Ecological studies have generally focused on the core or buffer zones of natural protected areas, leaving aside the studies of disturbed environments such as urban areas (Cursach et al. 2012). However, urbanization continues growing, which involves modification of the original environmental characteristics, changing as the urban area extends, causing loss of vegetation cover or modification of the abiotic components, such as soil, changing its physical and chemical properties, and altering the energy balance of the soil-atmosphere system leading to the formation of the urban heat island (Barradas 1987, Martínez Jiménez 2007). Cities are conformed by a mosaic of microclimates; therefore, different methods have been proposed to mitigate the urban heat island, such as the installation of water fountains in the city or the vegetation establishment (Ochoa-de la Torre 2009, Ballinas 2011, Ballinas & Barradas 2016). Urban vegetation is defined as any forested area influenced by the urban population.
Urban vegetation was used initially only for ornamental and aesthetic purposes; however, from the early sixties of the twentieth century in Germany and USA, some research on how the presence of vegetation in urban environments changed the microclimate with a resulting energy savings was conducted (Ochoa-de la Torre 2009). From this research, the presence of vegetation in the city has been linked to environmental and economic factors, such as the reduction of CO2 concentrations, removal of air pollution, reducing the use of air conditioning benefits, among others (Hernández-Palma 2008). Also, a decrease in temperature and increase in humidity are related to vegetation presence, which positively influence indices of human comfort/discomfort (Barradas 1991).
However, there is little research about urban vegetation (Barillas-Gómez 2004), which is reflected in the planning of green areas in cities, considering the attractive, the rapid growth or the cost of the species, as most important selection factors (Segura-Burciaga 2007), leaving aside the knowledge of the ecophysiological characteristics of species, which determine the survival of a species in the environment.
Understanding how the plant responds to environmental variables can help us predict its physiology. One of the most frequently used variables to evaluate the response of the plant to the environment is the stomatal conductance (gS). Stomatal conductance controls the water status of the plant and plays a role in photosynthesis (Jones 1992, Ramos-Vasquez & Barradas 1998). Stomatal behavior analyses are mainly based on the gS response to environmental factors such as photosynthetically active radiation (PAR), air temperature (TA), vapor pressure deficit (VPD) and water availability in the soil (Ramos-Vasquez & Barradas 1998). Water availability is evaluated by determining the soil water potential (ΨS) or leaf water potential (Ψ) which is proportional to the water status of the plant, and this in turn, the soil water status, which can be a good indicator of how a plant is resistant to drought and the amount of water available in the soil for the plant (Turner 1986).
The city of Puebla has a population of 1,539,819 inhabitants (INEGI 2011) and its urban area is expanding with an annual population growth rate of 2.3 % (INEGI 2004). However, like other cities, there is no a proper planning of green areas since in the selection criteria the ecophysiological characteristics of the target species is not included. Furthermore, Puebla is considered as one of the Mexican cities with less vegetation cover (Barillas-Gómez 2004, Morales-García de Alba 2009).
Taking this into account, the aim of this study was to evaluate the effect of urban microenvironment (TA, VPD, PAR) and leaf water potential (Ψ) on stomatal conductance (gS) of four tree species: Ficus retusa L., Fraxinus uhdei (Wenz.) Lingelsh., Ligustrum lucidum W.T.Aiton and Quercus rugosa Née, as part of the urban trees in the city of Puebla during the dry and wet seasons.
Materials and methods
Study area. The urban area of the city of Puebla, Mexico (18° 50’ 42” 19° 13’ 48” N, 98o 00’ 24” 98o 19’ 42” W) occupies an area of 157,933 km2. Most of the city has a flat topography with maximum and minimum elevations of 2,300 and 2,055 m, respectively. The predominant climate is temperate and humid, with a total annual precipitation of 751-1,000 mm rainfall from May to October, and an average annual temperature of 16 °C, with a warmer period (March, April, May) with temperatures up to 36.5 °C (Gobierno del Estado de Puebla 2007) with a marked effect of the urban heat island in the urban area with intensities up to 3.5 °C (Martínez-Jiménez 2007).
Study sites and selected species. Two contrasting sites, with presence and absence of the urban heat island, were selected using microclimate maps developed by Martínez-Jiménez (2007). Loma Linda site (18° 59’ 47.29” N, 98° 13’ 4.17” W, 2131 m a.s.l., Figure 1) shows the effect of urban heat island, and the area (1 km diameter) is predominantly paved and built (92 %) with just an 8 % of vegetation. Whereas in Clavijero site (19° 1’ 37.77” N, 98° 8’ 11.91” W, 2209 m a.s.l., Figure 1) there is no significant effect of the urban heat island; this area has 30-35 % of vegetation and 65-70 % pavement and buildings. Four tree species were selected: Ficus retusa L., Fraxinus uhdei (Wenz.) Lingelsh., Ligustrum lucidum W.T.Aiton and Quercus rugosa Née The first three species comprise more than 50 % of the trees in the city (Gobierno del Estado de Puebla 2007). Ficus retusa and L. lucidum are introduced species from Asia, F. uhdei is a native species and Q. rugosa, although is native to the Valle Poblano-Tlaxcala, it has been suggested for urban environments due to its slow growth could not constantly interfere with the street wiring (CONABIO 2012).
