Introduction
It is very common to observe mammals and birds consuming fragments of soil from certain forest sites where they live, a phenomenon known as geophagy (Diamond et al. 2008; Gilardi et al. 1999; Setzl et al. 1999). Such sites are known as mineral licks or in the Neotropics as saladeros (Voigt et al. 2007), salt licks (Tracy and Mc Naughton 1995), or natural mineral licks (Emmons and Stark 1979), as they contain mineral salts of sodium, calcium, potassium, or iron (Emmons and Stark 1979; Klaus and Schmid 1998; Lizcano and Cavalier 2004; Montenegro 2004; Mahaney et al. 2005; Link et al. 2012). Mineral licks can be classified as “open”, “wall” or “caves”, according to their characteristics (Montenegro 2004).
The mammals recorded visiting these mineral licks include chiropters, rodents, primates, and ungulates. In the case of birds, species of the orders Galliform (chickenlike birds locally known as pavas), Columbiform (pigeons), and in large numbers Psitaciform (parrots) have been recorded (Izawa 1993; Krishnamani and Mahaney 2000; Brightsmith 2004; Montenegro 2004; Voigt et al. 2007; Bravo et al. 2008; Tobler et al. 2009; Blake et al. 2010; Link et al. 2011; Link et al. 2012).
It has been proposed that geophagy represents a supplementary source of minerals (Davies and Baillie 1988; Klaus and Schmid 1998) that help reduce the absorption of toxins from food (Kreulen 1985; Diamond et al. 2008; Gilardi et al. 1999). It is also thought that geophagy allows ingesting antacid agents to regulate gastric pH (Davies and Baillie 1988). Soil consumption varies according to the species, geography, and climate (Davies and Baillie 1988; Setz 1999; Blake et al. 2010; Blake et al. 2011).
The Yasuní National Park, located in the Orellana and Pastaza provinces in the Ecuadorian Amazon region, is home to the Kichwa and Waorani indigenous peoples (Licona et al. 2011). For these communities, subsistence hunting is a primary activity to obtain protein from the consumption of prey such as monkeys and peccaries (Blake et al. 2013). However, overhunting, in addition to the illegal trade in wildlife facilitated by roads built by oil companies (Bass et al. 2010), has led to the decline of animal populations in the forest and, thus, in mineral licks near these communities (Suárez et al. 2009; Espinosa and Salvador 2017; Blake et al. 2013).
Hunting has a greater impact on mammals than birds, likely because the selective hunting of larger species (Benitez-López et al. 2017). Decades ago, in the Kichwa Añangu community located on the Napo River banks within the Yasuní National Park, wildlife was being overhunted. However, when its decline was noted, a tourism project was launched in 1998 as an alternative for socio-economic development. The book of the History of the Añangu Community states that the commitment to tourism is also a response to the exploitation of the Amazon for oil extraction (Renkert 2019). Another initiative implemented afterward as a community policy was the discontinuation of illegal wildlife hunting and trafficking to allow the recovery of wildlife populations. As a result, after 20 years, there is an evident recovery of landmark wildlife species, such as giant otters, jaguars, and large primates (Suárez and Zapata 2019).
The present work documented the species of large and medium-sized mammal species using three mineral licks in the Kichwa Añangu community area, using camera traps. In addition, capture frequencies and species composition in different climatic seasons were determined.
