Introduction
Traditionally, ethology has been defined as an approach to the study of animal behavior, derived from the discovery of instinctive movements (Eibl-Ebesfeldt 1975; Moreno and Muñoz-Delgado 2007; Breed and Moore 2012). This scientific discipline emerged from the interest in deepening the knowledge of animal behavior and understanding the variety of behaviors displayed by different species under different conditions (Carranza 2010). Ethology focuses on the study of behavior, understood as an exchange mechanism between the organism and its environment under natural conditions (Díaz 1994; Medawar and Medawar 1996).
The earliest ethological studies conducted in México were carried out by ecologists and taxonomists interested in behavioral aspects of animals and their environment (Herrera 1986). Mammals exhibit a wide variety of life histories that make them suitable models for conducting ethological studies (e. g., Soares et al. 2016). Eleven orders, 36 families, 169 genera, and 503 species of terrestrial mammals have been recorded in México (Álvarez-Castañeda et al. 2015). According to Guevara-Chumacero et al. (2001), very few studies on Mexican mammals have addressed behavior as the primary study field: only 0.6 % of the 1826 scientific articles published between 1890 and 1995 did so. The earliest clearly ethological studies on Mexican mammals addressed a diverse range of issues and taxa. For example, Russell and Findley (1954) described the swimming of a rodent of the genus Onychomys in the northern part of the country (State of Nuevo León), and Packard (1958) described carnivory behavior in the Mexican ground squirrel (Ictidomys mexicanus) in the State of Coahuila. Wimsatt (1969) described foraging and refuge selection by the common vampire bat (Desmodus rotundus) in the State of Tabasco, southern México, and Greenhall et al. (1971) documented the attack mode of this species on cattle in the State of Oaxaca. As these examples show, early ethological studies were observational and descriptive. These same study areas were explored in other Latin American countries prior to the adoption of experimental approaches (Jaffe et al. 2020). However, recent ethological studies from Latin America and other parts of the world (e. g., Europe and Australia) have used rigorous experimental methods based on hypothesis testing (Hoffmann et al. 2018; Morete et al. 2018; Wierucka et al. 2018; Mazza et al. 2020).
The available data indicate that ethological studies have recently become more prominent in México and Brazil than in other Latin American countries. However, an increase in the number of ethological studies over time is evident throughout Latin America, as interest in the academic discipline of animal behavior has grown in recent years (Jaffe et al. 2020).
Although México has been an important contributor to ethological studies, the progress made to date and direction taken by this type of studies in Mexican mammalogy have not been documented. Therefore, this study conducts an analytical review of the literature on ethological studies of native Mexican mammals. The objective of the study is to assess the wealth of information gathered to date and the path followed by this scientific discipline in México and based on this, to identify study subjects that need to be addressed by future efforts.
Materials and methods
Data gathering. An exhaustive search was conducted by consulting periodic journals on the bibliography concerning the ethology of mammals collected or observed in México between 1900 to 2018. We consulted the following websites: 1) Google Scholar (https://scholar.google.com/), 2) Clarivate Analytics - Web of science (https://clarivate.com/webofsciencegroup/) and 3) databases in bidiuam (https://bidi.uam.mx/bidi-ti/bases.html), such as: Biological Abstract, BioOne Complete, Current Contents Connect, EBSCO, Nature, ProQuest, Science, Scopus, and Scielo. The used search keywords were: mammals, México, behaviour, behavior, conduct, ethology, homeostasis, circadian cycles, learning, cognition, communication, movement, foraging, self-defense, mating systems, nesting, rearing, territoriality, social behavior, conservation and behavior; these words were also combined, and used in Spanish. Each article compiled was reviewed in detail to identify whether it had an ethological approach as its main objective.
