Introduction
Avocado (Persea americana Mill.) is classified into three botanical races or varieties, according to the morphological characteristics of the fruit: P. americana var. drymifolia, P. americana var. guatemalensis and P. americana var. americana (Guzmán et al., 2017; Ferrer-Pereira et al., 2017). Mexican criollo avocado belongs to drymifolia variety or Mexican landrace (Ayala & Ledezma, 2014). In addition, commercial varieties have been developed from crosses between landraces, such as the “Hass” avocado, which is a hybrid with predominantly guatemalensis and drymifolia features (Tremocoldi et al., 2018) while, “Fuerte” avocado is a hybrid of the drymifolia x guatemalensis varieties (Crane et al., 2013). Avocado is of great commercial importance due to the consumption of the fruit as food worldwide (Peraza-Magallanes et al., 2017; Ferrer-Pereira et al., 2017).
On the other hand, mistletoe (Family Loranthaceae) is a hemiparasitic tree plant with a wide distribution in urban and wooded areas (Díaz-Limón et al., 2016; Watson, 2017). In Mexico, mistletoe has a high harmful impact on the development and growth of forest and fruit trees and their susceptibility to other diseases (Azpeitia & Lara, 2006).
In avocado, in particular, damage has been observed mainly on trees of the var drymifolia (Coria et al., 2015), despite being resistant to pests and even being used as rootstocks for other varieties (Rincón-Hernández et al., 2011), while the commercial varieties “Hass” and “Mendez” display tolerance to the mistletoe attack (Coria et al., 2015).
A plant defense mechanism is the synthesis of chemical substances, known as secondary metabolites, for protection against invasive biological agents (Torres-Gurrola et al., 2011; Medina-Carrillo et al., 2017). A great variety of these compounds have been identified as useful for adaptation and survival of the plant, but are not essential for surviving (Wolfender et al., 2015; Lui et al., 2017). Some authors mention the hypothesis that plants that have a more significant amount and combination of secondary metabolites (SM) may have greater protection against pathogens and that it could be due to a specific compound or a mixture of several (Medina-Carrillo et al., 2017). However, Torres-Gurrola et al. (2011) reject the positive relationship between the diversity of chemical compounds and the defense against pathogens.
Because of the commercial importance of avocado at international level and the mistletoe attack against drymifolia avocado trees, used as rootstocks for their resistance to pests and diseases, the objective of this work was to evaluate the biochemical profile of drymifolia, “Hass” and “Mendez” avocados in order to make an exploration of secondary metabolites possibly related to mistletoe tolerance (Family Loranthaceae).
Material and Methods
In the present work, 27 drymifolia avocados, a Hass avocado and a Méndez avocado were studied, coming from four localities of Michoacan, which are, 11 from Ziracuaretiro, 10 from Tingambato, six from Uruapan and two from Cutzato. The avocado orchard has a age of 15 years. The diameter of the plants at the base is approximately 20-25 cm and is handled under a system conventional, with micro sprinkler irrigation.
Mature leaves were collected from avocado trees established in comercial orchards and backyard trees, which were kept at 4 °C until they were transferred to the laboratory. Five to seven young and healthy leaves were sampled. Each avocado tree was considered as an experimental unit.
Secondary metabolite isolation was performed by solidphase microextraction (SPME) in a 10 mL extraction vial with 200 mg of leaf tissue. Before metabolite extraction, the midrib and secondary veins were removed. The leaf was finely chopped with a scalpel. The determination of volatile compounds was accomplished by gas chromatography coupled to mass spectrometry (GC/MS).
For the adsorption of volatile compounds, a divinylbenzene/ carboxy/ polydimethylsiloxane (DVB/CAR/PDMS) fiber of 50/30 µm StableFlex/SS (1cm) (Supelco 57298-U) was used. The desorption was carried out in a BRUKER SCION 456-GC gas chromatograph with CombiPAL autosampler, equipped with a programmed temperature vaporization injector (PTV). Also, an HP-5MS Agilent 60 m × 0.250 mm × 0.25 µm capillary column (19091S-436) and a quadruple single mass detector of the BRUKER SQ were used.