Methods
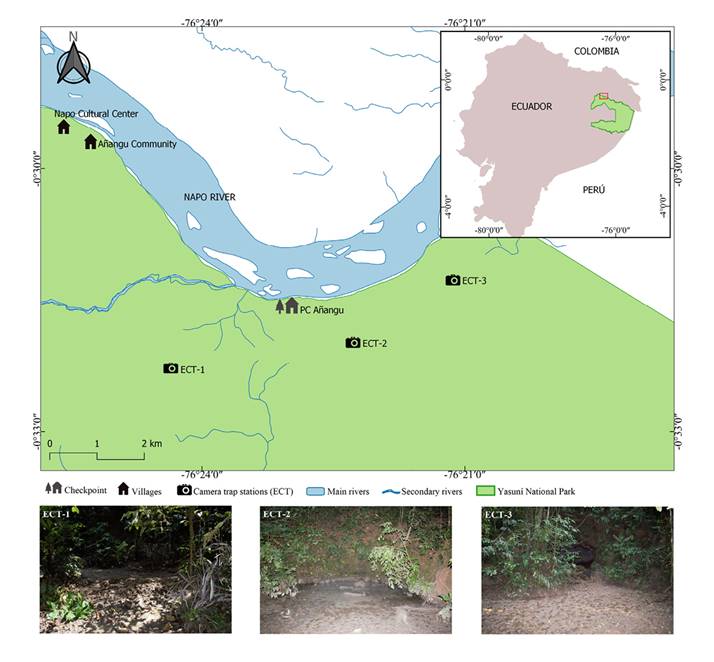
Study Area. Camera trap stations (ECT, for its acronym in Spanish) were installed in three open mineral licks of brown, marshy clay soils with a thin layer of decaying litter. ECT-1 (0° 32’ 15.818”, -76° 24’ 16.083”) was installed in a mineral lick of approximately 260 m2, ECT2 (-0° 31’ 58.068”, -76° 22’ 11.360”) in one of approximately 200 m2, and ECT-3 (0° 31’ 14.939”, 76° 21’ 4.527”) in one of about 150 m2. The three mineral licks were located within the territory of the Kichwa Añangu community in the Yasuní National Park, province of Orellana (Figure 1); this area is part of the Yasuní Biosphere Reserve, considered one of the most biodiverse regions worldwide (Bass et al. 2010). The study area is mainly covered by periodically flooded forests (varzea), non-flooded dryland forests, and marshlands where the buriti palm (Mauritius flexuosa) predominates. It is located within the Eastern Tropical zoogeographic region (Albuja et al. 2012), characterized by a humid tropical climate (Unesco 2010). According to the Nuevo Rocafuerte monitoring station of the National Institute of Meteorology and Hydrology (INHIM), in 2018, the total precipitation was approximately 3,300 mm and the average monthly temperature was 26.1 °C. Precipitation is concentrated between February and July and decreases the rest of the year, ranging between 2,881 mm and 3,942 mm (Pitman 2000; Albuja et al. 2012; Blake et al. 2012),
Sampling Design. We visited the mineral licks on three occasions to install the ECTs, which remained in operation for three periods of 30 to 40 days. The first period spanned from February to March, the second from June to July, and the third from November to December 2018; we recorded information from nine camera traps in total, three for each period.
The three mineral licks were georeferenced with a Garmin Oregon 650t GPS (Garmin Ltd.); the distance between them ranged from 2.5 to 6.4 km. Each ECT was equipped with one camera trap (Bushnell Trophy Cam HD, Aggressor model, Bushnell Corporation). Cameras were attached to tree trunks at the edge of each mineral lick, at a height of 0.5 to 0.75 m above the ground, and were oriented toward wildlife trails. These were set to capture three photographs each time the motion sensor was activated, with a 60-second interval between activations. The sampling effort varied between 61 and 98 camera trap days between mineral licks. The number of camera trap days was estimated from the time the camera started operating until the last image was captured (based on the date and time stamped on the pictures).
Data Analysis. The images captured were entered and processed in the Wild.ID software (https://www.wildlifeinsights.org/team-network). Based on the criteria proposed by Tobler et al. (2009), Blake et al. (2011), and Link et al. (2012), “independent” records were those images captured consecutively from clearly distinguishable individuals or individuals of the same species captured by the same camera within a 60-minute interval.
