Introduction
Small rodents are extensively used in ecoepidemiological research (“disease ecology”) by people who are not adequately familiar with the ecology and dynamics of the reservoir host populations. This can and has led in the past to misinterpretations of data and hence erroneous conclusions. Small rodent populations, or generally small mammal populations, are by no means homogenous groups. On the contrary, they are heterogeneous, and this heterogeneity changes seasonally, multiannually, and density- and/or phase-dependently. Many infection parameters and processes are related to the dynamics of the functional groups in the populations, i. e., subgroups that differ physiologically, behaviorally, and immunologically. Understanding host population structure and its varying demographic heterogeneity is a key factor in disease ecology, enabling more detailed analyses and drawing of realistic conclusions. In this commentary I will show why it is important to understand this dynamic heterogeneity. My conclusions are mostly based on our studies in high-latitude systems, but no doubt the conclusions can be applied to small rodents in other systems, and for other small mammal taxa as well.
What is a functional group? The idea of the functional group (functional category) as a behavioral/physiological population subunit was introduced to the small mammal literature by Myllymäki (1977 a, b). This concept has subsequently been expanded to pathogen/parasite research, e. g., by Haukisalmi et al. (1988, 1995), Haukisalmi and Henttonen (2000), and Cattadori et al. (2005). The basic idea as it relates to parasite/pathogen research is the division of a small mammal population into subgroups that are behaviorally, physiologically, and immunologically uniform. A particularly important attribute of this concept is that it is dynamic: population structure changes seasonally, multiannually, and density- and phase-dependently. When making comparisons over season and years, functional groups are the level at which parasite/pathogen comparisons should be made among host populations.
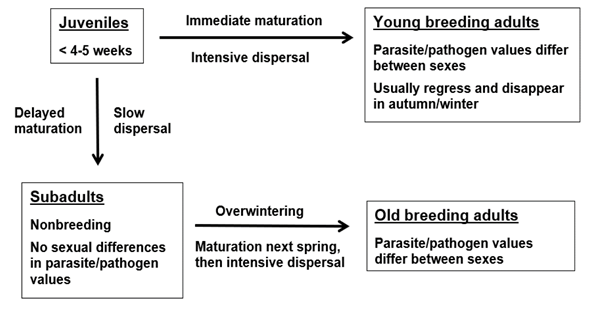
In small mammal populations, juveniles have two alternatives after they leave the natal nest (Figure 1). They can either mature immediately, with females in some species becoming pregnant at the age 3-4 weeks, or they can delay maturation and breeding to the next summer (e. g., Prévot-Julliard et al. 1999). The use of the words summer and winter are here equivalent with other breeding /nonbreeding seasons in other biomes. If juveniles mature immediately, they become young breeding adults, either males or females. If juveniles delay maturation, mostly due to late season or high density, they become subadults. These animals do not breed, they are clearly smaller than breeding adults, and most importantly, with respect to behavior and physiological immunology, they are “sexless”, and tolerated by breeding animals. Usually subadult males and females do not differ in parasite/pathogen infection values, unlike breeding adults either young or old. After overwintering, subadults mature in spring, males earlier than females, and they become old breeding adults, males and females. Maturation of subadults affects their behavior, physiology, and immunology, and leads rapidly to sexual differences in parasite/pathogen values characteristic of those of adults.
It is essential to understand that the functional group is not the equivalent of age. For example, young breeding adults in early autumn can be two months old (and potentially have parasite/pathogen infection values resembling those of overwintered adult breeding animals), but subadults who have delayed their breeding can be eight months old in late winter and still have parasite/pathogen values typical of a nonbreeding animal, with no differences between sexes. Parasite/pathogen infection values are often more closely associated with the functional group of an individual than its absolute age. Still, within a functional group like subadults from autumn to spring, levels of infection with parasites and pathogens can increase with time and age, but without differences between sexes (e. g., for the bank vole and Puumala orthohantavirus, see Voutilainen et al. 2016).
The seasonality of these events can occasionally vary, for example during phase-, climate- or mast-induced bouts of winter breeding in some rodent species (Tersago et al. 2009). Nonetheless, the parasite/pathogen patterns related to functional groups remain.