The injection sequence was programmed by preheating the fiber at 220 °C. For the adsorption of volatile compounds with DVB/CAR/PDMS fiber, a specialized automated SPME attachment integrated to the chromatograph autosampler was used; which allowed incubating the chopped leaf sample with the exposed fiber in the head space at 37 °C for 10 min with intermittent shaking at 250 rpm and cycles of 2 s turned on and 4 s turned off, to achieve the highest release of volatile compounds.
Afterward, the fiber performed the desorption of the analytes in the PTV injector at 180 °C for 2 min. The temperature ramp used in the GC was 50 °C for 3 min, 120 °C at 5 °C/ min for 3 min and 240 °C at 5 °C/min for 7 min. Ultrapure grade helium was used as a carrier gas at a flow rate of 0.8 mL*min-1. Finally, the analytes were identified by comparing the mass spectra reported in the NIST (Mass Spectral Search Program) Version 2.0 library included in the software.
Results and Discussion
In the analysis of the biochemical profile of the leaf tissue of avocado trees var. drymifolia, 111 different compounds were detected, in the tree of the variety “Hass” were 25 and in the tree of the variety “Mendez” 29 compounds (Table 1).
Table 1 Relative concentration of the secondary metabolites identified in drymifolia, Hass and Méndez avocado leaves, susceptible and tolerant to mistletoe.
| # | Secondary Metabolites identified | drymifolia Average conc. (%) | Hass Conc. (%) | Mendez Conc. (%) |
|---|---|---|---|---|
| 1 | Estragóle | 61.173 | 67.997 | 81.520 |
| 2 | Caryophyllene | 19.398 | 5.211 | 3.230 |
| 3 | (+)-4-carene | 7.532 | 6.835 | 2.676 |
| 4 | γ-terpinene | 6.929 | 7.998 | 2.655 |
| 5 | o-cymene | 1.154 | 1.819 | 0.781 |
| 6 | Copaene | 1.522 | 1.664 | 1.347 |
| 7 | Benzaldehyde, 2,5-bis | 0.069 | 1.500 | 1.360 |
| 8 | Caryophyllene oxide | 0.404 | 0.996 | 0.424 |
| 9 | 2H-2,4a-Ethanonaphthalene, 1,3,4,5,6,7-hexa- hydro-2,5,5-trimethyl- | * | 0.835 | * |
| 10 | β-phellandrene | 0.187 | 0.730 | 0.344 |
| 11 | Methyl eugenol | 0.273 | 0.626 | 1.610 |
| 12 | α-terpinene | 0.497 | 0.568 | 0.243 |
| 13 | β-cubebene | 0.997 | 0.564 | 0.186 |
| 14 | Anethole | 1.922 | 0.367 | 0.351 |
| 15 | α-cubebene | * | 0.327 | * |
| 16 | α-copaene | 0.337 | 0.322 | 0.209 |
| 17 | 2,4,6-octatrienoic acid | * | 0.237 | * |
| 18 | 2-carene | 0.151 | 0.219 | * |
| 19 | β-copaene | 0.049 | 0.213 | * |
| 20 | α-phellandrene | * | 0.206 | * |
| 21 | Eucalyptol | 0.266 | 0.182 | 0.096 |
| 22 | β-guaiene | 0.026 | 0.176 | 0.138 |
