Introduction
Today, the planting of trees in cities is done with the intention of obtaining environmental, social and economic benefits, also known as ecosystem services of urban forests (de Abreu-Harbich, Chebel, & Matzarakis, 2015; Moser et al., 2018; Pincetl, Gillespie, Pataki, Saatchi, & Saphores, 2012). The proper establishment of urban trees involves consideration of climatic and soil aspects as well as species (Jankovska et al., 2015; Koeser, Gilman, Paz, & Harchick, 2014); however, when climatic conditions are favorable and suitable tree species for the planting space are used, one of the main problems for optimal plant development is the condition of the soil (Day, Wiseman, Dickinson, & Roger, 2010; Ow & Yusof, 2018).
The World Reference Base for Soil Resources classifies urban soils as Technosols (Food and Agriculture Organization of the United Nations [FAO], 2015), which do not have a definite arrangement of layers (horizons) and possess material of anthropogenic origin (Hassan, 2018; Watson, Hewitt, Custic, & Lo, 2014). For this reason, it is common to find disturbed soils in urban areas due to constant remodeling, adaptation or construction of infrastructure (Guilland, Maron, Damas, & Ranjard, 2018; Pereira et al., 2016). Physical alteration of soil layers modifies general properties such as infiltration, aeration, microfauna, nutrients, texture, density, pH, and concentration of salts or carbonates (Pereira et al., 2016; Scharenbroch, Meza, Catania, & Fite, 2013; Tresch et al., 2018). This limits proper root growth and development, demerits plant vitality, and increases the risk of mortality (Ow & Yusof, 2018; Vidal-Beaudet, Galopin, & Grosbellet, 2018).
The trees set in urban environments goes through a process of adaptation to the new conditions of the planting site (Mohedano-Knight, Cetina-Alcalá, Chacalo-Hilu, Trinidad-Santos, & Gonzalez-Cossio, 2005), in which the vegetation presents some degree of physiological stress that affects its growth (Jankovska et al., 2015; Johnston & Hirons, 2014). This situation is aggravated by the use of young trees, a common practice in the interior of cities due to the ease of management and planting (Koeser et al., 2014; Roman, Battles, & McBride, 2013). Newly transplanted young trees are more vulnerable to physiological stress because they have small energy reserves to cope with stressful situations (Day et al., 2010; Ramírez, Handa, Posada, Delagrange, & Messier, 2018). As a result, success in urban afforestation is low, with mortality rates ranging from 30 to 50 % in the first year of planting (Percival & Fraser, 2005).
The use of amendments in urban soils is a viable strategy to improve their physical, chemical and biological characteristics (Ceveira & Lavado, 2006; Vidal-Beaudet, Forget-Caubel, & Grosbellet, 2015). The amendments cover well-root manures, fertilizers and the use of carbohydrates such as sucrose, glucose, fructose and starch (Percival, Fraser, & Barnes, 2004). The application of amendments of carbohydrates to the soil implies that small amounts of these are used by the microorganisms, thereby increasing the microfauna of the soil (Martínez-Trinidad, Watson, Arnold, & Lombardini, 2010; Percival & Fraser, 2005), being the biodiversity of the organisms that inhabit the soil, an important indicator of its quality (Schloter, Nannipieri, Sørensen, & Elsas, 2017). Soil microorganisms participate in important biochemical processes that contribute to improving soil properties and nutrient availability for plants (Ponge et al., 2013). Similarly, carbohydrate amendments such as sucrose, applied to the root system of trees, promote the growth of fine roots (Al-Habsi & Percival, 2006; Percival et al., 2004). Proper development of the root system in a newly planted tree is key to its survival in adverse environments such as those prevailing in a city (Day et al., 2010; Vidal-Beaudet et al., 2015). These factors improve settlement and increase the survival rate of urban woodland (Scharenbroch et al., 2013; Vidal-Beaudet et al., 2018). In this context, the objective of this study was to evaluate the effect of the application of carbohydrates (sucrose and glucose) to the soil on the growth and vitality of jacaranda trees (Jacaranda mimosifolia D. Don) planted in urban areas, as a management alternative during the initial stages of tree establishment.