Processing of data. The information contained in the references was reviewed and organized in a database using EndNote Plus v. X 7.5 (Niles & Associates, Inc). The fields used were: author, year, title, journal, volume, pages, language, nationality of the first author or corresponding author, federal entity, order, family, genus, species, location in geographical coordinates, state (wild, captive, semi-captive), subject of study, the Mexican institution affiliation of first or corresponding author, and the risk category according to the Mexican Official Standard (SEMARNAT 2010), the IUCN Red List of Threatened Species (Red List) and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES).
Study locations. For each article, the locality/localities where the observations were performed or the place where the data of the individuals studied in captivity was collected, and were registered for the Mexican state and the geographic coordinates (in decimal degrees). When not included in the publications, the mentioned localities were georeferenced in Google Earth (Lisle 2006). Using open-source Geographic Information System QGIS 2018 v. 3.4.4, a map of the localities was prepared, using as a base layer the State Political Division (2018), available in the Geoportal of the National Commission for the Knowledge and Use of Biodiversity (CONABIO).
Data analysis. To view research trends in detail, the data was split into periods of five years (Lustrums), except for the last period that covers three years. When the corresponding author was absent, the main author was considered as first author. In addition, the authors were classified as national or foreign according to the country of origin. A grouping was also made according to the origin of the journal (national or foreign).
Each publication was grouped into one of the 12 main ethological fields (Table 1) following Breed and Moore (2012). In addition, the publications were grouped according to the Mexican state where each study was carried out. In this work we follow the taxonomic nomenclature proposed by Álvarez-Castañeda (2015).
Table 1 List of ethological studies according to Breed and Moore’s categories (2012).
| Fields | Subfields |
|---|---|
| Homeostasis and time budgets | Biological clock and circadian cycles, homeostatic regulation, time budgets and trade-offs. |
| Learning | Learning and memory, social learning, play, learning and development. |
| Cognition | Concept of self, thinking, predicting, and solving problems, intelligence and social cognition, personality and behavioral syndrome, impulse control, animal emotions. |
| Communication | Evolution of communication, types of communication, out-of-control sexual selection and signaling, deception and honest communication, game theory and communication, interspecific signaling. |
| Movement | Searching, homing (ability to return to a territory after leaving it), migration, dispersal. |
| Foraging | Diet and food choice, obtaining food, food disposal, prey handling, parasite cycles, foraging and optimization theory, optimal patch choice, prey choice. |
| Self-defense | Cryptic behavior and camouflage, surveillance and alarm, mimicry and deviance, evasion, predator deterrence and response to attack, pathogen avoidance, deterrence behavior and disease. |
| Mating systems | Sexual selection, variation in mating event, choice of male, hormones and sexual behavior, hormones territoriality and aggression, sperm competition, forced copulations, models of good genes for choosing a male. |
| Nesting, rearing, and territoriality | Nests and nesting, parental investment, parental care patterns, hormones and parental care, parenting, and conflict of interest, begging and weaning conflict, sibling conflict, infanticide, aggression, and territoriality. |
| Social behavior, cooperation, and kinship | Altruism and self-interest, herds and hordes, cooperation, eusociality, social recognition. |
| Comparative social behavior | To elucidate the differences of a specific behavior in different species. |
| Conservation and behavior | Integration of the other fields to obtain useful information for conservation. |
Results
One hundred and sixty-seven published scientific articles were registered between 1900 and 2018, with no productivity between 1900 to 1953, and a low productivity in the first forty years (1954 to 1995) with only 16 papers. On the other hand, in the period between 1996 to 2018, there was a notable increase in published articles (n = 144; 90 % of the total; Figure 1). In general, Mexican authors (108 articles, 67.5 %) had a more active participation that foreign authors (52, 32.5 %, respectively).