To assess the completeness of the species inventory, the species richness estimators Chao1 and ACE were calculated for each mineral lick and a species accumulation curve was constructed for the data set using the EstimateS 9.1.0 program (Colwell and Coddington 1994; Colwell 2013). To determine whether this variation influenced the estimation of species richness between mineral licks as well as between seasons, an analysis was performed with rarefaction curves interpolated to the smallest sample (Krebs 1989; Colwell et al. 2012) using the program Past 3.03 (Hammer et al. 2001). The Jaccard similarity index was also calculated to evaluate the similarity of species composition between mineral licks as well as between climatic seasons (Moreno 2001).
The capture frequency (FC, for its acronym in Spanish) of each species was calculated by dividing the number of capture events by species for the sampling effort (number of camera trap days) in each mineral lick and climatic season and multiplying by 100 to facilitate comparisons with similar studies (Blake et al. 2011; Blake et al. 2013).
Capture frequency (FC) patterns were compared graphically through rank-abundance curves (Whittaker’s plots) based on Magurran (2004). The vertical axis measured capture frequencies and the horizontal axis measured the range of species recorded in each mineral lick (ECT), the total number of species of the whole study, and the number of species by climatic season.
Results
A total sampling effort of 249 camera trap days yielded 645 images, of which 398 were independent records of 16 species in 11 families and seven taxonomic orders. The number of independent records per mineral lick varied between 112 and 159, with 11 species recorded in the first and second mineral licks and 9 in the third (Table 1). The taxonomic order with the highest number of records was Rodentia, with three families and four species (Table 2).
Table 1 Camera-trap data in three mineral licks located in the Yasuní National Park, Kichwa Añangu community. Trap Camera Station (ECT).
| ECT-1 | ECT-2 | ECT-3 | Total | |
|---|---|---|---|---|
| Camera trap days | 61 | 98 | 90 | 249 |
| No. of captures | 303 | 137 | 205 | 645 |
| Species richness (Sobs) | 11 | 11 | 9 | 16 |
| Number of independent records | 159 | 112 | 127 | 398 |
| Chao 1 | 25.9 | 11.0 | 11.9 | 16.8 |
| ACE | 38.5 | 11.6 | 12.7 | 18.7 |
Observed Species - Species Richness Estimates. A total of 16 species (Sobs) were recorded, accounting for 95.2 % of the figure estimated with Chao1 (S = 16.8) and 85.6 % of the figure estimated with ACE (S = 18.7). Therefore, a representative sample was obtained in general terms. However, in ECT-1, the recorded species amounted to 42.5 % of the Chao1 estimate (S = 25.9) and 28.6 % of the ACE estimate (S = 38.5). In contrast, ECT-2 recorded 100 % of the Chao1 estimate (S = 11) and 94.8 % of the ACE estimate (S = 11.6). ECT-3 recorded 75.6 % of the species estimated by Chao1 (S = 11.9) and 70.9 % of the expected species according to ACE (S = 12.7; Table 1).
Capture Frequency of Mammalian Species. The species recorded most frequently over the three sampling periods were the red brocket (Mazama zamora; 155 independent records, FC = 62.2), white-lipped peccary (Tayassu pecari; 89 independent records, FC = 35.7), South American tapir (Tapirus terrestris; 72 independent records, FC = 28.9), and collared peccary (Pecari tajacu; 20 independent records, FC = 8.0). The species with the lowest number of records were the collared anteater (Tamandua tetradactyla), white-fronted Capuchin (Cebus yuracus), and green acouchi (Myoprocta pratti), with one independent record (FC = 0.4) in each case (Table 2).
