Introduction
Oaks are trees that belong to the Fagaceae family and one of the world's most important genera: Quercus. This genus is found in nearly all temperate forests of the northern hemisphere, as well as in some tropical and subtropical regions; some species are even found in drier habitats in Southeast Asia and Northeast Africa. In the Americas, oaks are found from Canada to Colombia, including Cuba. Two centers of diversity for the genus are recognized. One is Southeast Asia with over 125 species (Valencia, 2004), while the other is Mexico, particularly in rocky regions, where oaks are an important part of the temperate forests, where they stand out amongst plant communities. Most oaks (~95 %) are distributed between 1,200 and 2,800 m above sea level throughout Mexico, except in Peninsula de Yucatan.
Oaks as a natural resource in Mexico
The number of oak species in Mexico is not well known; some authors studying their distribution estimate around 253 species, while others calculate between only 135 and 150 species. The forest resource is important in the ecology and economy of Mexico, especially the state of Durango, where about 44 % (5,402,825 ha) of the total area is covered by temperate coniferous forests, namely pine-oak (Pinus-Quercus), oak-pine or oak forests (Luna-José, Montalvo-Espinoza, & Rendón-Aguilar, 2003). The oak family includes six to nine genera and about 600-900 species. In Mexico three variants of these genera have been recognized: red oak; also known as Erythrobalanus, Lobatae (white oaks or Leucobalanus and Protobalanus (intermediate oak) (Vázquez, Valencia, & Nixon, 2004). The white oaks are better distributed in mountainous areas, where they show greater ecological tolerance. The oak forests are located in protected canyons, where there are better humidity conditions. The proportion of red oaks increases towards damp places such as canyons or high slopes and edges of creeks. All oaks share a number of common biological characteristics: woody stems, leathery leaves (leathery or hard) and presence of acorns. Their growth form is like a common tree (with a height of 3-40 m) and some as shrubs (heights of 10- 60 cm), but never as grass. The leaves are characterized by different types of apex (tip of the leaf), leaf base, number of ribs, margin (or edge of the leaf), texture, sizes and colors, morphological properties that are used in taxonomy for their botanical classification (González-Elizondo, López-Enríquez, González-Elizondo, & Tena-Flores, 2002).
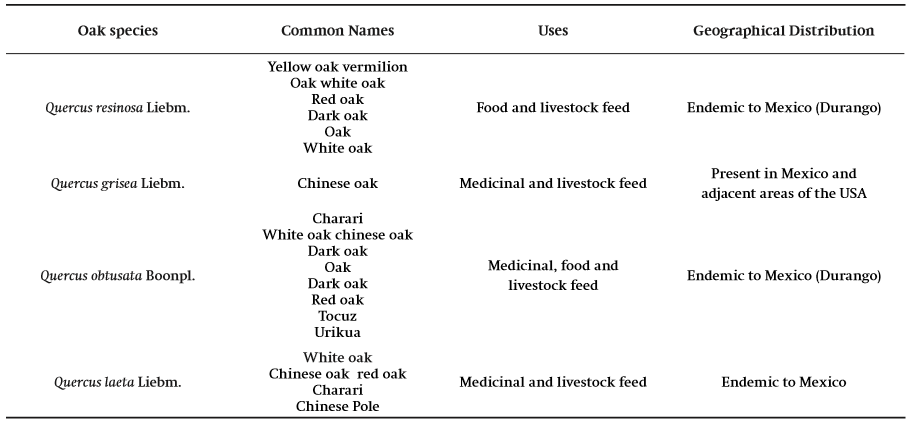
Scientific studies that have addressed the use of oak in Mexico mainly highlight its timber use due to its significant physical, mechanical and anatomical properties. Although this use is widely acknowledged its non-timber use has been undervalued, even though different ethnic and rural communities nationwide collect and process oak medicinal and food products as part of their culture (Luna-José et al., 2003). The study of the oaks has been given a low priority due to a lack of biological information and their complicated taxonomy. In Mexico there are many Quercus species that are used for non-timber purposes, highlighted by: Q. eduardii Trel., Q. sideroxyla Bonpl., Q. durifolia Seemen ex Loes, Q. resinosa Liebm, Q. laeta Liebm, Q. obtusata Boonpl. and Q. grisea Liebm. In Table 1 there are some oak species with reported non-timber uses.