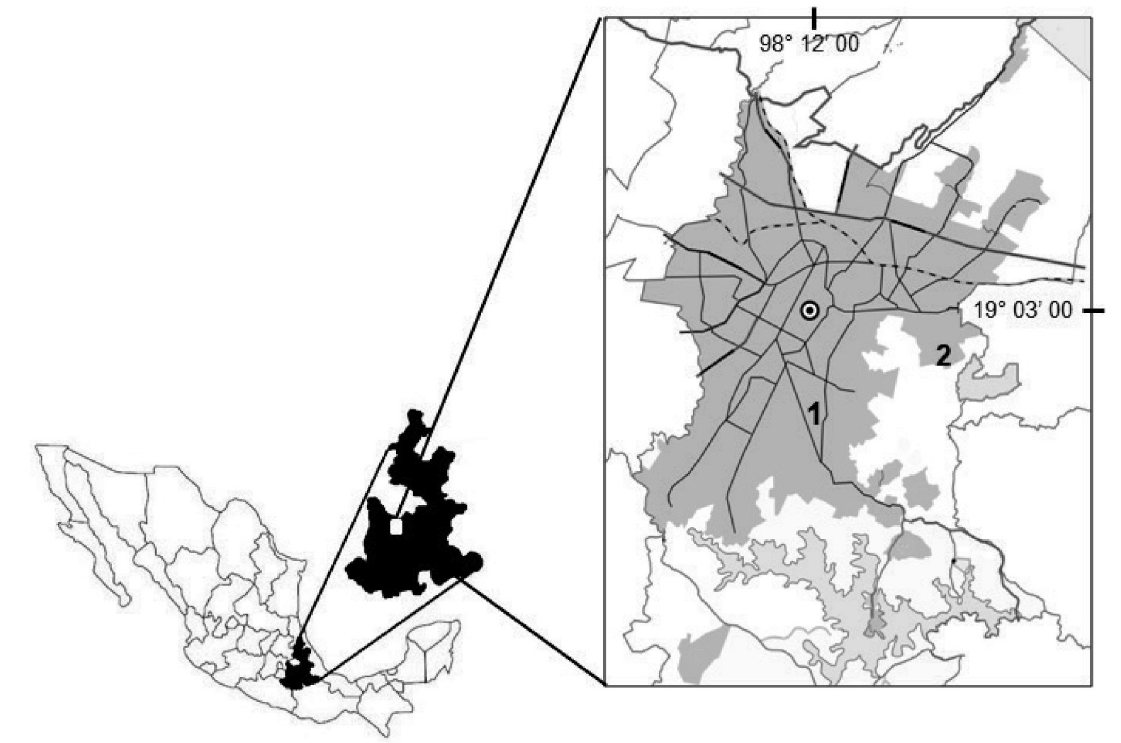
Figure 1 Location of the study sites at Loma Linda (1) and Clavijero (2), in the urban area of the city of Puebla, Mexico.
Experimental design in the field. Three saplings of each species were measured in each site, considering that all measurements must be performed as simultaneously as possible. Plant height was 0.80 to 1.2 m. Measurements were made during the dry (March-April 2013) and wet (October-November 2012 and August-September 2013) seasons. All measurements were performed for five non-consecutive days for each season per site, giving a total of ten days of measurement per site. Measures were taken every two hours from 0800 to 1600 hours (local hour, h).
Stomatal conductance (gS) was measured with a steady-state porometer (LI-1600, LI-COR, Ltd., Lincoln, Nebraska, USA) in three mature leaves randomly selected without signs of deterioration or herbivory and with apparent good health. Leaf water potential (Ψ) was measured on three leaves of each tree with a Scholander pressure bomb (PMS, Corvallis, Oregon, USA). At the same time, measurements of the microclimatic factors of air temperature (TA), photosynthetically active radiation (PAR) and relative humidity (RH) were performed. The leaf temperature (TL) was measured with fine thermocouples and PAR was measured with a quantum sensor (LI-190SB, LI-COR, Ltd., Lincoln, Nebraska, USA). Air temperature and RH were measured with a sensor HUMICAP (Vaisala, Helsinki, Finland) installed on the porometer. Estimation of vapor pressure deficit (VPD) was obtained from RH, TA and TL to the following equation: VPD = eSL RHeSA, where eSL and eSA are the vapor pressure saturation of the leaf and air, and are functions of TL and TA, respectively (Ramos-Vasquez & Barradas 1998).
Experimental design in the greenhouse. Two experimental groups including the four species were formed and were kept in the greenhouse of the Institute of Ecology, National Autonomous University of Mexico (UNAM), in order to determine whether there were differences in the ecophysiological responses between species under water stress conditions. All individuals were between 1.0 and 1.3 m height. Plants were placed in containers of 40 cm height by 30 cm in diameter. Individuals were divided into two groups: a control group (well-watered) of 10 individuals per species, and an experimental group of 10 individuals per species, subjected from well-watered plants to water stress by withholding water during 13 days.
Before the water stress experiment a pilot study was conducted, whose purpose was to establish the time of day when the maximum and minimum gS and Ψ values were registered. We measured the microclimatic (PAR, VPD, TA) and ecophysiological (gS, Ψ) variables in three leaves of ten individuals per species for seven consecutive days from 0700 to 1600 h. We observed the maximum and minimum gS and Ψ values at 0700 and 1400 h, respectively.