| 23 | Naphthalene, 2-methyl- | 0.074 | 0.172 | 0.070 |
| 24 | Naphthalene, 6-ethyl-1,2,3,4-tetrahydro- | 0.131 | 0.165 | 0.090 |
| 25 | Naphthalene, 1-methyl- | 0.029 | 0.071 | * |
| 26 | Isolongifolene | 1.232 | * | 0.738 |
| 27 | Humulene | 1.529 | * | 0.454 |
| 28 | Methyl benzoic dodecyl ester, 2,6-difluoro-3-acid | 0.479 | * | 0.321 |
| 29 | Isoledene | 0.736 | * | 0.314 |
| 30 | Naphthalene, 1,2,3,4-tetrahydro-1,6,8-trimethyl- | 0.254 | * | 0.276 |
| 31 | β-cadinene | 0.113 | 0.247 | |
| 32 | (±) cadinene | 0.017 | * | 0.092 |
| 33 | Thymol | * | * | 0.063 |
| 34 | α-elemene | 0.131 | * | 0.062 |
| 35 | (-)-aristolene | 0.087 | * | 0.055 |
| 36 | δ-selinene | 0.096 | * | 0.051 |
| 37 | (+)-epi-bicyclosesquiphellandrene | 1.335 | * | * |
| 38 | Limonene | 1.138 | * | * |
| 39 | 2 5-bis (trimethylsilyl)oxy benzaldehyde | 0.945 | * | * |
| 40 | β-pinene | 0.337 | * | * |
| 41 | 1,4-Benzenediol, 2,5-bis(1,1-dimethylethyl)- | 0.279 | * | * |
| 42 | Naphthalene, 2-buthyldecahydro | 0.201 | * | * |
| 43 | Mesitylene | 0.196 | * | * |
| 44 | Hydroxyacetophenone, 3,5-di-tert-butyl-4- | 0.140 | * | * |
| 45 | (±) cadinene, 1,4-benzenediol, 2,5-bis(1,1 -di- methylethyl)- | 0.183 | * | * |
| 46 | Naphthalene, 1,2,3,4-tetrahydro- 1,5-dimethyl- | 0.166 | * | * |
| 47 | Naphthalene, 1,2,3,4-tetrahydro-6,7-dimethyl- | 0.156 | * | * |
| 48 | (-)-calamenene | 0.144 | * | * |
| 49 | (+)-2-bornanone | 0.144 | * | * |
| 50 | Morpholine, 4-octadecyl- | 0.140 | * | * |
| 51 | 1,3-cyclopentadiene, 1,2,3,4,5-pentamethyl- | 0.135 | * | * |
| 52 | Aromadendrene oxide(2) / aristotele epóxide | 0.121 | * | * |
| 53 | Benzene 1,2,3,5-tetramethyl- | 0.063 | * | * |
| 54 | α-ylangene | 0.117 | * | * |
| 55 | Benzene 1,2,4,5-tetramethyl- | 0.058 | * | * |
| 56 | Naphthalene | 0.104 | * | * |
| 57 | Isolongifolene, 4,5,9,10-dehydro- | 0.086 | * | * |
| 58 | Benzene, 1-ethyl-4-methyl- | 0.084 | * | * |
| 59 | ƴ-terpineol | 0.081 | * | * |
| 60 | ρ-cimene | 0.070 | * | * |
| 61 | γ-muurolene | 0.068 | * | * |
| 62 | (+)-δ-cadinene | 0.067 | * | * |
| 63 | Benzene, 1,3-dichloro- | 0.065 | * | * |
| 64 | α-cadinene | 0.064 | * | * |
| 65 | Octanoic acid methyl ester | 0.063 | * | * |
| 66 | Calarene | 0.063 | * | * |
| 67 | Nootkatone | 0.057 | * | * |
| 68 | Benzene, 2-ethyl-1,4-dimethyl- | 0.055 | * | * |
| 69 | Benzene, 4-ethyl-1,2-dimethyl- | 0.054 | * | * |
| 70 | Agarospirol / Ciperene | 0.051 | * | * |
| 71 | Naphthalene, 1-isocyano- | 0.051 | * | * |
| 72 | Linalool | 0.048 | * | * |