Table 2 List of orders, families, and species recorded according to higher or lower rainfall, FC value and the threat category in IUCN, LRE=Libro Rojo de Ecuador (Red Book of Ecuador) and CITES.
| List | Higher rainfall | Lower rainfall | Total | IUCN | LRE | CITES | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feb-Mar | Jun-Jul | Nov-Dec | |||||||||
| No. Rec. | FC | No. Rec. | FC | No. Rec. | FC | No. Rec. | FC | ||||
| ARTIODACTYLA | |||||||||||
| Cervidae | |||||||||||
| Mazama zamora | 55 | 54.5 | 58 | 78.4 | 42 | 56.8 | 155 | 62.2 | DD | NT | * |
| Mazama murelia | 0 | 0.0 | 4 | 5.4 | 2 | 2.7 | 6 | 2.4 | LC | NT | * |
| Tayassuidae | |||||||||||
| Tayassu pecari | 47 | 46.5 | 27 | 36.5 | 15 | 20.3 | 89 | 35.7 | VU | CR | II |
| Pecari tajacu | 1 | 1.0 | 16 | 21.6 | 3 | 4.1 | 20 | 8.0 | LC | NT | II |
| CARNIVORA | |||||||||||
| Felidae | |||||||||||
| Leopardus pardalis | 0 | 0.0 | 3 | 4.1 | 7 | 9.5 | 10 | 4.0 | LC | NT | I |
| CINGULATA | |||||||||||
| Dasypodidae | |||||||||||
| Dasypus pastasae | 0 | 0.0 | 0 | 0.0 | 2 | 2.7 | 2 | 0.8 | LC | DD | * |
| Dasypus novemcinctus | 8 | 7.9 | 2 | 2.7 | 1 | 1.4 | 11 | 4.4 | LC | LC | * |
| PERISSODACTYLA | |||||||||||
| Tapiridae | |||||||||||
| Tapirus terrestris | 35 | 34.7 | 19 | 25.7 | 18 | 24.3 | 72 | 28.9 | VU | EN | II |
| PILOSA | |||||||||||
| Myrmecophagidae | |||||||||||
| Tamandua tetradactyla | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | LC | LC | * |
| PRIMATES | |||||||||||
| Atelidae | |||||||||||
| Alouatta seniculus | 0 | 0.0 | 0 | 0.0 | 11 | 14.9 | 11 | 4.4 | LC | LC | II |
| Ateles belzebuth | 0 | 0.0 | 0 | 0.0 | 2 | 2.7 | 2 | 0.8 | EN | EN | II |
| Cebidae | |||||||||||
| Cebus yuracus | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | LC | NT | * |
| RODENTIA | |||||||||||
| Dasyproctidae | |||||||||||
| Myoprocta pratti | 0 | 0.0 | 1 | 1.4 | 0 | 0.0 | 1 | 0.4 | LC | LC | * |
| Dasyprocta fuliginosa | 1 | 1.0 | 1 | 1.4 | 4 | 5.4 | 6 | 2.4 | LC | LC | * |
| Cuniculidae | |||||||||||
| Cuniculus paca | 4 | 4.0 | 0 | 0.0 | 5 | 6.8 | 9 | 3.6 | LC | NT | III |
| Erethizontidae | |||||||||||
| Coendou prehensilis | 1 | 1.0 | 1 | 1.4 | 0 | 0.0 | 2 | 0.8 | LC | DD | * |
The most frequent species in the three mineral licks were M. zamora, T. terrestris, T. pecari, and A. seniculus. These species obtained different capture frequencies in each ECT, thus attaining a different rank. These were the most abundant species and were relatively easy to capture with camera traps; the exception was A. seniculus, which appeared among the dominant species only in ECT-2 (Figure 2).
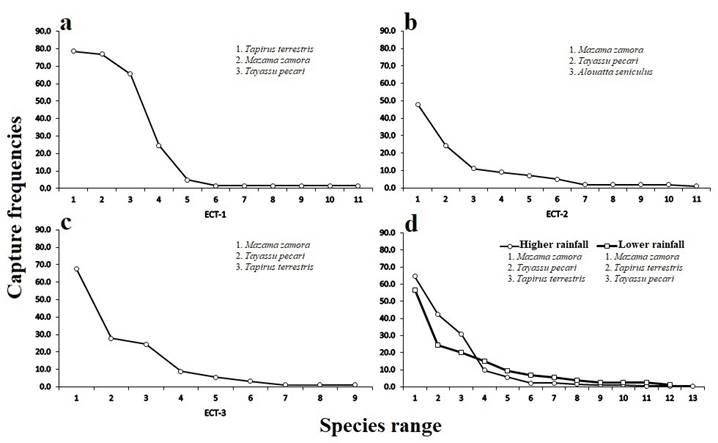
Figure 2 Range-abundance curves (Whittaker’s plots) with capture frequencies for the mineral licks a) ECT-1. b) ECT-2. c) ECT-3. d) Seasons of higher and lower rainfall.