Under water stress conditions, we measured daily the ecophysiological and microclimatic variables in at least three leaves per individual at 0700 and 1400 h. Also we performed diurnal measurements from 0700 to 1600 h, every two hours, at the beginning and at the end of the experiment. The measurement period ended when individuals showed signs of wilting or leaf loss. The control group was irrigated daily at 0630 h with a liter of water. The experimental group was irrigated only at the beginning of the experiment.
Statistical analyses. To determine whether there were significant differences among the ecophysiological variables, species, study sites (presence/absence of urban heat island effect), and seasons we used the non-parametric test Kruskal-Wallis. The effect of the physiological (Ψ) and microenvironment (PAR, TA, DPV) variables on gS in both study sites and in the greenhouse, was determined with a multiple regression analysis (Zar, 1986). Data were analyzed using a computer program (Statgraphics Centurion 16.1, Statpoint Technologies, Inc., Warrenton, VA, USA). Variance homogeneity was tested using Levene’s test. Statistical significance was considered at 95 % for all cases.
Results
Microclimatic variables in the field. Maximum PAR values were recorded at 1400 h, and were higher in the dry season (H = 73.752, df = 1, P < 0.001) and in Loma Linda (H = 18.583, df = 1, P < 0.001). The highest temperature was recorded between 1200 and 1400 h, whereas the lowest at 0800 h. As expected, temperature was higher in the dry season (H = 52.683, df = 1, P < 0.001) in Loma Linda (H = 25.122, df = 1, P < 0.001). Concomitant to TA, VPD was higher in the dry season (H = 81.361, df = 1, P < 0.001) and in Loma Linda (H = 38.737, df = 1, P < 0.001). This behavior is due to the season and the urban heat island effect. Maximum VPD values were observed between 1400 and 1600 h in both seasons and sites (Figure 2).
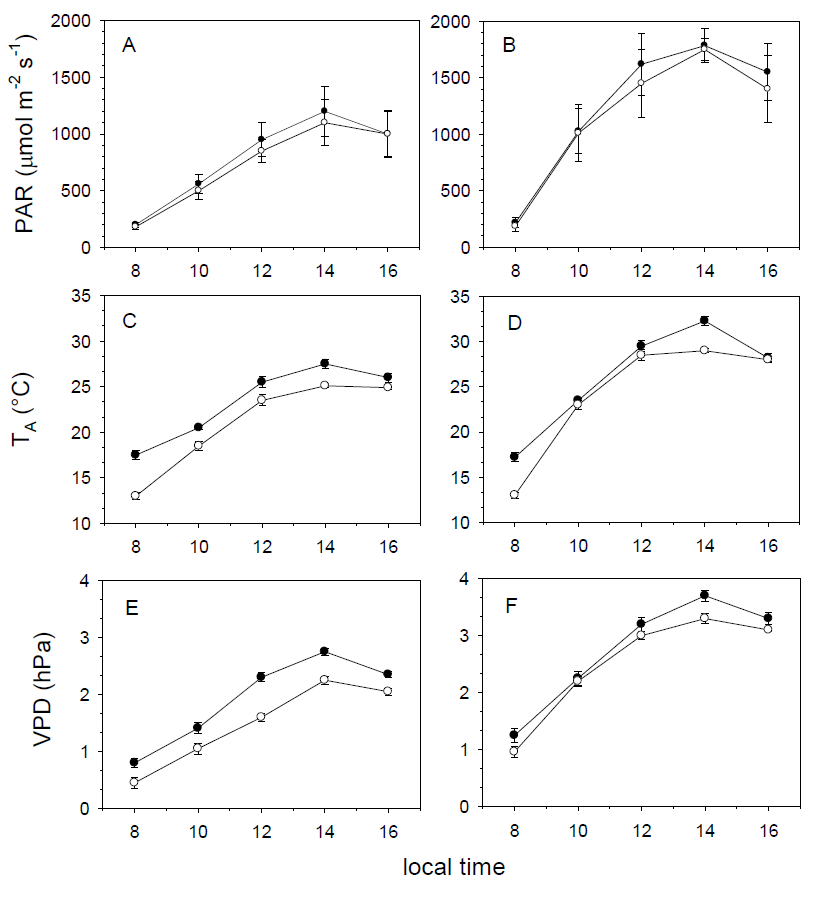
Figure 2 Diurnal variation of photosynthetically active radiation (PAR), air temperature (TA) and vapor pressure deficit (VPD) in Loma Linda (closed symbols) and Clavijero (open symbols) in the wet (A, C and E) and dry (B, D and F) seasons.
Ecophysiological variables in the field. For all species maximum gS and Ψ were recorded at 0800 h; whereas the lowest values were registered at 1400 h. They were also lower in the dry (H = 106.451, df = 1, P < 0.001) than in the wet season for all species at both sites (Figures 3 and 4).