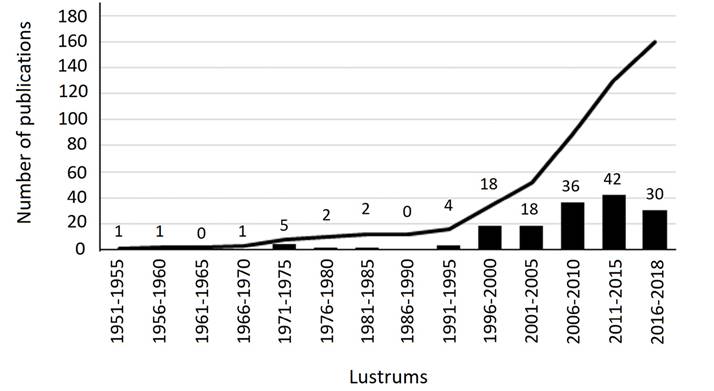
Figure 1 Articles published about ethological fields between 1951 and 2018 in 5-year periods (Lustrums). The bars and numbers on bars indicate the number of publications each five years, and the line indicates the cumulative increase in publications.
Studies were published in 51 different journals (40 included in the Journal Citation Reports 2018 of Institute for Scientific Information - ISI) from 14 countries, 7 of Mexican origin and 44 foreign (33 % corresponded to the United States). The journals with the highest number of publications were American Journal of Primatology (n = 16), Journal of Mammalogy (n = 15), and International Journal of Primatology (n = 14). Among the Mexican journals, Acta Zoológica Mexicana (nueva serie) and Therya had the highest number of publications with 9 publications, each (Table 2).
Table 2 List of journals according to number of published articles on ethological fields, country of origin and their presence in Journal Citation Reports (JCR) 2018. Number of articles (No.). Country of origin (C.O.). JCR (presence: OK; absence: -).
| Journals | No. | C.O. | (ISI) |
|---|---|---|---|
| American Journal of Primatology | 16 | U.S.A. | OK |
| Journal of Mammalogy | 15 | U.S.A. | OK |
| International Journal of Primatology | 14 | U.S.A. | OK |
| Acta Zoológica Mexicana (nueva serie) | 9 | México | - |
| Primates | 9 | Japan | OK |
| Therya | 9 | México | - |
| The Southwestern Naturalist | 6 | U.S.A. | OK |
| Folia Primatologica | 5 | Switzerland | OK |
| Behavioral Ecology and Sociobiology | 4 | Germany | OK |
| Ethology | 4 | Germany | OK |
| Mammalian Biology (Zeitschrift für Säugetierkunde) | 4 | Germany | OK |
| PLOS ONE | 4 | U.S.A. | OK |
| American Journal of Physical Anthropology | 3 | U.S.A. | OK |
| Biotropica | 3 | U.S.A. | OK |
| Journal of Zoology | 3 | England | OK |
| Revista de Biología Tropical | 3 | Costa Rica | OK |
| Revista Mexicana de Biodiversidad | 3 | México | OK |
| Revista Mexicana de Mastozoología (nueva época) | 3 | México | - |
| Western North American Naturalist | 3 | U.S.A. | OK |
| Anales del Instituto de Biología, UNAM. Serie Zoología | 2 | México | - |
| Acta Chiropterológica | 2 | Poland | OK |
| Animal Behaviour | 2 | England | OK |
| Biological Rhytm Research | 2 | England | OK |
| General and Comparative Endocrinology | 2 | U.S.A. | OK |
| Hormones and Behavior | 2 | U.S.A. | OK |
| Physiology and Behavior | 2 | U.S.A. | OK |
| Zoo Biology | 2 | U.S.A. | OK |
| American Midland Naturalist | 1 | U.S.A. | - |
| Animal Biology | 1 | Netherlands | OK |
| Animal Cognition | 1 | Germany | OK |
| Animal Conservation | 1 | England | OK |
| Behavioral Ecology | 1 | England | OK |
| Biology Letters | 1 | England | OK |