Rarefaction and Interpolation. It was found that by interpolating to the smallest number of independent records (112 records), ECT-2 was the mineral lick with the highest number of species (S = 11). This indicates that species richness in this site was significantly higher relative to ECT-3 (S = 8.6) under the same number of records since the confidence intervals did not overlap. However, the confidence intervals of ECT-1 (S = 9.2) overlapped with those of the other sites, so differences were non-significant (Figure 3).
Climate Temporality: Rarefaction and Interpolation, Capture Frequency and Species Composition. During the higher rainfall season, the sampling effort was 175 days/trap, resulting in 286 independent records of 13 species, ten families, and seven orders. In the season of lower rainfall, the sampling effort was 74 camera trap days, yielding 112 independent records of 12 species in eight families and six orders.
Between the two seasons, the rarefaction analysis interpolating to the lowest number of independent records (112) revealed that ten species were recorded during the higher rainfall season and 12 in the lower rainfall season. The 95 % confidence intervals overlapped, so no significant difference in species richness from climatic seasonality was observed (Figure 3).
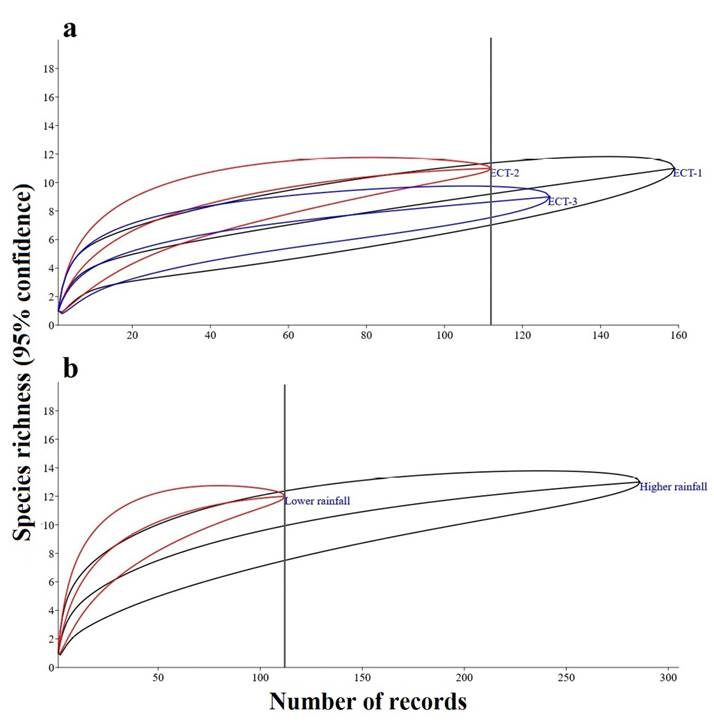
Figure 3 Rarefaction curves based on species richness for a standard sample of 112 independent records in a) ECT-1. ECT-2. ECT-3 mineral licks. b) Seasons of higher and lower rainfall, with a 95 % confidence interval in both cases.
In the higher rainfall season, the most frequent species were M. zamora (113 records, FC = 64.6), T. pecari (74 records, FC = 42.39), T. terrestris (54 records, FC = 30.9), and P. tajacu (17 records, FC = 9.7). In the lower rainfall season, the most frequent species were M. zamora (42 records, FC = 56.8), T. terrestris (18 records, FC = 24.3), T. pecari (15 records, FC = 20.3), and red howler (Alouatta seniculus; 11 records, FC = 14.9).