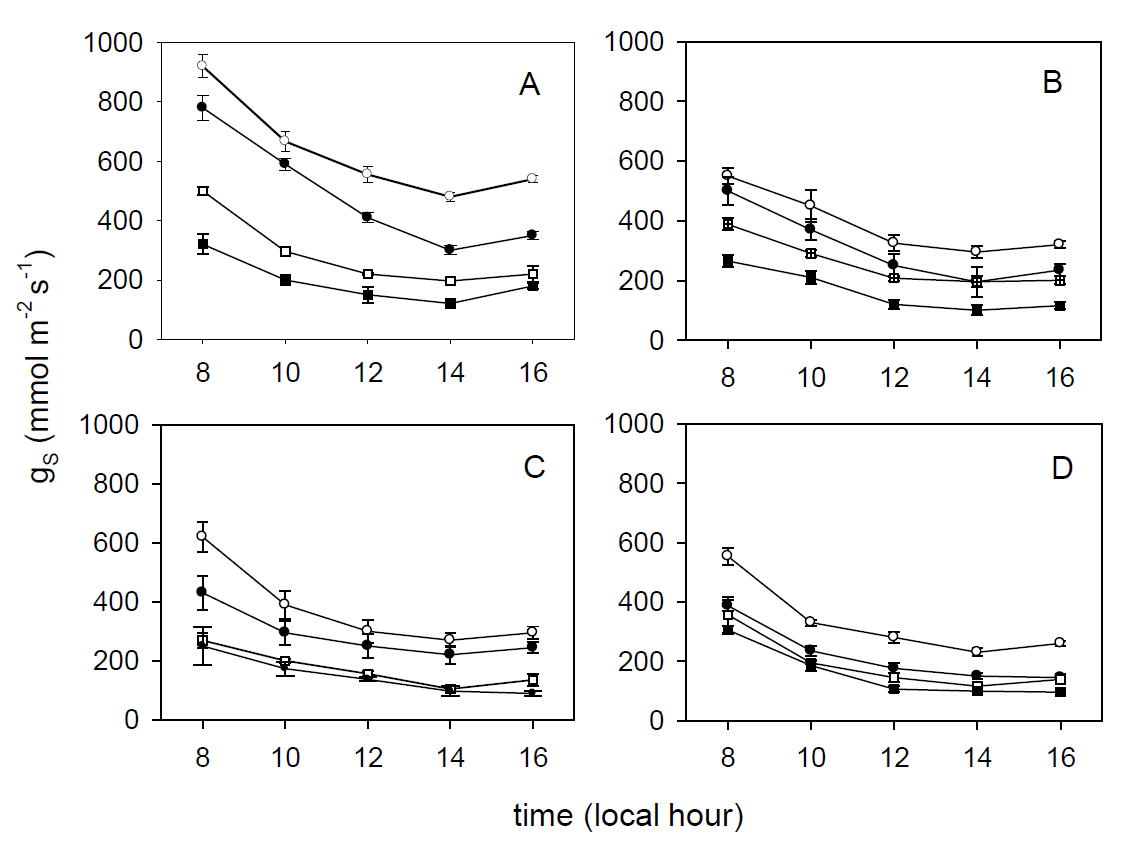
Figure 3 Stomatal conductance (gS) patterns for Ficus retusa (A), Fraxinus uhdei (B), Ligustrum lucidum (C) and Quercus rugosa (D) at Loma Linda (closed symbols) and Clavijero (open symbols) in the wet (circles symbols) and dry (squares symbols) seasons.
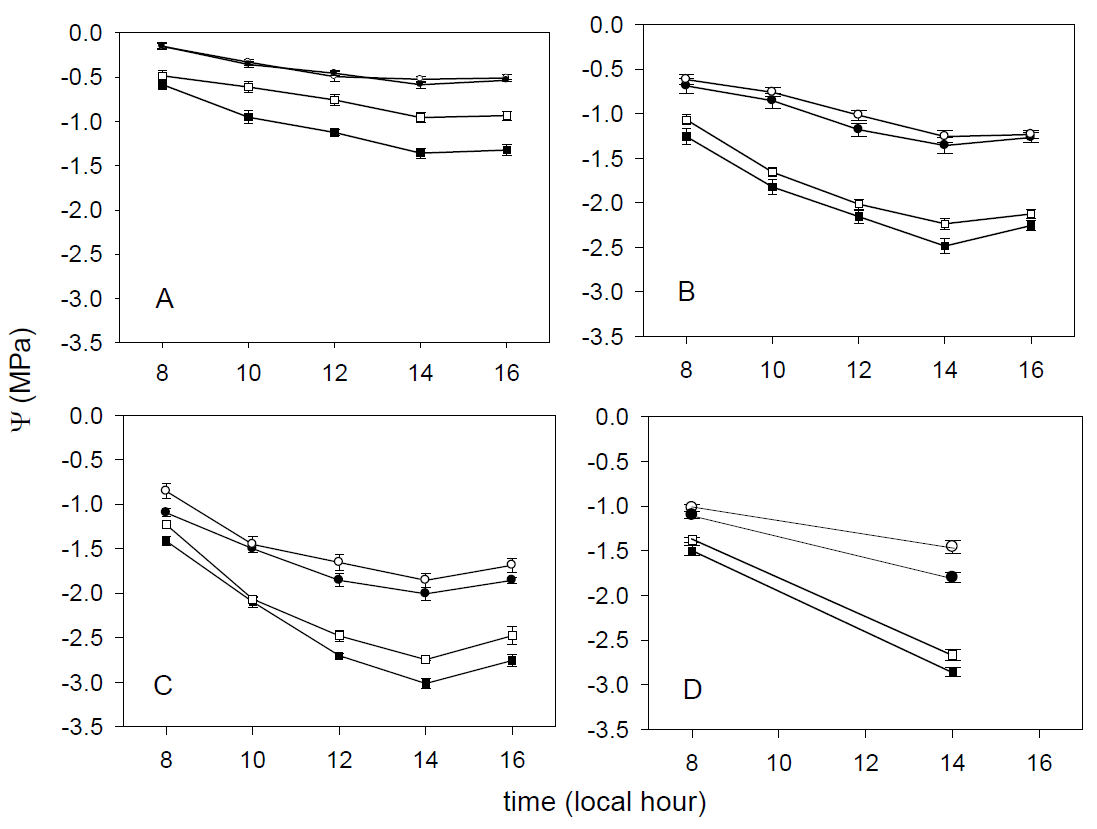
Figure 4 Leaf water potential (Ψ) patterns for Ficusretusa (A), Fraxinus uhdei (B), Ligustrum lucidum (C) -1.0 and Quercus rugosa (D) at -1.5 Loma Linda (closed symbols) and Clavijero (open -2.0 symbols) in the wet (circle -2.5 symbols) and dry (square symbols) seasons.