| Chiroptera Neotropical | 1 | Brazil | - |
| Chronobiology International | 1 | U.S.A. | OK |
| Cortex | 1 | Italy | OK |
| Current Zoology | 1 | China | OK |
| Ethology Ecology and Evolution | 1 | Italy | OK |
| Interciencia | 1 | Venezuela | - |
| Journal of Arid Environments | 1 | U.S.A. | OK |
| Journal of Comparative Psychology | 1 | Germany | OK |
| Journal of Zoo and Wildlife Medicine | 1 | U.S.A. | OK |
| Mammalia | 1 | Germany | OK |
| Mastozoología Netropical | 1 | Argentina | - |
| Natural Areas Journal | 1 | U.S.A. | - |
| Neotropical Primates | 1 | Brazil | - |
| Peer J. | 1 | England | OK |
| Salud Mental | 1 | México | OK |
| Scientific Reports | 1 | England | OK |
| Studies on Neotropical Fauna and Environment | 1 | England | - |
| Universidad y Ciencia | 1 | México | - |
In total the studies were based on 54 species from 16 families and 6 orders. The most studied species belonged to the order Primates and were Ateles geoffroyi, Alouatta palliata and A. pigra, with 43.6 % of the publications. Other orders were Rodentia (21.5 %), Chiroptera (15.4 %), Carnivora (15.4 %), Lagomorpha (2.1 %) and Cetoartiodactyla (2.1 %; Table 3). Of the 54 species, 10 are endemic to México and 17 species have some category of risk at the national or international level.
Articles were recorded for 23 Mexican states, with Veracruz (n = 31), Chiapas (n = 21), Quintana Roo (n = 20), totalling 43 % of all studies. States such as Aguascalientes, Nayarit, Sinaloa, Tlaxcala, among others, lack any studies (Figure 2). Sixty percent of the studies focused on foraging, movement, nesting, rearing and territoriality, social behavior, cooperation, and kinship, while only 2.4 % had as main objective the fields of learning and cognition (Figure 3).
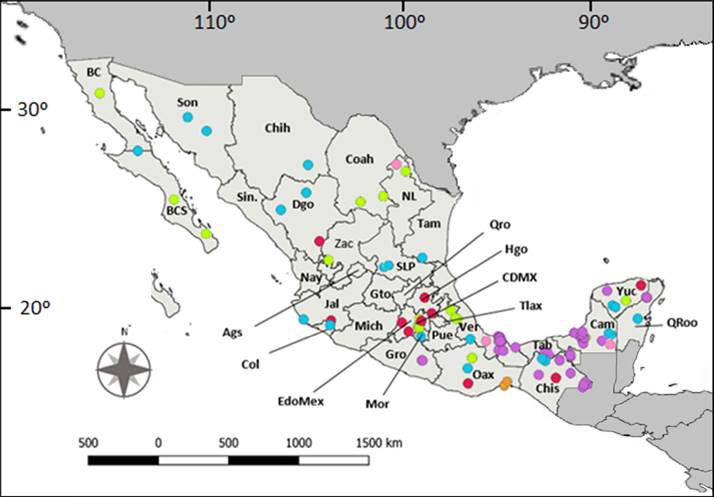
Figure 2 Map showing records of ethological studies carried out on native land mammals in México, categorized at order level. TThe colored circles represent orders and locations worked (n = number of locations): Primates (purple, n = 74); Rodentia (green, n = 33); Carnivora (blue, n = 25); Chiroptera (red, n = 22); Cetartiodactyla (pink, n = 3), and Lagomorpha (orange, n = 3). Acronyms (BC = Baja California; BCS = Baja California Sur; Son = Sonora; Sin = Sinaloa; Chih = Chihuahua; Dgo = Durango; Coah = Coahuila; Nay = Nayarit; Zac = Zacatecas; NL = Nuevo León; SLP = San Luis Potosí; Gto = Guanajuato; Ags = Aguascalientes; Jal = Jalisco; Col = Colima; Tam = Tamaulipas; Qro = Querétaro; Hgo = Hidalgo; CDMX = Ciudad de México; EdoMex = Estado de México; Tlax = Tlaxcala; Pue = Puebla; Ver = Veracruz; Mich = Michoacán; Gro = Guerrero; Mor = Morelos; Oax = Oaxaca; Tab = Tabasco; Chis = Chiapas; Cam = Campeche; Yuc = Yucatán; QRoo = Quintana Roo).