Table 1. Oak species with non-timber use in Mexico (Adapted from Arizaga, Martínez-Cruz, Salcedo-Cabrales, & Bello-González, 2009).
Nutraceuticals from oak
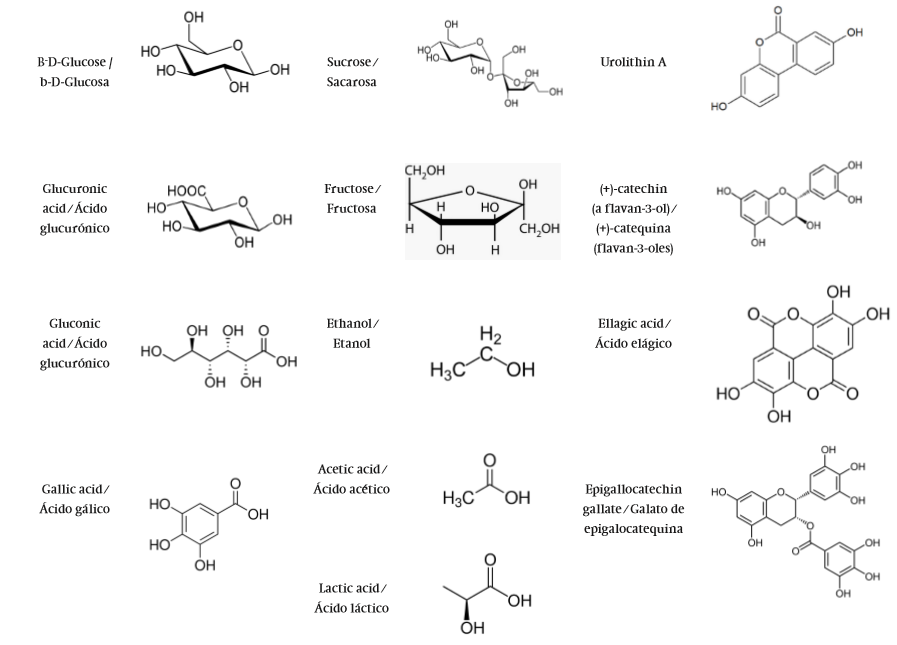
Plants have been widely used for medicinal purposes since ancient times. Most herbs have multiple physiological effects, because they produce a diversity of specialized bioactive phytochemicals, which correspond to the particular needs of the plant secondary metabolism. They should include essential and non-essential chemicals such as vitamins and polyphenols, which may be considered as part of the human food chain, and which also provide beneficial health effects (Biesalski, 2009). In medicinal plants, active phytochemicals are always biologically balanced. This balance is based on the presence of additional substances that potentiate each other, do not accumulate in the body and show limited undesirable effects (Wagner & Ulrich-Merzenich, 2009). Quite recently, these nutraceuticals have been recommended to treat the symptoms of common diseases or nutrition deficiencies. They are usually consumed by drinking an herbal infusion, defined as the drink produced from dried plant parts submerged in hot or boiling water for a few minutes. The use of herbal teas is popular due to their aroma, antioxidant properties and therapeutic applications (Manteiga, Park, & Ali, 1997). Ethnobotanical reports mention that infusions of some species of Quercus, in combination with other plants like yellow flowers from Solanum rostranum Dunal show anticarcinogenic effects in patients with gastric cancer, when they consumed them regularly (Alonso-Castro et al., 2011). More specific investigations in relation to anti-inflammatory studies of aqueous extracts from oaks were performed by Gharzouli, Khennouf, Amira, and Gharzouli (1999). They demonstrated the cytoprotective properties of aqueous extracts from Q. ilex Liebm. root bark compared to aqueous extracts from Punica granatum Linneo and Artemisia herba-alba Asso. leaves against damage caused by ethanol in the stomach, having tannic acid as a positive control. It has been documented that tannic acid together with other polyphenols such as quercetin and ellagic acid can inhibit the proton pump present in the parietal cells and thereby participate in protecting the stomach against harmful agents (Gharzouli et al., 1999). In this study, high oligomeric phenolic contents were obtained in the aqueous extracts of Q. ilex and P. granatum (2.33 to 4.41 mg·mL-1), while in the A. herba-alba leaves only monomeric flavonoids were found (0.33 to 0.51 mg·mL-1). Studies on the chemical composition of Quercus infectoria G. Oliver, traditionally used to treat wounds or burns associated with bacterial infections, have reported gallotannins and ellagic acid (Figure 1) in a 60 to 70 % proportion (Shariatifar, Fathabad, Khaniki, & Nasrabadi, 2014). These compounds have been reported to have astringent, antidiabetic, local anesthetic, antibacterial, antiviral and anti- inflammatory activities, as well as acting as good protection agents against oxidative damage of lipids and proteins (Charrier, Marques, & Haluk, 1992).