How to identify functional groups. Defining the functional group is based on the combination of breeding status and age. More detailed information is usually achieved at animal dissection, but many of the aging and breeding parameters can be seen externally in live animals, which helps in categorizing animals without a need to sacrifice them. There are several methods of age determination for small mammals (see e. g., Morris 1972), albeit nowadays often poorly known among modern digital ecologists, and still less known among medical people. Weight is often used as a proxy for age, but weight depends on maturation and breeding status which are density- and phase-dependent.
I detail some aging methods below. Different rodent genera differ with respect to the most appropriate aging methods. Several methods can be used for Myodes voles, including growth patterns of molar roots (Tupikova et al. 1968; Lowe 1971; Viitala 1971), and pelage (fur) growth patterns (molting) that can be used in live-trapping studies (Zejda and Mazák 1965; Viitala 1981). The fur growth analysis is not very precise for young breeding females over 1.5 to 2 months of age because the energy allocation to pregnancy and lactation delays molting, but it is usable in differentiating juveniles (< 4 weeks) from older animals in summer, and summer-born adults from overwintered adults in late summer - autumn, which is practical in live-trapping. Eye lens development has been used for Myodes (Kozakiewicz 1976; Takahashi and Satoh 1997) and also for Lemmus, Microtus, and Apodemus (Östbye and Semb-Johansson 1970; Adamczewska-Andrzejewska 1971; Hagen et al. 1980). Muskrats also have growing molar roots (Pankakoski 1980), whereas Microtus and Lemmus species have continuously growing (“rootless”) molars that cannot be used for age determination, and therefore their age determination must be based on fur growth patterns (Sýkora 1960; Koponen 1970) or eye lens development. Apodemus mice can be divided into age groups using, e. g., molar wear (Adamczewska-Andrzejewska 1972).
My own 50-year experience is that methods based on external characters used in live-trapping studies usually work well enough for basic definition of functional groups, though some experience is needed. The problem now seems to be that many scientists do not even know that such methods exist. If animals are sacrificed for tissue sampling and museum specimen preparation, then more detailed information can easily be collected.
The distinction between juveniles and subadults or maturing young adults is rather easy using hair growth patterns. Young rodents have dull thin fur until the age of four weeks and thereafter start to grow their second fur (called, depending on the genus, post-juvenile fur or adult 1 fur). This is externally visible first as new short silky hairs emerging from the skin, first on the ventral side and later on the dorsal side, lasting about two weeks, during weeks 5 to 6 of age. These patterns are visible on the inside of the skin as black pigment figuration. The new emerging fur is eventually clearly longer than the original juvenile fur.
In early summer and/or early in the increase phase of a population cycle the distinction between juveniles and young adults, which then mature and grow quickly, is occasionally more difficult because juveniles can start to sexually mature while still having juvenile fur, i. e. even if still having the juvenile fur and being < 4 weeks old, but their behavior changes to that of a breeding type.
Table1 An example of how a parasite/pathogen analysis can give different results depending if it is based on pooled samples or functional groups. Pooled samples seem to suggest a difference between treatments, but it is only because of the different age structures. There is no difference between functional groups, i. e. at the individual level. Overwintered (OW), subadults (SUB).
| Control | Experimental | |||
|---|---|---|---|---|
| OW | SUB | OW | SUB | |
| Number of individuals | 20 | 60 | 60 | 20 |
| Prevalence/functional group | 15.0% | 5.0% | 15.0% | 5.0% |
| Population mean prevalence | 16.3% | 38.8% |
The behavior of young small rodent males changes when their testicles reach a length of 5 to 7 mm and they disperse. This coincides with a change in the behavior of adult males towards young maturing males, expelling them from natal sites. Depending on the genus, the same change can occur in young females when the vagina opens. In Myodes species, breeding females have exclusive territories, and maturing young females have to disperse whereas in Microtus young maturing females can stay in home ranges overlapping those of their mothers. If the maturation is delayed, young subadults can stay at their natal home ranges. Subadults are not territorial and do not induce territorial behavior in adults, and they can usually stay at their natal sites on overlapping home ranges.
Spring maturation is accompanied with intensive dispersal because territories/home ranges of adults are clearly larger than home ranges of subadults. This spring dispersal, which in boreal conditions may already begin under snow, is often forgotten in models analyzing density-dependent dispersal because it takes place before the “summer” field season.