Table 3 List of species found in ethological studies according to order, family, and national and international risk categories. A Number of published articles; B Endemic (presence: OK; absence: -); C In NOM 059 Semarnat 2010 categories (E: Probably extinct in the wild; P: endangered; A: threatened; absence: -). D In IUCN categories (EN: endangered; LC: least concern; VU: vulnerable; NT: near threatened; absence: -). E In CITES Appendices (I: Highly endangered; II: may become endangered; absence: -).
| Order | Family | Species | A | B | C | D | E |
|---|---|---|---|---|---|---|---|
| Primates | |||||||
| Atelidae | Ateles geoffroyi | 37 | - | P | EN | I, II | |
| Alouatta pigra | 25 | - | P | LC | I | ||
| Alouatta palliata | 23 | - | P | EN | I | ||
| Rodentia | |||||||
| Cricetidae | Neotomodon alstoni | 12 | OK | - | LC | - | |
| Neotoma mexicana | 1 | - | - | LC | - | ||
| Microtus mexicanus | 2 | - | - | LC | - | ||
| Onychomys leucogaster | 1 | - | - | LC | - | ||
| Peromyscus aztecus | 1 | - | - | LC | - | ||
| Peromyscus melanocarpus | 1 | OK | - | EN | - | ||
| Peromyscus melanophrys | 2 | OK | - | LC | - | ||
| Peromyscus mexicanus | 1 | - | - | LC | - | ||
| Peromyscus melanotis | 1 | OK | - | LC | - | ||
| Peromyscus yucatanicus | 2 | - | - | LC | - | ||
| Reithrodontomys megalotis | 2 | - | - | LC | - | ||
| Sigmodon leucotis | 1 | - | - | LC | - | ||
| Heteromyidae | Chaetodipus siccus | 3 | OK | A | LC | - | |
| Dipodomys merriami | 1 | - | A | LC | - | ||
| Heteromys gaumeri | 2 | - | - | LC | - | ||
| Heteromys irroratus | 1 | - | - | LC | - | ||
| Sciuridae | Cynomys mexicanus | 1 | OK | P | EN | I | |
| Ictidomys mexicanus | 2 | OK | - | LC | - | ||
| Ictidomys spilosoma | 1 | - | - | LC | - | ||
| Tamiasciurus mearnsi | 1 | OK | A | EN | - | ||
| Xerospermophilus perotensis | 2 | OK | A | EN | - | ||
| Chiroptera | |||||||
| Phyllostomidae | Artibeus jamaicensis | 6 | - | - | LC | - | |
| Anoura geoffroyi | 2 | - | - | LC | - | ||
| Order | Family | Species | A | B | C | D | E |
| Artibeus lituratus | 1 | - | - | LC | - | ||
| Carollia sowelli | 1 | - | - | LC | - | ||
| Desmodus rotundus | 6 | - | - | LC | - | ||
| Diphylla ecaudata | 1 | - | - | LC | - | ||
| Glossophaga commissarisi | 1 | - | - | LC | - | ||
| Leptonycteris yerbabuenae | 3 | - | - | NT | - | ||
| Emballonuridae | Balantiopteryx plicata | 1 | - | - | LC | - | |
| Molossidae | Molossus rufus | 1 | - | - | LC | - | |
| Nyctinomops laticaudatus | 1 | - | - | LC | - | ||
| Mormoopidae | Pteronotus gymnonotus | 1 | - | A | LC | - | |
| Pteronotus mesoamericanus | 1 | - | - | LC | - | ||
| Vespertilionidae | Aeorestes cinereus | 1 | - | - | LC | - | |
| Antrozous pallidus | 1 | - | - | LC | - | ||
| Lasiurus blossevillii | 1 | - | - | LC | - | ||
| Lasiurus borealis | 1 | - | - | LC | - | ||
| Carnivora | |||||||
| Canidae | Canis lupus baileyi | 6 | - | E | LC | I, II | |
| Canis latrans | 5 | - | - | LC | - | ||
| Urocyon cinereoargenteus | 1 | - | - | LC | - | ||
| Felidae | Lynx rufus | 3 | - | - | LC | II | |
| Puma concolor | 7 | - | - | LC | - | ||
| Panthera onca | 6 | - | P | NT | I | ||
| Mephitidae | Conepatus leuconotus | 1 | - | - | LC | - | |