| 73 | Naphthalene, 5-ethyl-1,2,3,4-tetrahydro- | 0.046 | * | * |
| 74 | Calarene epoxide | 0.044 | * | * |
| 75 | Benzaldehyde, 3,5-di-tert-butyl-4-hydroxy- | 0.040 | * | * |
| 76 | β-neoclovene | 0.040 | * | * |
| 77 | Benzene, 1-ethyl-2,4-dimethyl- | 0.034 | * | * |
| 78 | 4-epi-cubedol | 0.037 | * | * |
| 79 | Benzene, 1-methyl-4-propyl- | 0.029 | * | * |
| 80 | Thujopsene-(i2) | 0.027 | * | * |
| 81 | Aristolene epoxide | 0.026 | * | * |
| 82 | Ledene alcohol | 0.025 | * | * |
| 83 | Benzene, 1,2,4-trimethyl- | 0.022 | * | * |
| 84 | α-longipinene | 0.020 | * | * |
| 85 | Benzene, 1-ethyl-3,5-dimethyl- | 0.043 | * | * |
| 86 | 5-dodecanol acetate | 0.015 | * | * |
| 87 | ƴ-eudesmol | 0.015 | * | * |
| 88 | S-octahydrophenanthrene, 9-methyl- | 0.014 | * | * |
| 89 | δ-neoclovene | 0.013 | * | * |
| 90 | Benzene, 1-ethynyl-4-metoxy- | 0.013 | * | * |
| 91 | 3-carene | 0.013 | * | * |
| 92 | Ciperene | 0.012 | * | * |
| 93 | Cubedol | 0.012 | * | * |
| 94 | β-nootkatone | 0.011 | * | * |
| 95 | Cembrene | 0.011 | * | * |
| 96 | Benzene, 1,2,3,4-tetramethyl- | 0.011 | * | * |
| 97 | 1,4-benzenediol, 2,6-bis(1,1-dimethylethyl)- | 0.008 | * | * |
| 98 | 4-terpinenyl acetate | 0.007 | * | * |
| 99 | Verbenone | 0.007 | * | * |
| 100 | Phenylethyl Alcohol | 0.006 | * | * |
| 101 | Benzene, 1-methyl-2-propyl- | 0.004 | * | * |
| 102 | α-muurolene | 0.003 | * | * |
| 103 | Camphor | 0.003 | * | * |
| 104 | Caryophyllene-(l-1) | 0.002 | * | * |
| 105 | ƴ-selinene | 0.002 | * | * |
| 106 | Carveol | 0.002 | * | * |
| 107 | Guadiol | 0.002 | * | * |
| 108 | (+)-nerolidol | 0.002 | * | * |
| 109 | α-gurjunene | 0.002 | * | * |
| 110 | α-irone | 0.002 | * | * |
| 111 | β-eudesmol | 0.002 | * | * |
| 112 | cis 3-pinanone | 0.001 | * | * |
| 113 | Terpinyl formate | 0.001 | * | * |
| 114 | Phenol acetate, 2,5-dimethyl- | 0.0004 | * | * |
| 115 | Durene | 0.0003 | * | * |
| 116 | Pinocarvone | 0.0003 | * | * |
*Undetected compounds.
A greater number of secondary metabolites (SM) was identified in avocado trees var. drymifolia, compared to previous reports. Torres-Gurrola et al. (2011) determined the biochemical profile of drymifolia avocado trees in leaf tissue, to identify the relationship with the incidence of gall-forming insects, in which they reported 33 secondary metabolites. In another study on the chemical composition of drymifolia avocado trees, from the germplasm banks in Uruapan, Cupatitzio and Apatzingán, in the State of Michoacán, 64 compounds in 247 trees evaluated were reported (Rincón-Hernández et al., 2011).