In additional studies our research team elucidated the potential of oak as a sustainable source of high value-added products, as a result of their nutraceutical properties. Antioxidant and anticarcinogenic activities of phytochemicals present in oak leaf infusions, the mechanisms of cancer prevention with oak phytochemicals having therapeutic potential in in vitro human cell models (Rocha-Guzman et al., 2009) and in in vivo models using Sprague-Dawley rats, have been reported (Moreno-Jiménez et al., 2014; Rocha-Guzmán et al., 2012). We have also identified anti-topoisomerase activity in in vitro models using mutated yeast and antimicrobial activities against enteric pathogens (Sanchez-Burgos et al., 2013). Some alternatives have been explored for the preparation of foodstuffs containing phenolic compounds from oaks that have demonstrated a high cardioprotective potential, particularly with Q. resinosa species (Rivas-Arreola et al., 2010). Recently, a significant gastroprotective effect against damage induced by food chemicals and non-steroidal anti-inflammatory drugs (NSAID) in human intestinal cell models was shown (Sanchez-Burgos et al., 2013). The age or maturity degree of leaves determines the content of total extractable phenolics; Makkar, Dawra, and Singh (1991) conducted studies in several Quercus species noting that younger leaves showed grossly two to threefold higher phenolic contents (20 to 284 mg·g-1) than mature ones (8 to 125 mg·g-1). Apparently, the polymerization degree increases with age, as well as the protein and phenolics contents; at the same time their biological activities change as leaves become mature. Thus, the degree of polymerization of polyphenols determines the antioxidant capacity and various features of the plant material that will be reflected in their biological response.
Oak as an herbal infusion for the production of Kombucha
Our research team studied the acceptability of herbal infusions from different Quercus species, finding that oak leaves with higher phenolic content (3 to 13 mg·g-1) have lower acceptability (Rocha-Guzman et al., 2012). Such is the case of Q. resinosa herbal tea, which despite its proven healthy phenolic content, it is not totally accepted by general public. This is due to the astringency phenomenon, where polyphenols present bind the proline-rich proteins from saliva in the mouth, giving rise to an insoluble precipitate. This stimulus also affects saliva rheological properties, possibly leading to friction and reduced lubricity. Vázquez-Cabral et al. (2014) used the microbiome Kombucha (Chinese fungus tea) to ferment oak leaf infusions, increasing their sensory acceptability without detriment to their nutraceutical properties. Results from this study showed increased antioxidant capacity and sensory acceptability, by reducing the distinctive astringency flavor of this herbal tea. Kombucha is a slightly acidic beverage that is produced by fermentation of black tea and sugar through a symbiotic association of bacteria and yeast that form the "tea fungus." The drink dates back to about 220 BC in China, where it was recognized by the Tsin dynasty for its energizing and detoxifying properties (Dufresne & Farnworth, 2000), and it has been consumed worldwide for its prophylactic and therapeutic properties. This fermentation product is known throughout the world by different names: in Russia, where it has been prepared for two centuries, it is called "Tea Kvass," whereas in Japan it is known as "kōcha kinoko." Traditionally, for a product to be considered Kombucha, it has to be made only from sweetened black or green tea because they possess the nitrogen source needed by microorganisms, which are released during tea decoction as purines and xanthine derivatives, such as caffeine, theophylline and stimulating alkaloids of the central nervous system (CNS) (Frank, 1995). The extensive research on black/ green tea and their effects on health is a good reference to understand the complex mechanisms involved in the physiological activity of tea and Kombucha (Dufresne & Farnworth, 2000). Yeast cells hydrolyze sucrose to glucose and fructose, producing ethanol as a metabolite (Figure 1) (Reiss, 1994; Sievers, Lanini, Weber, Schuler-Schmid, & Teuber, 1995). Whereas acetic acid bacteria convert glucose to gluconic acid and fructose to acetic acid, there are other metabolites produced such as lactic acid, glucuronic acid and glycerol. The oxidation of tea polyphenols during fermentation leads to the formation of catechins, theaflavins, teaflavinic acid and proanthocyanidin polymers as well as the synthesis of B vitamins and folic acid (Dufresne & Farnworth, 2000). Phenolic compounds are involved in various functions, such as nutrient uptake, protein synthesis, enzyme activity, photosynthesis, formation of structural components and defense against adverse environmental factors. Diet polyphenols present a significant antioxidant/radical scavenging property and the mechanisms by which they express their beneficial effects against various common chronic diseases, such as cardiovascular disease, diabetes, and cancer are not totally clear, but appear to involve their interaction with molecular signaling pathways and related machinery that regulate cellular processes such as inflammation (Gonzalez et al., 2011).