| Procyonidae | Bassariscus astutus | 1 | - | A | LC | - | |
| Lagomorpha | |||||||
| Leporidae | Lepus flavigularis | 4 | OK | P | EN | - | |
| Artiodactyla | |||||||
| Cervidae | Odocoileus virginianus | 3 | - | - | - | - | |
| Tayassuidae | Tayassu pecari | 1 | - | P | VU | II |
Discussion
The publication rate of ethological studies of Mexican mammals varied considerably over the study period (1900 to 2018). We are aware of the probability that some gray literature articles might have not been considered, although it should not affect the trends presented. No production was recorded between 1900 to 1953, while very few studies were published (n = 16) between 1954 and 1995. This is consistent with the findings of Guevara-Chumacero et al. (2001) who found that only 0.6 % of the 1826 articles on Mexican terrestrial mammals that were published during the period from 1890 to 1995 dealt with behavioral studies. The number of publications increased noticeably since 1996 (Figure 1); one hundred and forty-four were published during 1996 to 2018. This increase, mostly in the last decade of the 20th century, is the result of increasing specialists in mammalogy and their engagement in interdisciplinary collaborations (Ramírez-Pulido et al. 2017), mainly in areas such as ecology and taxonomy (Cordero 1994) throughout México. In the second period, foreign authors had a more important role of participation than national authors. While in the third period, the national authors were the main authors of the studies (figure not shown). Institutions such as the Instituto de Neuroetología of the Universidad Veracruzana, Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz, El Colegio de la Frontera Sur-Campeche, as well as Facultad de Estudios Profesionales Superiores Iztacala, Estación de Biología Tropical Los Tuxtlas, and the institutes of Biology and Ecology of Universidad Nacional Autónoma de México (UNAM), have played a key role in this growing trend.
A large proportion of papers (82.5 %) were published in international, non-Mexican journals, most of which (84 %) are indexed in the Journal Citation Reports - ISI. Not surprisingly, journals specialized in primatology (e. g., American Journal of Primatology, International Journal of Primatology, Primates, Folia Primatologica, and others) stand out, as over half of all articles have focused on the order Primates.
Primatology studies of native Mexican species began with several ecological and behavioral studies carried out at Los Tuxtlas Biological Station, in the State of Veracruz (e. g., Estrada 1984). Eighty-four percent of the original vegetation of the Los Tuxtlas region has been either lost or fragmented (Dirzo and García 1992; Dirzo et al. 2009); consequently, local populations of primate species have also been significantly reduced (Escobedo-Morales and Mandujano 2007). The proper design of conservation strategies requires up-to-date information on aspects such as the geographic distribution (Estrada 1982; Estrada and Coates-Estrada 1988) and ethology (Estrada et al. 1999; Juan et al. 2000) of the species. As a result, Veracruz is the Mexican state where the highest number of ethological studies (n = 31) have been conducted.