Differences in gS were found between sites and among species in the wet (H = 129.563, df = 1, P < 0.001) and dry season (H = 84.769, df = 1, P < 0.001) (Figure 3). Similarly, we found differences in Ψ between sites, being in Clavijero higher in the wet (H = 187.093, df = 1, P < 0.001) and dry season (H = 128.462, df = 1, P < 0.001) (Figure 4).
Stomatal conductance presented significant differences among species at both sites and seasons (H = 140.391, df = 1, P < 0.001) (Ficus retusa > Fraxinus uhdei > Quercus rugosa = Ligustrum lucidum, Figure 3). Leaf water potential had significant differences among species, sites and seasons (H = 324.361, df = 1, P < 0.001) (F. retusa > F. uhdei = Q. rugosa > L. lucidum, Figure 4).
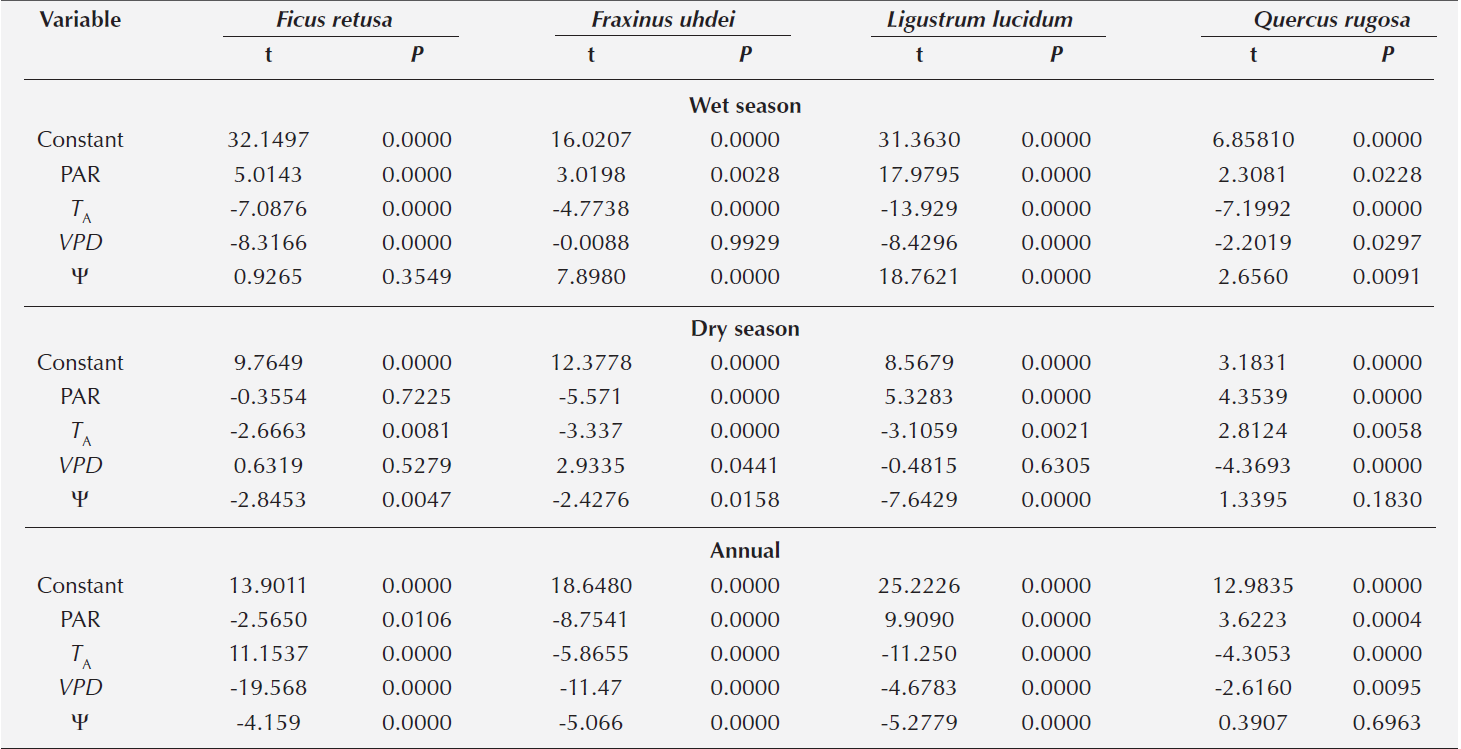
Effect of the microenvironment in the field on gS. When correlating the microclimatic factors (TA, PAR, VPD) and Ψ with gS, we found a strong relationship with all factors for Quercus rugosa and Ligustrum lucidum in the wet season, whereas for Ficus retusa and Fraxinus uhdei we only found a relationship with TA and PAR. In the dry season, all microclimatic factors affected the gS for F. uhdei, whereas for L. lucidum and Q. rugosa, no relationship with VPD and Ψ was found; gS for F. retusa only correlated to TA and Ψ. However, after unifying data from the two seasons, we observed for F. retusa, F. uhdei and L. lucidum a correlation between gS and all microenvironmental factors and Ψ. Quercus rugosa showed no relation to Ψ (Table 1).