Materials and methods
Study setting
The assay was set in the urban green area named "Alameda Texcoco" in Texcoco de Mora, whose coordinates are 19° 31' 11.07" N and 98° 52' 29.68" W, at an average altitude of 2 250 m. The climate is temperate semi-dry, with an average annual temperature of 15.9 °C and a precipitation of approximately 650 mm per year (Moreno, 2007). The soil is a Vertisol type with clayed texture, moderate drainage, neutral to slightly acidic pH, organic matter between 1.12 and 2.03 % and electrical conductivity between 0.53 and 0.71 dS·m-1 (Gutiérrez & Ortiz, 1999; Segura, Gutiérrez, Ortiz, & Gómez, 2000). Thirty-six jacaranda trees with a normal diameter of 0.05 m and a height of 2 m, on average, were planted in holes of 0.5 m long x 0.4 m high approximately with separation of 6 m between plants. The treatments were completely randomized by factorial arrangement 3 x 3 (0, 60, 80 g·L-1 glucose; 0, 60, 80 g·L-1 sucrose) (Table 1).
Table 1 Carbohydrate treatments in jacaranda trees (Jacaranda mimosifolia) planted in "Alameda Texcoco", State of Mexico.
| Glucose (g·L-1) | Sucrose (g·L-1) | Treatment |
|---|---|---|
| 0 | 0 | G0S0 (Control) |
| 0 | 60 | G0S6 |
| 0 | 80 | G0S8 |
| 60 | 0 | G6S0 |
| 60 | 60 | G6S6 |
| 60 | 80 | G6S8 |
| 80 | 0 | G8S0 |
| 80 | 60 | G8S6 |
| 80 | 80 | G8S8 |
In the first application of the treatments, the surface layer of the soil was removed in order to remove the vegetation around the trunk; subsequently, Faena® herbicide was applied every two months to avoid the presence of vegetation. The treatments were applied as drenches of 10 L of solution directly to the soil at a distance of 0.5 m from the base of each tree, after transplantation (Martinez-Trinidad et al., 2010), with seasonal applications for an entire year.
Plant parameters
Tree growth was measured seasonally with a diameter tape (Forestry Suppliers Inc., Jackson, MS, USA), taking into account the increase in diameter of the trunk (cm), from an indelible mark made at 0.1 m above the ground, and the increase in total height of each tree (m) with a retractable tape (Truper®). The foliage color of each tree was determined at the beginning of the trial as a reference point (before applications), and seasonally (Mohedano-Caballero et al., 2005) through pictures taken with a 12.1 megapixel digital camera (Sony DSC-w290®). These were obtained between 9 and 11 o´clock with a west orientation and only in the seasons where the trees had foliage. In each picture, the number of green pixels was analyzed using the “histogram” module of the ImageJ® computer software (National Institutes of Health, Wayne Rasband, USA) version 1.50i (Abràmoff, Magalhães, & Ram, 2004; Schneider, Rasband, & Elieiri, 2012). The dry matter of roots of each tree was quantified through two holes made in the soil (0.05 m diameter x 0.1 m length), North and South, at a distance of 0.5 m from the base of the tree, which were filled with sand (Martinez-Trinidad et al., 2010). This was collected every two and a half months, placed in plastic bags inside a cooler at 4 °C and immediately transported to the laboratory. Samples were carefully washed to separate the roots from the sand; subsequently, the plant material was dried in a forced air stove at 65 °C for 72 h until a constant weight was obtained (Zhang, Xie, & Li, 2016), which was determined on an analytical balance (Mettler AJ 150®, USA).
Tree vitality was assessed through the recording of chlorophyll fluorescence (Fv/Fm) with a Pocket PEA portable fluorimeter (Hansatech Instruments Ltd., UK), with a detection time of 1 s, light emission at a wavelength of 650 nm and an intensity of 3 500 µmol·m-2·s-1 (Zhang et al., 2016). In order to make the measurements, 10 randomly chosen leaves around the crown of each tree were adapted to darkness with fluorimeter clips for a period of 10 minutes (Morales-Gallegos, Martinez-Trinidad, Gómez-Guerrero, Razo-Zárate, & Suárez-Espinosa, 2019). The starch content of the roots was determined in samples collected randomly from below the base of the trunk, which were macerated in liquid nitrogen and stored at -20 °C until its processing in the laboratory. Two alcoholic extractions of sugars were performed from the collected tissues, each with 40 mL of ethanol at 80 % (v/v) for 20 min at 100 °C (Quentin et al., 2015). The extraction obtained was diluted in 10 mL of distilled water for quantitative determination; the samples were worked in triplicate and evaluated by hydrolysis of the precipitate of the alcoholic extraction process with the enzyme diastase (SIGMA®) (Palevitz & Newcomb, 1970). The anthrone method was applied to the resulting hydrolysate to determine the starch content based on the glucose content present in the sample (Witham, Blaydes, & Devlin, 1971).