In “Hass” avocado trees, a smaller number of SM was identified (25), compared to a previous study, in which 34 compounds were detected in the volatile fraction of the hexane extract of foliar tissue, of which 32 were identified and two were not identified. (García-Rodríguez et al., 2016). On the other hand, so far there are no reports in the literature related to secondary metabolites identified in leaf tissue of avocado trees “Mendez”.
On average, four secondary metabolites were the most abundant in the drymifolia, “Hass” and “Mendez” varieties.
The relative concentrations of these compounds are: estragole 61.2, 68.0 and 81.5 %, karyophylene 19.4, 5.2 and 3.2 %, (+) - 4-carene 7.5, 6.8 and 2.7 % and γ-terpinen 6.9, 8.0 and 2.7 % in varieties drymifolia, “Hass” and “Mendez”, respectively (Table 1). These four compounds represent the 95.0, 88.0 and 90.1 %, of the total SM identified in the volatile profile of avocado leaf tissue for drymifolia, “Hass” and “Mendez” avocados, respectively. The high similarity in the observed abundance of these four compounds, among the varieties of avocado studied, suggests that the possible mistletoe tolerance could be related to other compounds or factors.
For avocado drymifolia, Rincón-Hernández et al. (2011) reported the presence of estragole (75-86 %), β-caryophyllene (1.4-3-1 %), β-pinene (1.3-1-8 %) and α-pinene (0.8-1.5 %).
In the case of the «Hass» variety, García-Rodríguez et al. (2016), reported a lower abundance of estragole, on average 1.7 %, compared with the 68.0 % reported in this study. In contrast, the compounds persin (17.9 %), 2 - [(8Z, 11Z) -heptadeca-8,11-dienyl] furan (15.8 %), squalene (13.9 %) and nonacosan (11.9 %) had higher concentrations, while, they were not identified in the present work. The difference in abundance may be due to the chemotype of the materials studied in each study (García-Rodríguez et al., 2016).
In avocado materials, var. drymifolia, a high diversity of relative abundance was observed among the 27 individuals studied, mainly in the most abundant compounds. The ranges of abundance of the compounds estragole, karyophylene, (+) - 4-carene and γ-terpinen were: 0.39 to 97.00 %, 0.56 to 71.50 %, 0.05 to 27.81 % and 0.60 to 28.63 %, respectively. In the «Hass» and «Mendez» varieties, only one individual from each variety was studied, so that it was not possible to determine the ranges of the concentrations, however, low variability was observed in these commercial varieties, probably because they are propagated in a clonal manner (García-Rodríguez et al., 2016). On the other hand, in the “Hass” variety four secondary metabolites were identified, 1,3,4,5,6,7-hexahydro2,5,5-trimethyl 2H-2,4a-ethanonaphthalene (0.84 %), α-cubebeno (0.33 %), 2,4,6-octatrienoic acid (0.24 %) and α-felandrene (0.21 %), which were not detected in the drymifolia and “Mendez” variety (Table 1).
There are few reports related to defense activities of the MS. The estragole compound has antibacterial, antioxidant (Iscan, 2017), anticancer, genotoxic (Alhusainy et al., 2012; Basaglia et al., 2014) and callus inducer for in vitro plant regeneration (Ibrahim et al., 2011). The karyophylene compound has antibacterial and antifungal activity (Selestino-Neta et al., 2017). There are no reports of biological activity for the compounds carene and γ-terpinen.
Also, in the avocado variety «Mendez,» the thymol compound was identified (0.06 %), which was not detected in drymifolia and “Hass” varieties (Table 1). The relative abundance of these compounds was less than 1 %. However, since it was not found in var. drymifolia, a susceptible variety to mistletoe attack, these SM and their combination, represent an opportunity to be studied in subsequent projects, to identify their possible relationship with tolerance to mistletoe.
