tree species of Mexican temperate forests (Cornejo-Tenorio & Ibarra-Manríquez 2007, Cortés-Flores et al. 2013). In this sense several authors such as Borchert (1994) and Seghieri et al. (2009), have suggested that the apparent paradox between water stress conditions and the increase of flowers, is due to the fact that before the beginning of their production, there is a considerable loss of leaves that avoids the loss of water by evapotranspiration, this explanation coincides with our observations in the field. On the other hand, dry season flowering can have an adaptive meaning, since the most intense activity of pollinators has been registered for this season and it is also when there usually is an important decrease of insect pests (Wright & van Schaik 1994, Bolmgren et al. 2003, Kilkenny & Galloway 2008).
Plant reproductive phenology patterns are also modified by changes in temperature. A clear example of this is confirmed by the temperate tree species, in which the proportion of fruits rises up above the mean, during the season of the highest temperatures (Wright & van Schaik 1994, Kramer et al. 2000). In this study the relation between fruit phenology of Sambucus nigra subsp. canadensis and the temperature showed a significant and positive relation. About this Atkinson & Atkinson (2002) have reported that S. nigra subsp. nigra increases the percentage of its fruits in sites with the highest temperatures.
Anther factor that controls plant phenology is light. The maximum flowering periods of temperate forest understorey species have been registered during the season of most light availability and least cloudiness, which corresponds to the beginning of spring, before the increases in canopy cover (Wright & van Schaik 1994, Cornejo-Tenorio & Ibarra-Manríquez 2007). This argument is supported on the results of this research, since there is a positive relation between the canopy openness and the amount of light with the flower production of Sambucus nigra subsp. canadensis.
It has been proposed that the micro-environmental differences in light are conducive to variability in the fructification times between the individuals that grow up in a single area (Williams-Linera 2003). For example, in Sambucus nigra subsp. nigra and Sambucus racemosa, it has been reported that their establishment and fruit production increases in sites with the highest light availability (Atkinson & Atkinson 2002, Abe et al. 2008, Schaefer & Braun 2009). These results coincide with what we found in this study, since in micro-environment one, which showed the highest canopy openness as well as light amount, the individuals of S. nigra subsp. canadensis showed the highest fruit percentages.
Micro-environmental differences in soil chemical properties can influence the reproductive phenology of plants (Valdez-Hernández et al. 2010). The relation between reproductive phenology and chemical soil characteristics has been little studied. However, an increase in flower percentages in soils with highest nitrogen and humidity has been reported (Galen et al. 1999, Zhang et al. 2013). In this study the highest percentages of Sambucus nigra subsp. canadensis flowers were found in the most humid sites, with highest soil organic matter. The close relation between these characteristics, could be due to the fact that in the decomposition process organic matter is produced and the release of nitrogen by microorganisms is favored, this interaction also promotes the formation of organo-mineral complexes (Liebig et al. 2004).
Although micro-environmental conditions influence the flowering and fructification patterns, the adaptive response is different between the individuals in a population, and it can be independent of environmental factors (Danuso et al. 2012, Post et al. 2001). In this work, the study site includes micro-environmental conditions that did not show a direct relation with the reproductive phenology for all the individuals. For example reproductive phenology of the individuals in group three, did not show any relation with the evaluated micro-environmental variables. Other random factors, or the genetic constitution of individuals, plant diseases, pollination interactions, dispersal and predation can influence the variability of reproductive patterns (van Schaik et al. 1993, Cuevas-García et al. 2013, Alvarado et al. 2014). Likewise, the temporal variability between years can display the relation between environmental factors and the production of reproductive structures at a finer scale (Sherry et al. 2007). The results of our study only consider the environmental variability of one year.
On the other side, in ecosystems that are under continuous anthropic disturbances, micro-environmental changes occur rapidly and they can change plant reproductive phenology (Post et al. 2001, Wolkovich & Cleland, 2010). With respect to this Post et al. (2001) point out those reproductive patterns synchrony tends to be small in conditions of frequent disturbances. Wider distribution and higher abundances of Sambucus nigra subsp. canadensis have been found in perturbed areas (Santibáñez-Andrade 2009). This coincides with our results, because small values of synchrony were registered in the three micro-environments. Since patterns of flowering and fructification are a reflex of the opportunities for pollination and dispersal (Augspurger 1983, Pires et al. 2014) and taking into account the predictions proposed by Post et al. (2001), for species under anthropic disturbances, the small values of synchrony indexes of S. nigra subsp. canadensis suggest that pollinators and dispersers are available during most of the year and they do not present a marked seasonality.
This study is the first approach to the reproductive ecology of Sambucus nigra subsp. canadensis in Mexico. The importance of this research is based in knowing the reproductive dynamics of a disturbance indicator species that shows high importance values in these areas (Santibáñez-Andrade 2009). According to our results, S. nigra subsp. canadensis shows different flowering and fructification patterns in the evaluated micro-environments. The micro-environment with the highest values of nitrogen, organic matter and soil moisture content was the one with the highest percentages of flowers, and the micro-environment with the highest amounts of light was the one that showed a high proportion of fruits. The relation between a higher fruit proportion and high amounts of light has already been reported for its sister subspecies, S. nigra subsp. nigra, which is considered as one of the main invasive and weed species of Europe (Atkinson & Atkinson 2002, Kabuce & Priede 2006). For Atkinson & Atkinson (2002) this close relation is one of the main characteristics that promotes its colonization in temperate forests under anthropic disturbances, since the generation of forest gaps is one of the main perturbations promoted by deforestation.
The data obtained in this work do not exclude the possibility that Sambucus nigra subsp. canadensis can turn into a weed or invasive species, if the ecological dynamics of this community is perturbed by anthropic disturbances. However, in order to determine a species as invasive and/or weed, it is necessary to know its biogeographic origin, its distribution area and to evaluate the dynamics of its reproductive success in the long term, through its reproductive phenology, germination and survival (Richardson et al. 2000, Wolkovich & Cleland 2010, Danuso et al. 2012). In the case of S. nigra subsp. canadensis little is known about its ecology. Therefore it is necessary to continue the study of its reproductive phenology in order to determine how micro-environmental differences can influence other characteristics of its life history, such as germination and survival, as well as to understand the ecological role of this species in Mexican temperate forests.











 nova página do texto(beta)
nova página do texto(beta)