Soil Variables
Soil respiration was determined using the alkaline trap method (Anderson, 1982). To this purpose, 60 g of soil was taken from each plantation hole; the sample was kept at 4 °C in a cooler while transported to the laboratory. The samples were then dried at 30 °C until a constant weight was obtained, and were incubated in 500 mL glass vials. To homogenize the moisture of the soil samples, the field capacity constant was determined to serve as a reference to estimate the amount of water to be added, which in this case was 30 mL. The soil was deposited inside each vial and a test tube (Pyrex®) containing 5 mL of NaOH was placed on top. The vials were let to balance for 24 h and 15 d; at the end of each period the CO2 of the atmosphere of the vial which passed to the NaOH solution was evaluated (Anderson, 1982). The total soil carbon was quantified by the method of wet oxidation with potassium dichromate (K2Cr2O7) in acidic medium. To do this, 0.5 g of dry soil was sifted through 0.5 mm mesh and placed in a 500 mL Erlenmeyer flask, then 10 mL of K2Cr2O7 (1N) and 20 mL of phosphoric acid (H3PO4) were added; the mixture was stirred and allowed to stand for 20 min. Subsequently, 200 mL of distilled water, 10 mL of H3PO4 and 25 drops of diphenylamine indicator were added and titrated with ferrous sulfate (FeSO4) at 0.5 N (Eyherabide, Sainz, Barbieri, & Echeverria, 2014). Finally, soil moisture was measured with a FieldScout® TDR-300 time domain reflectometer (Spectrum Technologies, Inc., USA) with 20 cm long electrodes (thin root growth area). Humidity was measured twice a month during the evaluation of the experiment (approximately one year).
Statistical analysis
A completely randomized experimental design was used with eight treatments and one control (purified water); each treatment had four replications. Assumptions of normality were verified with the Kolmogorov-Smirnov test (n ≥ 30 and α = 0.05) and homogeneity of variances (Levene test). The information was analyzed through the sum of Type III squares of the GLM (generalized linear model) procedure of the SAS® statistical software in its version 9.4 (SAS Institute Inc., 2013). When significance was found between treatments, a comparison of means was performed with the Tukey Honestly-significant-difference test (HSD) with α = 0.05. When the assumptions were not met in the data, the Kruskal-Wallis nonparametric method was used, and the means were compared with the Wilcoxon rank-sum test.
Results and discussion
Increase in height and diameter
After 371 d, no statistical evidence was found that carbohydrate amendments affected the increase in height or diameter in jacaranda trees (P > 0.05). Percival et al. (2004) and Percival and Fraser (2005) point out that the application of carbohydrates directly to the soil stimulates the development of the root system to a greater extent; however, the effects on the aerial part of the tree (height, diameter and active foliage) may not be evident in the short term. Good root development, after planting, helps support and improves the ability of the plant to dispose of soil resources; nonetheless, in many cases, the benefits are quantifiable in the long term (Day et al., 2010).
The results of this work are contrary to those observed in birch trees (Betula pendula Roth.), which positively responded to the application of 70 g·L-1 sucrose to the soil, stimulating the growth of both roots and shoots (Percival & Fraser, 2005). A disadvantage of sugars placed in the soil is that some of them can be used by the microbiota or may be lost by the effect of rain or irrigation (Martinez-Trinidad, et al., 2010), which may explain, in part, the lack of significance between treatments for the variables of increase in height and diameter in this trial. Moreover, it has been found that the application of carbohydrates injected directly to the tree trunk of jacaranda (J. mimosifolia) and evergreen oak (Quercus virginiana P. Miller) favorably promotes growth in height and diameter (Martinez-Trinidad, Watson, Arnold, Lombardini, & Appel, 2009; Morales-Gallegos et al., 2019).
Dry matter of roots
There was a significant effect on dry matter of jacaranda tree roots (P ≤ 0.05) with treatment of glucose with 80 g·L-1 and sucrose with 80 g·L-1 (G8S8) (Figure 1) with an average of 0.034 g, this being the highest treatment in carbohydrate concentration. As for the control (G0S0), the dry matter of roots reached an average of 0.006 g.