Bacteria and fungi in Kombucha form a powerful symbiosis, which is able to inhibit the growth of potential contaminating bacteria (Balentine, Wiseman, & Bouwens, 1997; Liu, Hsu, Lee, & Liao, 1996). The main bacteria found in tea fungus are: Acetobacter xylinum Brown (Balentine et al., 1997), Bacterium gluconicum Hermann (Reiss, 1994), Acetobacter aceti Pasteur and Acetobacter pasteurianus Kozulis & Parsons (Liu et al., 1996). Common yeasts identified in tea fungus are: Schizosaccharomyces pombe Lindner, Saccharomycodes ludwigii Hansen, Kloeckera apiculata Reess, Saccharomyces cerevisiae Meyer, Zygosaccharomyces bailii Barnett, Brettanomyces bruxellensis Kufferath & Von Laer, B. lambicus Koff & Van Laer, B. custersii Florenz and Candida and Pichia species (Balentine et al., 1997; Liu et al., 1996; Mayser, Fromme, Leitzmann, & Gruender, 1995). Consequently, Kombucha drink has shown antimicrobial activity, as have other fermented beverages produced from other herbal infusions such as Thymus vulgaris L., Lippia citriodora Palau, Rosmarinus officinalis L., Foeniculum vulgare Mill. and Mentha piperita L. (Battikh, Bakhrouf, & Ammar, 2012). These Kombucha analogues have shown strong antimicrobial potential, particularly against Candida strains, which make them promising health beverages. Interestingly, the antimicrobial potential of Kombucha drinks is not only due to the acidity or their organic acids (gluconic, glucuronic, lactic, and acetic acids), but also to other biologically-active metabolites (polyphenols, vitamins, aminoacids, antibiotics, micronutrients), which are biosynthesized during the fermentation process, improving the beverage nutraceutical profile.
The fermentation process involves as a first step the action of yeasts fermenting glucose and fructose to ethanol, which continues oxidation to acetic acid by the action of acetic acid bacteria. The main source of carbon in this process is sucrose. This sugar is hydrolyzed by the enzyme invertase from yeast in the Kombucha consortium, leaving fructose and glucose available. Yeasts metabolize sugar with initial preference for fructose to produce ethanol. Glucose not initially metabolized by yeast is used by bacteria in the consortium and via the action of catalase, it is converted to glucono delta lactone acid, which transforms spontaneously to gluconic acid (Figure 1). Another way of metabolizing glucose is by its oxidation to glucuronic acid. The physiological significance of this organic acid is based on its ability to be conjugated to various endogenous and exogenous substances (i. e., xenobiotics), forming glucuronides, which are produced through a reaction catalyzed by the enzyme UDP-glucuronyl transferase. Small hydrophobic molecules containing oxygen, nitrogen, sulfur or carboxyl functional groups may be chemically conjugated with glucuronic acid to produce more polar and acidic glucuronides that are more water-soluble at physiological pH than the precursors, altering their own metabolism, transport or excretion properties.
Some of the positive effects attributed to Kombucha consumption, based mostly on testimonials and personal observations, are: antibiotic properties, intestinal and gastric regulation, glandular activity, relief from rheumatic ailments, gout control, hemorrhoids treatment, cholesterol level and atherosclerosis regulation, blood toxin cleansing, and as a control for diabetes, nervousness, and aging problems. However, few properties have been demonstrated by scientific and experimental studies. In 1995, the FDA (Food and Drug Administration) reported two cases of severe illness in which the consumption of Kombucha was involved. One individual died of intestinal tract perforations caused by severe acidosis, and the other person, who survived, mentioned that she had increased the fermentation time from 7 to 14 days. These cases were investigated, and it was concluded that consumption of Kombucha is not harmful at low doses of 100 mL·day-1. The FDA recommends that the fermentation period does not exceed 10 days, because the increasing acidity may lead to potentially harmful levels for consumers (Nummer, 2013).