Table 1 Multiple regression of stomatal conductance (gS) versus photosynthetically active radiation (PAR), air temperature (TA), vapor pressure deficit (VPD) and leaf water potential (Ψ) of four tree species in the wet and dry season, and annual period in the city of Puebla, Mexico.
Ecophysiological variables and microclimate in the greenhouse. Values of TA, PAR, VPD in the greenhouse were similar for both conditions (irrigated and stressed). Maximum values were recorded at 1400 h (35.0 °C), and the minimum at 0700 h (15.0 °C) and both patterns (irrigated and stressed) were similar. At the end of the experiment Ficus retusa, Fraxinus uhdei and Ligustrum lucidum presented wilting signs. For all species, control and experimental groups, maxima gS and Ψ values were registered at 0700 h, whereas minima values were registered at 1400 h. In well-watered conditions, there were significant differences among species for gS (H = 494.752, df = 3, P < 0.001) (F. retusa > F. uhdei = Q. rugosa > L. lucidum). With respect to Ψ, significant differences among species were found (H = 1088.068, df = 3, P < 0.001) (L. lucidum < Q. rugosa < F. uhdei < F. retusa).
In the stressed group, significant differences among species were found in gS (H = 610.410, df = 3, P < 0.001) and Ψ (H = 246.897, df = 3, P < 0.001). Throughout the experiment, gS was higher at 0700 h than at 1400 h; however, as days passed, differences among species declined.
Leaf water potential at 0700 h was similar for all species; however, at 1400 h differences among species were found during the beginning of the experiment, as days passed differences were subsequently declining (Figure 5A, B). Similarities between gS and Ψ concerning grouping pattern were observed. Values for Ficus retusa were significantly higher, followed Fraxinus uhdei, whereas the lowest values were found in Quercus rugosa and Ligustrum lucidum, which did not differ from each other (Figure 5C, D).
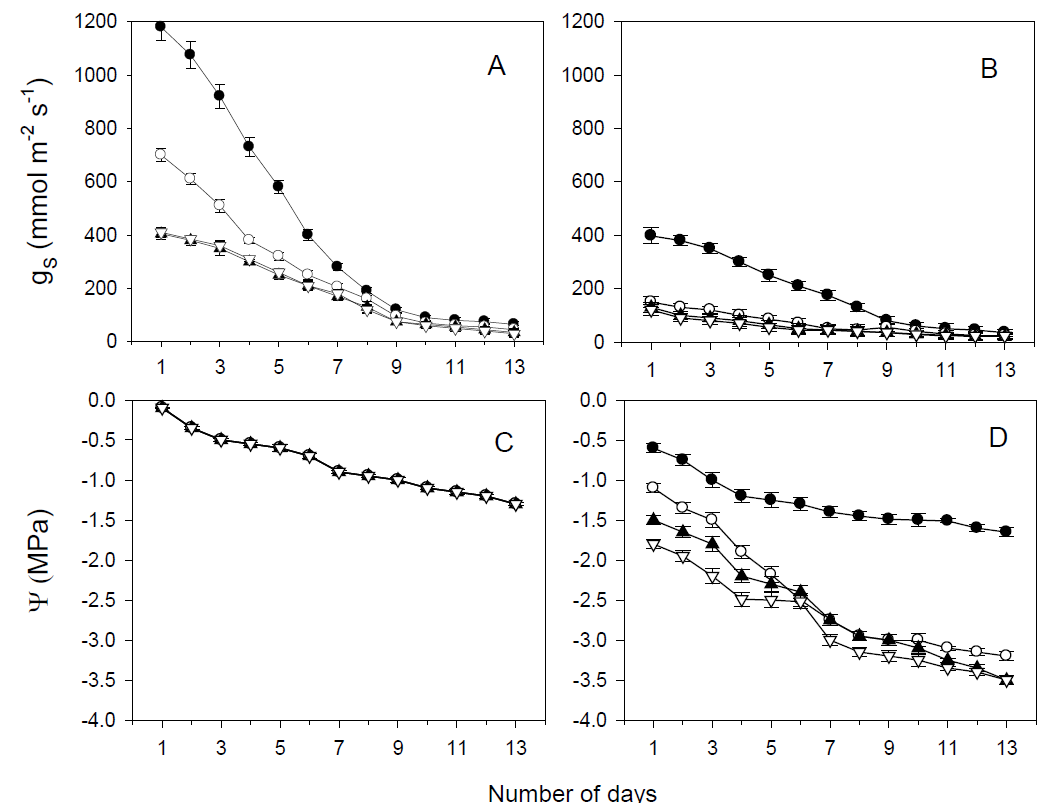
Figure 5 Stomatal conductance (gS; A, B) and leaf water potential (Ψ; C, D) during water stress experiment withholding water for 13 days for Ficus retusa (black circles), Fraxinus uhdei (open circles), Ligustrum lucidum (black triangles) and Quercus rugosa (open triangles) at 0700 h (A, C) and 1400 (B, D) local hour.