When the range-abundance (FC) curves are compared between the two seasons, M. zamora is the predominant species in both seasons, followed by T. pecari and T. terrestris. These were the most abundant species that were observed most frequently in mineral licks (Figure 2).
Similarity. ECT-1 (C. albifrons, T. tetradactyla, and M. pratti) and ECT-2 (C. prehensilis, A. seniculus, and A. belzebuth) showed three species that were not observed in any other mineral lick; ECT-3 had no unique species. According to the Jaccard similarity index, stations ECT-1 and ECT-2 shared 37.5 % of the recorded species. For their part, ECT-1 and ECT-3 shared 53.8 % of species, the same percentage shared by ECT-2 with ECT-3.
The higher rainfall season recorded four unique species (C. yaracus, C. prehensilis, T. tetradactyla, and M. pratti) and the lower rainfall season recorded three unique species (A. seniculus, A. belzebuth, and D. pastasae). Altogether, the two climatic seasons yielded 56.3 % of species similarity, according to the Jaccard index.
Table 3 Comparison of capture frequencies (FC) of four species reported by Blake et al. (2013) versus the figures obtained in the present study.
| Species | Blake et al. 2013 | Present study | |
|---|---|---|---|
| TBS (FC) | YRS (FC) | ECT-Total (FC) | |
| Mazama zamora | 211.2 | 57.7 | 62.2 |
| Tapirus terrestris | 37.1 | 25.5 | 28.9 |
| Pecari tajacu | 48.2 | 5.5 | 8 |
| Tayassu pecari | 28.1 | 0 | 37.5 |
Discussion
Fauna Recorded in Mineral licks. According to the richness estimators Chao1 (S = 16.8) and ACE (S = 18.7), a considerable sample was obtained to determine the richness of species visiting mineral licks. However, species richness would probably increase with a higher sampling effort as the species accumulation curve (Sobs = 16) shows no asymptotic trend. Whittaker’s plots are similar for the three mineral licks, showing equal species capture frequencies and the same dominant species (Figure 2). On the other hand, rarefaction curves evidenced a non-significant difference in the species recorded in the three mineral licks (Figure 3), thus stressing the importance of conserving mineral licks as sources of mineral supplements for the visiting fauna. As in previous studies carried out in the Neotropics (Tobler et al. 2009; Blake et al. 2011; Blake et al. 2013), the red brocket, collared peccary, white-lipped peccary, and South American tapir were the species most frequently recorded in these habitats, also being commonly hunted (Blake et al. 2013).
Comparing the capture frequencies of four species with those obtained by Blake et al. (2013) in two locations -one disturbed from the proximity of a road and under hunting pressure (YRS) and the other being hard to access and under minimum hunting pressure (TBS)-, the capture frequencies in the present study are in between those observed in TBS and YRS (Table 3); the exception was T. pecari, which showed a higher capture frequency. It is worth mentioning that this was one of the most heavily hunted species in the Añangu community area some 20 years ago (Suárez and Zapata 2019). For this reason, we assume that in the mineral licks included in this study, animal populations that were previously overhunted may be undergoing a recovery process; this deserves to be further explored in future research.