Jayabalan, Subathradevi, Marimuthu, Sathishkumar, and Swaminathan (2008) found that total phenolic content in black tea gradually increased (from 80 to 100 µg·g-1) over fermentation time (0 to 18 days), obtaining a high level of antioxidants with the ability to capture free radicals and reactive oxygen species. A similar case was observed by Vázquez-Cabral et al. (2014), who observed that phenolic content (~1 mg·g-1) was related to fermentation time, although sensory acceptance was impaired. The latter was attributed to the reduction in sensory acceptability from the sucrose biotransformation metabolites that interfere with taste as well as the presence of flavan-3-ols such as catechin and epicatechin. It is possible that the metabolic conversion of phenolic compounds in oak infusions by the Kombucha microbiome is due to glucuronidation of flavonoid compounds. This may increase their bioavailability, being oak leaves a rich source of ellagitannins and flavonoids, which have shown important biological activities in both in vitro and in vivo assays.
Conjugation of polyphenols and their bioavailability
Bioavailability can be understood as the integration of various processes by which a fraction of a nutrient or drug ingested is available for digestion, absorption, transport, use and disposal (Hurrell & Egli, 2010). An important aspect concerning the bioavailability of polyphenols is their chemical stability during gastric digestion and intestinal course. The total intake of polyphenols involves a complex interaction that includes the biochemistry of polyphenol glycosides, their aglycone metabolism and the transport rate of each form. Most flavonoids entering the systemic circulation in conjugated form are either sulfated or glucuronidated.
Most biological activities in vitro are performed using the aglycone forms of polyphenol glycosides. Particularly, most of the flavonoids, except catechins, are present in plants and foods as β-glycosides. It is recognized that glycosylation influences the chemical, physical and biological properties of polyphenols. Once ingested and before entering general circulation, these glycosides can undergo hydrolysis (Berrin, McLauchlan, Needs, & Williamson, 2002; Németh, Plumb, Berrin, & Juge, 2003). Flavonoids once hydrolyzed may undergo conjugation by methylation, sulfation or glucuronidation reactions, because they show high conjugation capacity at their generally very low plasma concentrations (Manach, Scalbert, Morand, & Remesy, 2004). Catechins undergo a wide biotransformation, including glucuronidation, which increases their bioavailability. Bioconversion of flavan-3-ols such as methylation or other conversion reactions may occur at the enterocytes or later in liver cells (Tapiero, Tew, Nguyen Ba, & Mathé, 2002). Within the enterocytes, the aglycones become glucuronides that can pass through their basolateral membranes, travel from there to the vascular system and then to the systemic circulation; or they may also be transferred back to the luminal compartment by P-glycoprotein or multidrug resistance proteins.
Glucuronidation is one of the most common reactions used by the liver to produce polar (hydrophilic) metabolites. Glucuronidation involves the transfer of glucuronic acid components from UDP-glucuronic acid to a substrate by means of any UDP-glucuronosyltransferase. It is known that UDP-glucuronic acid is an intermediate in the process and is formed in the liver. The resulting substances from glucuronidation are identified as glucuronides and are more soluble in water. Therefore, this system is used by the human body to allow subsequent removal through urine or feces. Hormones may also be glucuronidated to allow easier transportation throughout the body. The conjugation of xenobiotic molecules with hydrophilic molecular species such as glucuronic acid is known as Phase II Metabolism.
In vitro experiments using flavonoid aglycones or glycosylated conjugates (Lolito, Zhang, Yang, Crozier, & Grei, 2011) have demonstrated that quercetin glucuronidation or quercetin sulfation affects their inhibitory outcome on the expression of adhesion molecules, whereas methylation retains its anti-inflammatory activity. The highly documented unconjugated epigallocatechin gallate (EGCG) is the most abundant form detected in plasma, with a ratio ranging from 77 to 90 % of intake. On the other hand, other catechins appear highly conjugated with glucuronic acid and/or sulfate groups (Rahman, Biswas, & Kirkham, 2006).