Effect of the microenvironment in the greenhouse on gS. For the control group we observed for all species a correlation between gS and all microenvironmental variables (TA, PAR, VPD). Under conditions of water stress for all species we observe that gS did not relate to PAR; Quercus rugosa did not correlated to TA either (Table 2).
Discussion
Microclimatic variables. Photosynthetically active radiation was highest at 1400 h and in the dry season. Differences in PAR between seasons affected the stomatal behavior, because having intermittent cloudy days in the rainy season, gS fluctuations were higher.
Regarding TA, previous studies determined the presence of the urban heat island in the city of Puebla (Martínez-Jiménez 2007), concluding that the heat island is present in the center, extending to the south of the city; while cooler areas are on the east side of the metropolitan area. Temperature observations of this study corroborate the information above, finding in Loma Linda significantly higher temperatures. Temperature differences at both seasons are possibly given by dissimilarities in development, having in Loma Linda a higher proportion of built area compared to Clavijero. Urbanization is a determining factor for the formation of urban heat island, because the greater the building area the greater the heating, as the original atmosphere-soil characteristics are changed, contributing to the imbalance in the energy balance of the system causing the phenomenon of urban heat island (Barradas 1987, Martínez-Jiménez 2007, Ochoa-de la Torre 2009).
Vapor pressure deficit was higher at 1400 h and in the dry season at both sites and in the greenhouse. These results coincided with previous studies (Goldstein 1986, Ramos-Vasquez & Barradas 1998, Esperón-Rodriguez & Barradas 2015). The VPD variation during day involves fluctuating temperature and humidity in the atmosphere. When the temperature was increased, in the field and in the greenhouse, VPD was higher, indicating that there was a greater evaporative demand, as the surrounding atmosphere is more eager for water, inducing stomatal closure to prevent excessive loss of water (Jones 1992, Squeo & León 2007).
Ecophysiological variables. Maximum gS and Ψ for all species were recorded at 0800 h during the wet season whereas the minimum values were recorded at 1400 h. Under greenhouse conditions, the gS and Ψ diurnal variation followed a similar pattern to that observed in the field in all species. These results might be due to changes in TA, PAR and VPD between seasons and sites (Goldstein 1986, Ramos-Vasquez & Barradas 1998, Esperón-Rodriguez & Barradas 2014, 2015).
Differences in gS among species observed followed the relation Ficus retusa > Fraxinus uhdei > Ligustrum lucidum = Quercus rugosa. Stomatal conductance is closely related to biomass productivity and hence the rate of growth (Hogan et al. 1995). In this case F. retusa is the species with the highest relative growth rate, followed by F. uhdei, L. lucidum and finally Q. rugosa. Leaf water potential was higher at 0700 h at Clavijero, in both seasons and for all species, in agreement with previous studies, which mentioned that the minimum values ??are recorded at 1400 h in the dry season, while higher values occur around 0800 h in the wet season (Goldstein 1986, Ramos-Vasquez & Barradas 1998, Squeo & León 2007, Esperón-Rodriguez & Barradas 2014, 2015).
In the early morning hours the highest Ψ might be related with low PAR and TA. At this time the evaporative demand is lower (Goldstein et al. 1986). As the day passes, the demand for water from the atmosphere increases, taking water from the plant and soil, therefore the amount of water available in the soil decreases, so the plant needs more strength to prevent water loss, causing stomata closing (Jones 1992, Squeo & León 2007). Maximum values in the wet season could be due to a greater availability of water in the soil, therefore, plants require less strength to get water. However, in the dry season there is less water availability.
Differences in Ψ between sites were found in both seasons for all. This may be due to differential urbanization degree between sites, because having more pavement surface in Loma Linda, the evaporative demand increases, reducing water availability in the substrate, which is reflected in Ψ, which was significantly lower than in Clavijero. The pattern observed was that Ficus retusa had the highest Ψ, followed by Fraxinus uhdei, Ligustrum lucidum and Quercus rugosa. The drought resistance is associated with low Ψ (Turner, 1986; Curran et al. 2010, Esperón-Rodríguez & Barradas, 2015). Quercus rugosa and L. lucidum could be considered the most drought resistant species, whereas F. retusa and F. uhdei were less tolerant.
Relationship between gS and the microenvironmental variables. The microenvironmental variables effect on gS was very difficult to establish in any season probably due to the low variability of data, finding hard to observe a clear pattern in the relationships; however, after combining data from both season, we could see clearer effects of that relationship.
However, analyzing the annual period all the measured environmental variables and Ψ had an effect on gS for all species, except Quercus rugosa, where Ψ was not significant. From the multiple regression of the control group in the greenhouse was found that all variables affected the gS response in all species; whereas for the experimental group no relationship between gS and PAR was observed in all species, and for TA for Q. rugosa.