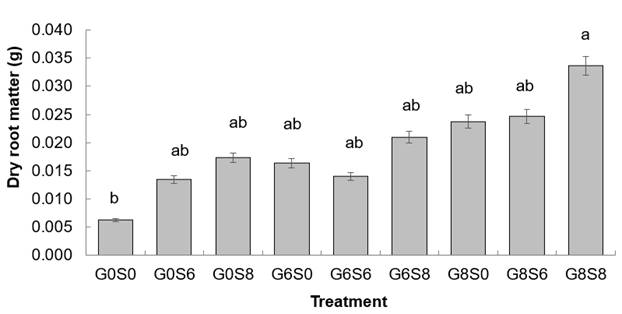
Figure 1 Dry matter of roots of jacaranda trees (Jacaranda mimosifolia) with carbohydrate amendments to the soil. The bars indicate the standard error of the mean. Equal letters mean statistically similar means (P ≤ 0.05) using Tukey's HSD test. Carbohydrate concentrations in the treatments: G0, G6 and G8 = 0, 60 and 80 g·L-1 glucose, respectively; S0, S6 and S8 = 0, 60 and 80 g·L-1 sucrose, respectively.
The application of sugars to the soil tends to initially stimulate root growth (Martinez-Trinidad et al., 2010; Percival et al., 2004), mainly due to the improvement in microbial activity and its interaction with the rhizosphere (Percival et al., 2004). An adequate development of fine roots may favor, in the future, the settlement of the plant and, consequently, a better growth (Day et al., 2010). Previous studies have demonstrated the positive effect of sucrose on root system development in trees such as horse chestnut (Aesculus hippocastanum L.), cherry (Prunus avium L.) and oak (Quercus robur L.) (Percival et al., 2004).
Foliage Color
The green color of jacaranda tree foliage was not statistically significant (P > 0.05). In a study of Pinus greggii Engelm., Mohedano-Caballero et al. (2005) used the same method to determine green pixels of the leaves from trees which were stressed by transplantation (up to 90 % in root cutting) and no significant differences were found either; the authors attributed this to a possible effect of continuous regrowth throughout the study, which prevented significant differences in the color of the tops of this tree species. In stressful conditions, whether biotic or abiotic, the plant is able to move its energy reserves as required; for example, for the production of out-of-season regrowth (Valenzuela, Maillard, González, & González, 2013; Wiley, Casper, & Helliker, 2017). Similarly, in this assay, there was continuous regrowth possibly induced by stress at the planting site, which could affect the evaluation of green color throughout the trial.
Chlorophyll fluorescence
The fluorescence of chlorophyll showed no statistically significant differences in any treatment. The Fv/Fm value ranged from 0.713 in the glucose-free treatment with 60 g·L-1 sucrose (G0S6) to 0.735 in the sucrose-free 80 g·L-1 glucose treatment (G8S0). Some authors claim that the values of Fv/Fm between 0.78 and 0.85 are considered indicators of good health or lack of stress in vegetation (Callow, May, & Johnstone, 2018; Johnstone, Moore, Tausz, & Nicolas, 2013; Uhrin & Supuka, 2016). Taking into account the above-mentioned interval as an indicator of good health, jacaranda trees, planted in an urban space with carbohydrate amendments, had values below what is indicated as optimal. This may be because the trees presented physiological stress due to the process of adaptation to the planting site (Mohedano-Caballero et al., 2005). Similar data were recorded in a trial where carbohydrates (glucose) were injected into the tree trunk of jacaranda (Morales-Gallegos et al., 2019).
Starch in roots
According to Figure 2, at the end of the evaluation period (371 d), significant statistical differences (P ≤ 0.05) were found in starch content in roots of jacaranda trees treated with carbohydrate amendments. The highest values correspond to the treatment of 80 g·L-1 glucose and 80 g·L-1 sucrose (G8S8) with 54.8 mg·g-1 MS. This is consistent with previous studies in other species where sucrose stimulated the growth of the root system (Martinez-Trinidad et al., 2010; Percival et al., 2004; Percival & Fraser, 2005).

Figure 2 Starch content in roots of jacaranda trees (Jacaranda mimosifolia) at the end of the evaluation period (371 d) with carbohydrate amendments to the soil. The bars indicate the standard error of the mean. Equal letters mean statistically similar means (P ≤ 0.05) using Tukey's HSD test. Carbohydrate concentrations in the treatments: G0, G6 and G8 = 0, 60 and 80 g·L-1 glucose, respectively; S0, S6 and S8 = 0, 60 and 80 g·L-1 sucrose, respectively.
Urban environmental conditions play an important role in the variability of carbohydrate concentration in plant tissues (Maselli & Silveira, 2017), because large amounts of sugars are invested in tissue generation and defense. It has been observed, for example, that the species of silver maple (Acer saccharinum L.) and Norway maple (Acer platanoides L.), tend to mobilize their carbohydrate reserves for shoot production after a pruning event (stress) (Ramirez et al., 2018). In this work, starch values in roots remained at their highest point at 322 d (Figure 3), more than a month before regrowth (mid-April 2018).