The tolerance of avocado trees varieties “Hass” and “Mendez” to mistletoe attack could be explained by the presence of 1,3,4,5,6,7-hexahydro-2,5,5trimethyl 2H-2,4a-ethanonaphthalene, α-cubebeno, 2,4,6-octatrienoic acid, α-felandrene and thymol or, the combination of several compounds, which are not found in drymifolia trees (Torres-Gurrola et al., 2011).
In avocado trees var. drymifolia, 80 compounds were identified, with concentrations in a range of 0.0003 to 1.335 % (Table 1), that were not detected in the trees of the “Hass” and “Mendez” varieties. The susceptibility or tolerance of avocado trees could depend not only on the biochemical profile of the individual but also on other complex factors such as the combination of compounds, physiological processes, the presence of diseases, nutritional deficiencies and the different stages of plant development.
Another particular group of 27 secondary metabolites, with relative concentrations of less than 2 %, were present in two or three of the varieties under study (Figure 1). These compounds can be of special attention because, 2,5-bis benzaldehyde and β-guaiene had 21.7X and 6.8X times more abundance, respectively in «Hass» vs. drymifolia. Additionally, 15 other compounds presented 0.2 to 4.3X times more abundance in “Hass” vs. drymifolia avocados (Table 2).
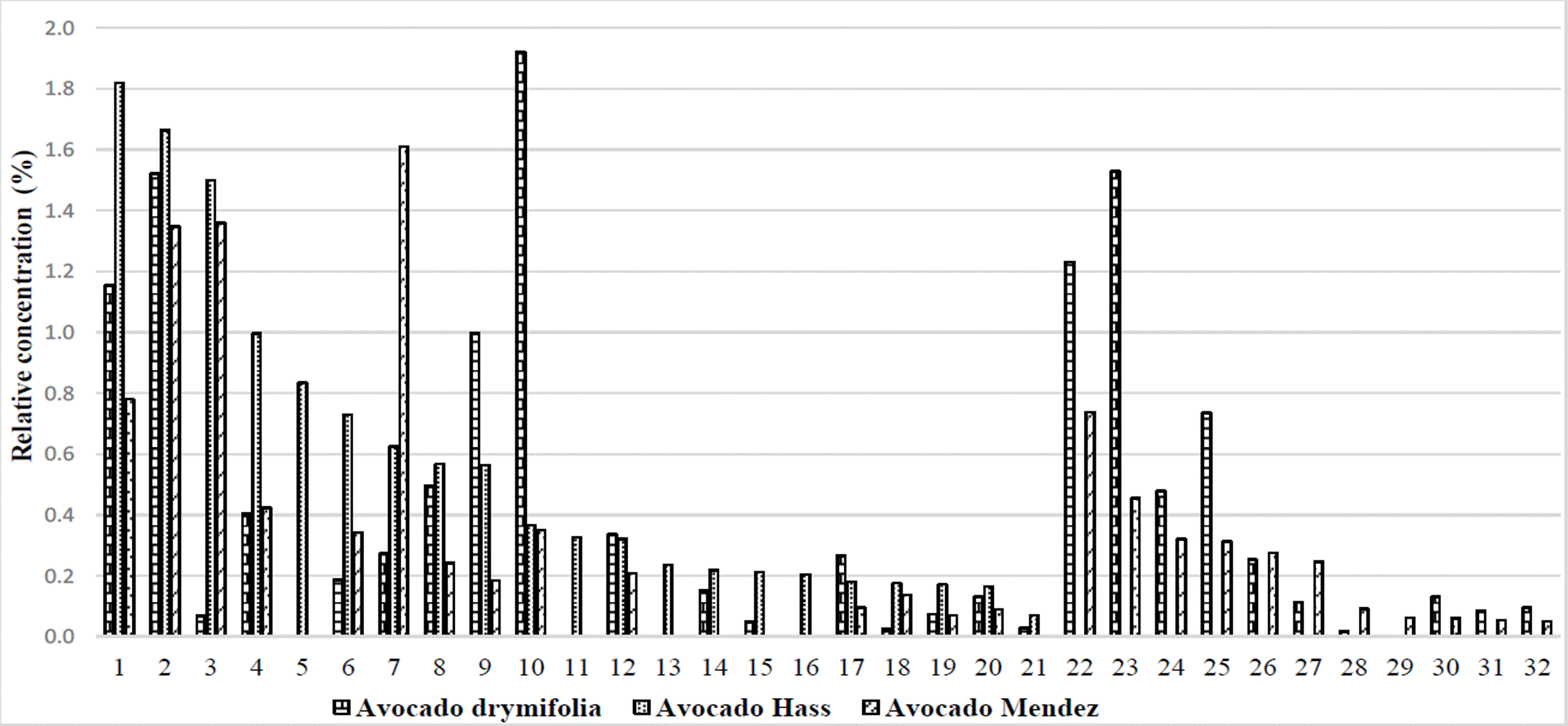
Figure 1 Relative abundance of secondary metabolites identified in the drymifolia, “Hass” and “Mendez” avocados. 