Young maturing voles can be teenager-type runners for a week or two, and depending on the genus and space available, thereafter settle down as breeding adults. These behavioral changes can occur when voles are three weeks old or after overwintering half a year later. Later in summer, live-trapped young and old adults can be distinguished by fur length, or dead animals by molar and fur patterns. When subadults mature in spring, they grow and enter breeding condition, which can be seen externally also in live animals.
Examples of common errors. Seasonality. Many parasite/pathogen values have a seasonal pattern. Prevalence can increase with age. Prevalence is typically low in late summer - early autumn, but can approach 100% in old animals in the next summer (Voutilainen et al. 2016). If geographic comparisons are made on the basis of samples collected in different seasons, potential differences are possibly due to seasonality and different age structures, not due to geography or other causes. This is a common, elementary mistake.
Some opportunistic parasites infect only old overwintered voles in their second summer when they are soon to disappear from the host populations (Haukisalmi et al. 1988). The proportion of this host functional group in the whole population can be only 5 %, but more than 50 % of these voles are infected. This period in late summer is rather short. Unless there is a concentrated effort on this specific functional group in a specific season, these opportunistic parasites would be missed, or considered rare at the whole population level, leading to a misinterpretation of their role. It is rare to find these parasites in other functional groups. For example, knowing that certain parasite taxa may almost solely occur in old overwintered animals in late summer, researchers must plan the sampling time to detect occurrence in these individuals.
Sampling. Instead of one pooled sample for parasite/pathogen studies, sampling should be designed so that main functional groups with their specific infection values are adequately represented. This, by necessity, increases the total sample size. It may even require extra effort to sample a functional group with low density. Indeed, if one thinks that, e. g., 50 rodents is a good sample, these thoughts may dissipate when this 50 is divided into functional groups: one rodent in the first group, two in a second, 47 in the third, and zero in the fourth.
Population structure. Even if samples are collected in the same season, erroneous conclusions are easily drawn without considering population structure. The same applies to experimental manipulations. Let’s take a simple example (Table 1). We can have two different years, or a simultaneous experimental manipulation and its control. The populations to be compared consist of old overwintered individuals and subadults. Total densities are the same in the two populations, but there is a clear difference in population structure. Both functional groups have their specific parasite/pathogen prevalence, which is the same in experimental and control groups. The common way to analyze this kind of material is to clump all animals together and estimate one prevalence value for the whole population, which in this case seems to suggest that parasite/pathogen prevalence is greater in the experimental group. However, if one analyzes the data per functional group, there is no change in parasite/pathogen prevalence within functional groups, i. e. at the individual level. The only thing that has changed is the proportion of functional groups in the sample, not the parasite/pathogen prevalence per functional group. The correct conclusion might be that the manipulation increased the abundance of old animals, but did not affect parasite/pathogen prevalence at the individual level. Or this kind of result could be a typical phase-dependent impact on the population structure between cyclic increase and peak years.
We encounter the same problem when the age of animals is based on proxies, not on real age determination. It is common to use weight as a proxy for age. Weight is sufficient to separate subadult and breeding rodents in late summer - autumn. However, as clarified above, there are two types of adults in late summer - autumn populations of small rodents: young adults born earlier the same summer, and old overwintered animals. They are all breeding adults, above some weight limit, but the young adults typically are 2 to 3 months old, and the overwintered ones are 12 to 14 months old. For example, in cyclic vole populations, adults in the autumn of the increase phase are mostly young individuals due to rapid maturation, while in the peak phase the proportion of old ones is much higher due to the delayed maturation of young animals at high densities (Prévot-Julliard et al. 1999). If the functional groups (and real age) have been properly identified, this age difference can have a drastic impact on parasite/pathogen values of young and old adults. If the “age” determination of these adults is based only on a proxy like weight and age groups are pooled, then, e. g., phase-dependent effects on the real parasite/pathogen values in different adult functional groups remain unknown.
Parasite/pathogen infection values can be sensitive to further structuring within young and old adults. Haukisalmi et al. (1995) analyzed parasite prevalences in breeding females of two sympatric vole species. Young adults differed from old ones, but prevalences also depended on whether females were pregnant and lactating, or only lactating. In addition, these differences were opposite in young and old females, and they were opposite between species.