Glycosylation influences the chemical, physical and biological properties of polyphenols. Sugar removal from glycosylated flavonoids by the action of glycosidases and consequent hydrophilic action induces passive diffusion of the aglycone through intestinal microvilli. Dietary polyphenols are substrates of beta-glucosidases, UDP-glucuronosyltransferases or catechol-O-methyl transferases in the small intestine, and also of various phase I and II enzymes in the liver (Rechner et al., 2002). As for the bioconversion of flavan-3-ols and flavonols, two important flavonoid subclasses associated with human health, conjugation reactions (methylation, sulfation and glucuronidation) can occur both in enterocytes and in hepatic cells. Flavanols such as (-)-epicatechin are often acylated especially by gallic acid. The resulting galloyl substitutions apparently do not affect the partition coefficients of compounds and their bioavailability as dramatically as glycosidation does (Tapiero et al., 2002). The flavan-3-ols apparently pass through biological membranes and are absorbed without deconjugation or hydrolysis. Thus, when consuming (+)-catechin, at 2 g doses, its plasma fraction varies from 0.2 to 2 % of the total consumed, detecting it still after 30 min as unconjugated catechin and after 120 min as methylated catechin. After 8 h, 40 % of the catechin was detected in urine as methylated, sulfated and glucuronidated derivatives. The flavan-3-ols do not alter the levels of endogenous antioxidants and bioconversion products in the presence of phenolic acids such as vanillic, 4-hydroxybenzoic, 3,4-dihydroxybenzoic, and 3-methoxy-4-hydroxyhippuric (Rodrigo, Miranda, & Vergara, 2011).
Other model compounds like ellagic acid and ellagitannins have shown antitumor activity in in vitro and in vivo assays. They have been proposed as pro-phytoestrogens because the bioavailability of metabolites derived from ellagitannins of the colonic microflora, known as urolithins (hydroxydibenzopyran-6-one derivatives), have shown anti-estrogen activity in in vitro models (Larrosa, González-Sarrías, García-Conesa, Tomás-Barberán, & Espín, 2006). Azorín-Ortuño et al. (2008) evaluated in vivo the safety of an oak-flavored milk powder, but it was not possible to detect ellagitannins and derived metabolites in liver, kidney and uterus of rats due to the low amount of administered ellagitannins (0.094 mg·day-1). Nevertheless, it was possible to identify urolithin A glucuronide in urine and urolithin A in feces, confirming that their production as metabolites derived from the microflora is independent of the structure or concentration of the ellagitannins. Espín et al. (2007) evaluated the bioavailability and metabolism of ellagitannins in Iberian pigs, and their distribution in different tissues in order to provide information on bioavailability in humans. They showed that ellagitannins release ellagic acid under physiological conditions in vivo, which is gradually metabolized in the intestine to produce urolithin D and C, ending with urolithin A and B. When the metabolites are absorbed, the first glucuronidation may occur in the intestinal cells, finding their way to the aorta; once in the liver, they are metabolized to yield glucuronides and sulfates, which may be secreted in bile. The presence of ellagic acid in bile and urine, and its absence in intestinal tissues, suggested that it may be absorbed in the first part of the gastrointestinal tract.
Using other analogue herbs such as rosemary (R. officinalis) has aroused great scientific interest, due to its major diterpene constituents: carnosic acid and carnosol. They confer biological significance to the plant, including anti-inflammatory, antiobesogenic, antiangiogenic and anticancer activities. Romo-Vaquero et al. (2013) conducted a study on the metabolic profile of intestinal lumen, liver, plasma and brain in female Zucker rats treated with an oral extract, noting that conjugation of carnosic acid and carnosol is mainly due to glucuronidation. The presence of carnosic acid glucuronide in liver and plasma was confirmed, although the incidence of methylated carnosic acid was more abundant in plasma than in intestine and liver.
Conclusions
Non-timber resources such as oak leaves are waiting for sustainable use to the benefit of human health. However, it is still necessary to clarify the bioconversion of polyphenols in fermentation systems using the Kombucha fungus, particularly in oak infusions, which would provide valuable information on the mechanisms of substrate consumption. In this way, an open panorama can be displayed to a wide source of forest herbal materials to be used as analogues of Kombucha such as oak leaf infusions with presumed high bioavailability and bioactivity.











 texto en
texto en