1) o-cymene, 2) Copane, 3) 2,5-bis benzaldehyde, 4) Caryophyllene oxide, 5) 1,3,4,5,6,7 hexahydro-2,5,5-trimethyl 2H- 2,4a-Ethanonphthalene, 6) β-phellandrene, 7) Methyleugenol, 8) α-terpinene, 9) β-cubebene, 10) Anetole, 11) α-cubebene, 12) α-copaene, 13) 2,4,6-octatrienoic acid, 14) 2-carene, 15) β-copaene, 16) α-phellandrene, 17) Eucalyptol, 18) β-guaiene, 19) 2-methyl-naphthalene, 20) 6-ethyl-1,2,3,4-tetrahydro-naphthalene, 21) 1-methyl-naphthalene, 22) Isolongifolene, 23) Humulene, 24) 2,6-Difluoro-3-methylbenzoic acid duodecyl ester, 25) Isoledene, 26) 1,2,3,4-tetrahydro-1,6,8-trimethyl naphthalene, 27) β-cadinene, 28) (±) cadinene, 29) Thymol, 30) α-elemene, 31) (-) - aristolene, 32) δ -selinene.
Table 2 Secondary metabolites in Hass and Méndez avocados with higher concentration in relation to drymifolia.
| # | Secondary metabolites | H/d (X) |
M/d (X) |
|---|---|---|---|
| 1 | Benzaldehyde, 2,5-bis | 21.74 | 19.71 |
| 2 | β-guaiene | 6.77 | 5.29 |
| 3 | β-copaene | 4.35 | * |
| 4 | β-phellandrene | 3.90 | 1.84 |
| 5 | Caryophyllene oxide | 2.47 | 1.05 |
| 6 | Naphthalene, 1-methyl- | 2.45 | * |
| 7 | Naphthalene, 2-methyl- | 2.32 | 0.94 |
| 8 | Methyl eugenol | 2.29 | 5.90 |
| 9 | o-cymene | 1.58 | 0.68 |
| 10 | 2-carene | 1.45 | * |
| 11 | Naphthalene, 6-ethyl-1,2,3,4-tetrahydro- | 1.26 | 0.69 |
| 12 | α-terpinene | 1.14 | 0.49 |
| 13 | Copaene | 1.09 | 0.89 |
| 14 | α-copaene | 0.96 | 0.62 |
| 15 | Eucalyptol | 0.68 | 0.36 |
| 16 | β-cubebene | 0.57 | 0.19 |
| 17 | Anethole | 0.19 | 0.18 |
| 18 | (±) cadinene | * | 5.43 |
| 19 | β-cadinene | * | 2.18 |
| 20 | Naphthalene, 1,2,3,4-tetrahydro- 1,6,8-trimethyl- | * | 1.09 |
| 21 | Methyl benzoic dodecyl ester, 2,6-difluoro-3-acido | * | 0.67 |
| 22 | (-)-aristolene | * | 0.63 |
| 23 | Isolongifolene | * | 0.60 |
| 24 | δ-selinene | * | 0.53 |
| 25 | α-elemene | * | 0.47 |
| 26 | Isoledene | * | 0.43 |
| 27 | Humulene | * | 0.30 |
X: Quantity of times more. H, Hass. d, drymifolia. M, Mendez.