Toxicological studies. Understanding population structure is also important in toxicological studies, where pooled population samples are commonly used. A pooled sample can include animals of very different ages, e. g. from 1 mo to 16 mo, and depending on the cyclic phase, one sample can be dominated by young animals, another one by old animals. Toxin accumulation certainly depends on age. Still, even though young non-breeding subadults and breeding adults are separated (which is seldom done), the problem of young and old adults remains. In short-lived animals, a difference in age from 2 to 4 mo to 14 to 16 mo makes a difference for accumulation of toxins.
Organ weight. Organ weights, like those of spleen, liver, etc., have often been used as proxies for stress, disease, toxins, and evolutionary processes. However, organ weight does not have a linear relation to animal body size or weight, and there can be abrupt and large differences between functional groups. Therefore, analyses of pooled samples of organ weights without reference to population structure may result in something that depends more on the hidden sample structure than on the biological phenomena being studied.
Season of study. Many, if not most field studies, are performed in “summer” time. Small rodent populations are at that time characterized by intensive breeding and by the presence of all functional groups. However, it is often forgotten that “summer” may comprise only 1/3 of the year, and still most hypotheses on parasite/pathogen transmission are derived from this limited period. These ideas are not wrong as such, but they may not be applicable for periods outside of the breeding season when the population structure is different, e. g. during winter when breeding adults are not usually present. The idea that old males with high testosterone values are responsible for most of the parasite/pathogen transmission in the population is popular. But if old males are present in the population only for 1/3 of the year, what happens during the other 2/3 of the year? In addition, seasonality and photoperiod have strong impacts on immunity.
Transmission route of pathogens depends on the functional group. During the breeding season, adult males can be territorial and aggressive, and transmission via biting can be common. Breeding males also are more active and move over larger areas. On the other hand, overwintering subadults are docile, and obviously most transmission in the nonbreeding season takes place indirectly via the environment, as suggested for Puumala orthohantavirus (Voutilainen et al. 2016).
Dilution. The impact of biodiversity on zoonotic diseases is a rapidly developing field (for a recent review, see Keesing and Ostfeld 2021). Dilution impact is often considered in this context. Transmission routes depend on the behavior of functional groups, and their occurrence is strongly seasonal. Treating the whole population as a homogeneous unit throughout the seasons will lead to omission of important aspects of seasonality in population structure and behavior. Not many studies on direct dilution effects have taken this into consideration. However, Voutilainen et al. (2012) analyzed the transmission of Puumala orthohantavirus in bank voles in a multispecies small mammal community separately in spring (all voles and shrews were breeding overwintered individuals) and autumn (mostly non-breeding subadults). They found that dilution impact was clear in spring populations consisting of breeding territorial voles and shrews, while in autumn in nonbreeding, non-territorial populations dilution was not observed.
Dispersal. Dispersal in small mammal populations is commonly observed, and has attracted a lot of attention (for reviews, see e. g., Anderson 1989; Lidicker 1995; Bjørnstadt et al. 1999; Krebs 2013). Dispersal is strongly seasonal and related to the functional groups; dispersers are not just random subsets of the population. Understanding the roles of dispersing subunits of the population and their potential in parasite/pathogen spread adds to our understanding of relevant patterns in disease ecology. This is relevant for dispersal of rodent-borne zoonotic pathogens, and can be used to predict risk periods, e. g., for rodent-borne pathogens. Dubois et al. (2017) and Razzauti et al. (2013) analyzed the dispersal of bank voles and Puumala orthohantavirus using genetic markers. There are three periods of intensive dispersal: 1) during the maturation and territorial formation in spring when the hantavirus prevalence is approaching its seasonal peak in old overwintered voles, though densities can be rather low at this time; 2) in early - mid summer when maturing young voles disperse from the territories/home ranges of their parents, but at this time virus prevalence in young animals is low, and this event is phase-dependent; and 3) in late autumn with freezing temperatures when subadult bank voles look for overwintering habitats and commonly enter human settlements - and this is the main human epidemic season at high latitudes.
Much more understanding would be gained if disease ecological studies on rodents were based on proper characterization of the dynamic heterogeneity of reservoir populations and identifying the population subunits relevant for the maintenance and spread of zoonotic parasites and pathogens. Using the concept of functional groups is a natural way to deal with population subgroups with distinctive physiological, immunological, and behavioral characteristics. Understanding the dynamic demographical background of parasite/pathogen infections and transmission would reduce noise in conclusions and help to avoid misinterpretations stemming from the pooling of materials.











 nueva página del texto (beta)
nueva página del texto (beta)