Also, Quercus rugosa did not show a Ψ effect on gS in the field. This might be because the substrate in the field never was completely dry (Goldstein et al. 1986), we could assume that Q. rugosa has different mechanisms for obtaining water or mechanisms to avoid water loss (Curran et al. 2010).
With respect to PAR, it was not correlated to gS in all species under water stress in the greenhouse, which could be due to lack of water availability. Water absence causes plants to close their stomata to prevent loss regardless their response to PAR. Stomatal opening is related to various environmental factors, but some factors have more weight than others, such is the case of Ψ (Squeo & Leon 2007). PAR is needed but to a limited extent, because excess radiation can affect stomatal apparatus, therefore plants are gradually closing the stomata as PAR increases (Goldstein et al. 1986, Ort 2001). The effect of PAR on gS appears to be related to the amount of CO2 available in the substomatal cavity (Squeo & León 2007).
Air temperature had a significant relationship with gS for all species in the greenhouse and in the field. PAR is linked to temperature, so high gS of the early morning hours decreases as the maximum daytime temperature is reached, because at high temperatures, stomata close (Baruch & Fisher 1988, Goldstein et al. 1986, Squeo & León 2007). VPD was always associated with gS for all species in both greenhouse and field, finding a linear relationship between these two variables. The trend observed was that the lower VPD the greater gS, this because when VPD is higher, the greater the evaporative demand, therefore the plant reacts by closing the stomata to prevent water loss.
Differences among species. The gS response to microenvironmental variables for Ficus retusa indicates a high stomatal control, this because any change of the variables directly affects the gS, especially Ψ. This species was the first one to show signs of wilting in the water stress experiment, and it also recorded the higher Ψ. Stomatal conductance was also the highest for this species, which is consistent with its growth rate (Hogan et al. 1995). Ficus retusa is a native species of moist warm environments of southeastern Asia to northern Australia (Gilman & Watson 1993), so it is adapted to conditions where water scarcity is rare, low Ψ are typical of plants adapted to arid environments (Turner, 1986).
Fraxinus uhdei showed high stomatal sensitivity to changes of all variables. In the morning in the field and under well-watered conditions, the gS was significantly higher than in Ligustrum lucidum and Quercus rugosa; however, at 1400 h under water stress, the gS response was similar among these species. This behavior may be due to adaptation mechanisms to drought, developed by the species to prevent water loss. Therefore, F. uhdei was considered as a drought-avoider species with high gS sensitivity to microenvironmental changes, because the range between the minimum and maximum gS was wider than in other species (Goldstein et al. 1986). The natural distribution of the species includes gallery forests, pine-oak and cloud forest, from Sinaloa to Chiapas, Mexico (CONABIO 2014) with a dry season, so F. uhdei may have some tolerance to water stress, presenting low Ψ, but it is also an avoider species, as it is a deciduous species.
For Ligustrum lucidum Ψ and gS were significantly lower than Ficus retusa and Fraxinus uhdei, suggesting that this species is better adapted to water stress conditions. The species is native to eastern China and south-west Japan, from subtropical humid all to cold and dry climates (Aragón & Groom 2003). Therefore, the species can grow in cold and dry environments, so it could be assumed that it developed mechanisms to prevent excessive water loss, presenting low values Ψ and gS, thus being a drought tolerant species. Of the four species studied, is the only species considered as an invasive species, so it is not recommended for reforestation (Aragon & Groom 2003).
Quercus rugosa was the only species with no relationship between gS and Ψ, except under water stress conditions in the greenhouse. In the field, Q. rugosa might have metabolic level mechanisms that allow them to live under water scarcity conditions, suggesting a drought tolerant species, and an avoider species being deciduous. This coincides with the widespread distribution of Q. rugosa, from west Texas and southern Arizona, USA, to Chiapas, Mexico (CONABIO 2012) including within its range from semi-dry to moist environments, is likely that this species has developed mechanisms to tolerate and avoid drought.
It is clear that the four studied species respond satisfactorily to the urban environment even in the city center where the microenvironment is relatively more stressing than the rural surroundings, since these species showed an effective control on gS and Ψ to face water stress. However, from these results it is possible just to recommend Ficus retusa and Fraxinus uhdei as suitable species to reduce the urban heat loads produced by the heat island through the cooling potential given by transpiration as these two species presented the highest stomatal conductance values (Barradas 1991, 2000).
Transpiration is a mechanism where the heat loss is about 2.442 MJ per kg of evaporated water (Jones 1992), therefore the higher gS the higher transpiration and the higher cooling potentiality of plant species. Nevertheless, in an urban environment as the presented in the city of Puebla it is very probably that soil water should be scarce mainly in the dry season and at the city center, when these two species showed low Ψ values indicating a high water stress for both species and Fraxinus uhdei as a deciduous species could remain with no leaves in the warmest period (March, April and May). Taking this into account, it could be recommendable to build tree arrangements including the four species in a proportion where the species which registered the highest stomatal conductance dominate. Therefore, it is important to design such tree arrangements considering both urban and physiological characteristics in the future to take advantage of the urban vegetation to mitigate the urban heat island. As an important result, it is necessary to state that it is not enough to just reforest the city, but it must be by using the most suitable species.











 nova página do texto(beta)
nova página do texto(beta)