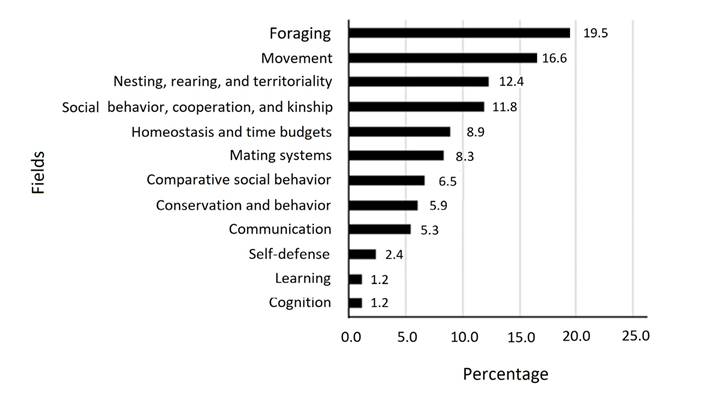
Figure 3 Percentage of published ethological articles organized by field based on Breed and Moore (2012).
Most records of ethological studies are concentrated in the southeast part of the country, roughly matching the geographic distribution of Mexican primates (Figure 2). Ateles geoffroyi, Alouatta palliata, and A. pigra were the most common species studied, with more than 40 % of the publications. In addition, the study of primates such as Ateles and Alouatta is key for understanding the development and evolution of the human species (e. g., Emery Thompson 2019) and is a cornerstone for conserving the tropical ecosystems they inhabit, given their role as umbrella species (Rodríguez-Luna et al. 2013). Finally, primates are charismatic species whose knowledge can contribute to attract support for conservation projects (Hill 2002).
Although with a much modest contribution in publications, the orders Rodentia, Carnivora and Chiroptera follow the order Primates ethological studies. The volcano mouse (Neotomodon alstoni), an endemic species from central México, is the most studied rodent because its social nature and omnivorous diet facilitates its study in captivity (e. g., Luis et al. 2000, 2012, 2017). The puma (Puma concolor), the jaguar (Panthera onca) and the Mexican wolf (Canis lupus baileyi) were the most heavily studied carnivores because they are emblematic, charismatic species and because are in some risk category (the last two; e. g., Servín 1991, 1997; Servín and Huxley 1991; Hernández-SaintMartín et al. 2015; De la Torre et al. 2017). The Jamaican fruit-eating bat (Artibeus jamaicensis) has attracted attention due to its dynamics of harem groups presented (Ortega and Arita 1999; Ortega and Maldonado 2006), and the vampire bat (Desmodus rotundus) for attacking cattle (Wimsatt 1969; Greenhall et al. 1971). Half of México’s land mammal orders (Didelphimorphia, Cingulata, Perissodactyla, Pilosa, Soricomorpha) are not the subject of ethology studies.
Ethology became established as a scientific discipline on its own right worldwide in 1973 (Moreno and Muñoz-Delgado 2007). Prior to that year, only six articles had been published in México, most of them authored by foreign researchers. Those studies examined various ethological aspects of mammals. Russell and Findley (1954) documented the first observation of swimming of a myomorph rodent of the genus Onychomys from the State of Nuevo León. Packard (1958) described the carnivorous behavior of the Mexican ground squirrel (Ictidomys mexicanus) preying on a rabbit (Sylvilagus sp.) in the State of Coahuila. Other studies described various aspects of the common vampire bat, Desmodus rotundus: foraging behavior and refuge selection in the State of Tabasco (Wimsatt 1969), mode of attack on cattle in the State of Oaxaca (Greenhall et al. 1971), biting and feeding habits in captivity at the Instituto Nacional de Investigaciones Pecuarias in México City (Greenhall 1972), and the relationship between the feeding periods and absence in relation to moonlight in Oaxaca and San Luis Potosí (Crespo et al. 1972).