Figure 3 Variation of starch content in roots of jacaranda trees (Jacaranda mimosifolia) with carbohydrate amendments to the soil, during an annual growth cycle (June 2017 to June 2018). Carbohydrate concentrations in the treatments: G0, G6 and G8 = 0, 60 and 80 g·L-1 glucose, respectively; S0, S6 and S8 = 0, 60 and 80 g·L-1 sucrose, respectively.
Soil respiration and total carbon
Net soil respiration did show significant differences (P ≤ 0.001). This ranged from 2.15 mg C-CO2·m-2 dry soil·h-1 in the water-only treatment (G0S0) to 5.08 mg C-CO2·m-2 dry soil·h-1 in the highest carbohydrate concentration treatment (G8S8). Microbial populations in soil are often quite sensitive to changes in general conditions, so they provide information about their alterations in an early and reliable manner (Paolini, 2017). An increase in CO2 production in soil may indirectly indicate the activity of microorganisms oxidizing soil organic matter under aerobic conditions, and the growth of their populations accelerating nutrient cycling (Schloter et al., 2017). This is because part of the carbohydrates placed in the soil serve as easily assimilable carbon and energy reserve (Ziter & MacDougall, 2013).
In a study with carbohydrate amendments under semi evergreen oaks (Q. virginiana), there was an increase in microbial activity (soil respiration as CO2); however, this only lasted while carbohydrates were metabolized by soil microorganisms (Martinez-Trinidad et al., 2010). It is important to mention that, although the net mass of CO2 was different between treatments, proportionally, the stimulation in respiration is low if the total soil carbon is considered. In this case, respiration was less than 1 % and there were no statistical differences between treatments (Table 2). Compared to some forest soils, the consumption of 1 % of soil carbon can be reached in the first 20 d in fertile soils and in more than 100 d in low fertility soils (Gómez-Guerrero & Doane, 2018).
Table 2 Soil respiration and total carbon in jacaranda trees (Jacaranda mimosifolia) in the "Alameda de Texcoco", State of Mexico.
| Treatment | CO2 (mg C-CO2·m-2 dry soil·h-1) | SE | TC (%) | SE |
|---|---|---|---|---|
| G0S0 | 2.152 d | ±0.61 | 0.563 a | ±0.12 |
| G0S6 | 3.145 cd | ±0.36 | 0.605 a | ±0.04 |
| G0S8 | 4.785 ab | ±0.96 | 0.509 a | ±0.20 |
| G6S0 | 3.437 bcd | ±0.66 | 0.628 a | ±0.17 |
| G6S6 | 3.915 abc | ±1.09 | 0.551 a | ±0.24 |
| G6S8 | 4.772 ab | ±0.94 | 0.909 a | ±0.31 |
| G8S0 | 3.627 bc | ±0.28 | 0.432 a | ±0.15 |
| G8S6 | 4.585 abc | ±0.67 | 0.759 a | ±0.28 |
| G8S8 | 5.085 a | ±0.89 | 0.914 a | ±0.25 |
CO2 = net soil respiration; TC = total soil carbon; SE = standard error of the mean. Equal letters mean statistically similar means (P ≤ 0.05) using Tukey's HSD test. Carbohydrate concentrations in the treatments: G0, G6 and G8 = 0, 60 and 80 g·L-1 glucose, respectively; S0, S6 and S8 = 0, 60 and 80 g·L-1 sucrose, respectively.
Soil moisture
Soil moisture was kept constant throughout the trial mainly due to scheduled irrigation. The values ranged from 31 to 46.9 % volumetric water content, finding the highest values in the rainy season without becoming statistically significant (P ≤ 0.05) with the days outside the rainy season (dry season).
Conclusions
Carbohydrate amendments to the soil stimulate the development of fine roots of trees planted in urban spaces; however, aerial characteristics such as foliage color or increase in height and diameter of trees were not altered, possibly due to the short evaluation time (about one year). The study of aspects related to the development of the aerial part of the trees, initially modifying the conditions of the root system, implies a longer evaluation time than considered in this trial. The results indicate that amendments with carbohydrates such as glucose and sucrose have an indirect positive effect on soil respiration and therefore on roots, possibly due to increased microbial activity that improves soil characteristics.











 texto en
texto en 