Similarly, the 2,5-bis benzaldehyde compound showed 19.7X times more abundance in avocado “Mendez” vs. drymifolia, and 23 other compounds presented 0.2 to 5.9X times more abundance in avocado “Mendez” vs. drymifolia (Table 2).
The difference in abundance of the 2,5-bis benzaldehyde compound between commercial varieties and var. drymifolia, suggests the possibility of exploring in more detail the role of this secondary metabolite regarding the tolerance to mistletoe attack from the Loranthaceae family.
There are no reports of these metabolites related to mistletoe tolerance, however, in the work on “Hass” avocado trees, reported by García-Rodríguez et al. (2016), they identified the MS α-cubebeno (4.83 %) in higher relative abundance than in the present work (0.33 %). This compound presents antioxidant, antibacterial activity (Nivas & Gaikwad, 2014) and attracts beetles carrying pathogenic fungi for the plant (Kendra et al., 2016). Besides, the α-felandreno and β-copaeno compounds (Table 2) have antimicrobial activity (De Santi et al., 2017), β-guaieneo has antiseptic activity (Camacho-Romero et al., 2017) and β-felandreno has antibacterial, antifungal and antioxidant properties (Gupta et al., 2017; Fathy et al., 2017). Concerning the compounds 2,5-bis benzaldehyde, 1,3,4,5,6,7-hexahydro-2,5,5-trimethyl 2H-2,4a-ethanonaphthalene, and 2,4,6-octatrienoic acid, there are no reports of any activity in plants.
It is important to note that most of the research done on avocado SM was carried out on different parts of the fruit, such as the seed, pulp and skin (Ikhuoria & Maliki, 2007; Rodríguez-Carpena et al., 2011; Ceballos & Montoya, 2013; Dabas et al., 2013; López-Cobo et al., 2016; Figueroa et al., 2018; Melgar et al., 2018) and focused on activities related to human health (Yasir et al., 2010).
Conclusions
In the volatile profile identified in the drymifolia avocado trees, “Hass” and “Mendez,” the SM estragole, karyophylene, (+) - 4-carene and γ-terpinen had the highest relative concentrations. However, being present in all three types of materials, these compounds were discarded as protective agents against mistletoe attack.
In trees of the drymifolia variety, a higher number of secondary metabolites was identified compared to the varieties “Hass” and “Mendez.” This is the first report of a high number of secondary metabolites in the drymifolia material.
The secondary metabolites, 1,3,4,5,6,7-hexahydro-2,5,5trimethyl 2H-2,4a-ethanonaphthalene, α-cubebeno, 2,4,6-octatrienoic acid, and α-phelandrene are present in «Hass» avocado trees but not in drymifolia and “Mendez” varieties, while the thymol compound was identified only in the “Mendez” variety.
The 2,5-bis benzaldehyde compound was observed with higher relative abundance in avocado trees “Has” and “Mendez,” 21.7 and 19.7X times respectively, compared to var. drymifolia. In order to determine the effect of these compounds present in “Hass” and “Mendez” exclusively, it is necessary to carry out isolation and characterization activities on the establishment and development of germination of mistletoe seeds under in vitro conditions.
This work is the first approach to the volatile profile in avocado leaves var. drymifolia (susceptible) and “Hass” and “Mendez” (tolerant) to the mistletoe attack (family Lauranthaceae) and can contribute to a better understanding of the defense systems of the crop against parasitic agents and optimize control strategies.











 texto en
texto en 