Other recorded species, such as the nine-banded armadillo (Dasypus novemcinctus) and the ocelot (Leopardus pardalis), are rare in mineral licks (Blake et al. 2011), but both have been recorded more frequently along trails (Blake et al. 2012, 2013; P. Macas-Pogo, personal observation). Ocelots are usually attracted by bats that visit certain mineral licks and are part of their diet (Tinoco and Camacho 2015; Contreras-Moreno et al. 2019) and have also been seen hunting amphibians (P. Macas-Pogo, personal observation). The red howler (A. seniculus) and the yellow-bellied spider monkey (A. belzebuth) are two of the most hunted primate species (Mena et al. 2000), so much so that their populations have been decimated in some areas of the Yasuní National Park (Franzen 2006). This study recorded these two species only in ECT-2 and with low capture frequencies (FC = 4.4 and FC = 0.8, respectively), especially the yellow-bellied spider monkey, a frequent visitor to mineral licks (Blake et al. 2010; Link et al. 2011). The black agouti (Dasyprocta fuliginosa) is another species preferred by hunters; this work recorded it at the three study sites. However, compared to data from studies performed by Blake et al. (2011) and Blake et al. (2013), the number of records is minimal, as is the number of other small species; this could be related to the sampling effort or even to the position of camera traps at the time of installation.
Climatic Seasonality. Several research studies on geophagy indicate that climatic seasonality influences the use of mineral licks (Jones and Hanson 1985; Atwood and Weeks 2003; Link et al. 2012) and that animals prefer visiting these sites on sunny days with no mist, wind, or rain (Brightsmith 2004). By contrast, according to Link et al. (2011), they display reduced activity in days of heavy rains. However, the rarefaction analysis indicated no significant differences between climatic seasons for the species recorded. The species composition was similar in the two seasons, with a Jaccard index higher than 0.5 (56.3 % similarity); the red brocket, white-lipped peccary, and South American tapir were the dominant species in both seasons. The slopes of Whittaker’s plots are steep, suggesting a low evenness of species (Figure 2).
The species recorded only in the lower rainfall season and those captured exclusively in the higher rainfall season with one or two records are insufficient to determine whether the use of mineral licks is related to the season of the year. Factors such as water accumulation in mineral licks are attractive to some individuals (Link et al. 2011). The consumption of fruits, seeds, or plants that produce secondary metabolites varies seasonally (Brightsmith 2004; Voigt et al. 2007) and induces mammals to search for mineral salts.
For the inhabitants of Añangu, based on a self-mandated internal regulation, the discontinuation of wildlife overhunting and, most importantly, wildlife trafficking, has yielded favorable results since today the fauna that can be observed is attractive for tourists visiting the area. A central factor in preventing wildlife trafficking has been that there are neither main roads nor alternate roads near mineral lick sites, and thus the hunting pressure has dropped significantly. This contrasts with areas crossed by the Maxus road where, according to Suárez and Zapata (2018), wildlife has been severely affected and the indirect effects of roads and the oil industry have been underestimated. Besides, excess hunting by the Waorani has led to the local extinction of the hunted species (Mena et al. 2000; Franzen 2006; Espinosa et al. 2014; Blake et al. 2013).
In conclusion, the results presented herein evidence the richness of mammalian species that use these mineral licks as an important source of minerals, which are undoubtedly essential for the normal development of organisms. As mentioned earlier, there is no marked climatic seasonality in this region; therefore, this factor is not a driver for peccaries, brockets, and tapirs when visiting a mineral lick since they were recorded in the three mineral licks over the three sampling periods studied, being the species with the highest capture frequencies. Besides, we recorded species unique to two of the three mineral licks to each climatic season. Therefore, the use of a given mineral lick may also be determined by home range, the particular behavior of each species, hunting pressure, or other factors that deserve further investigation in future research.
Mineral licks might be an important resource for local human populations if these are given proper use as subsistence hunting sites or even as sites for wildlife-watching tourism. It is evident that the sustainable practices adopted by the Añangu community support the sustainable management of these sites, making a positive contribution to the conservation of biodiversity in the Yasuní National Park. These mineral licks represent areas that should be valued for their role in the ecosystem, mainly in the diet of the fauna using them. Additional studies are needed to advance our understanding of their characteristics and contributions to forest dynamics. In addition to continuing the application of sustainable tourism practices, the local community should be advised to implement clear guidelines setting restrictions, visiting hours, and behavior of visitors of mammalian mineral licks to ensure their protection.











 nova página do texto(beta)
nova página do texto(beta)