Thirteen scientific papers were published between 1973 and 1995. For example, Gould (1975) recorded, described, and compared vocalization patterns related to precocial and altricial conditions of pups of eight bat species, including two from México (the lesser long-nosed bat, Leptonycteris yerbabuenae, from Sonora, and the black mastiff bat, Molossus rufus, collected by R. Horst and recorded at his laboratory). The mating behavior of four deer-mouse species of the genus Peromyscus from Oaxaca and Campeche was described by Dewsbury (1979). The diurnal roosting and resting behavior (in tree holes) of the Mexican fruit bat (Artibeus jamaicensis) at Chamela Biological Station, Jalisco, was described by Morrison (1979). Estrada (1984) and Estrada and Coates-Estrada (1985) studied the frugivory habits and range of habitats used by the mantled howler (Alouatta palliata) in Los Tuxtlas, Veracruz. Servín (1991) described 37 social behavioral patterns (classified into friendly, submissive, playful, sexual, and aggressive-defensive behaviors) displayed by five Mexican wolves (Canis lupus baileyi) over 15 months in captivity in the State of Durango. Servín and Huxley (1991) determined the seasonal and annual foods habits of coyote Canis latrans by analizing 330 scats collected on the buffer zone at the Michilia Biosphere Reserve, finding that mammals (e. g., rodents and ungulates) and fruits (e. g., Juniperus deppeana and Arctostapphyllos pungens.) were the most consumed food categories. In the same Biosphere Reserve, Servín and Huxley (1995) determined the home range of coyotes (Canis latrans), registering an average annual of 9.1 km2.
The most productive period by far was between 1996 and 2018, with 90 % of the articles retrieved were published over these years. The fields most frequently addressed were foraging with 33 articles (19.5 %), movement with 28articles (16.6 %) and nesting, rearing, and territoriality, and social behavior, cooperation, and kinship with 20 and 21 articles (12.4 % and 11.8 %), respectively.
Thirty-three studies focused on foraging, examining for example food preferences and selection strategies of spider monkeys and howler monkeys at Los Tuxtlas, Veracruz (Dunn et al. 2009, 2010, 2012), Palenque, Chiapas (Amato et al. 2014), Catemaco, Veracruz (Reynoso-Cruz et al. 2016), and the Yucatán Peninsula (Pinacho-Guendulain and Ramos-Fernández 2017), as well as the use of non-conventional sources of water (e. g., streams) in Veracruz (Serio-Silva and Rico-Gray 2000) and Campeche (Duarte-Dias et al. 2014). The response of monkeys to variations in food availability was studied in a controlled environment at the Hilda Ávila de O’Farrill Management Unit, Veracruz (Rangel-Negrín et al. 2015) and in fragmented habitats at Los Tuxtlas, Veracruz (Estrada et al. 1999; Juan et al. 2000; Asensio et al. 2007), Balacán, Tabasco (Pozo-Montuy and Serio-Silva 2006), and the Lacandona tropical rainforest, Chiapas (Chaves et al. 2012; Benitez-Malvido et al. 2016). Horner et al. (1998) described that southern long-nosed bats, Leptonycteis curasoae, from the State of Sonora visited between 80 to 100 cactus flowers daily to feed on nectar and acquired 40 kilojoules of energy. Frick et al. (2009) documented, based on 143 working nights at 14 sites in Baja California, the first known example of an insectivorous bat, the pallid bat Antrozous pallidus, displaying facultative nectarivorous habits. Hernández-Hernández et al. (2018) found that the endemic Perote ground squirrel, Xerospermophilus perotensis, feeds opportunistically but, under certain conditions, selects plant species that provide a better-quality diet; Luna-Casanova et al. (2016), for his part, determined the preference of Tehuantepec jackrabbit (Lepus flavigularis) in Oaxaca to establish feeding and resting sites in the pasture with the presence of cattle. The food habits, based on scats, of different carnivores were also determined, for example; the coyote (Canis latrans; Grajales-Tam et al. 2003), the puma (Puma concolor), and jaguar (Panthera onca; Aranda and Sánchez-Cordero 1996), and cacomixtle, (Bassariscus astutus; Nava et al. 1999), in different states such as Baja California Sur, Campeche, Hidalgo and San Luis Potosí.
Studies on movement (n = 28) focused on the use of space and movement patterns of animals, mostly in the Central American spider monkey (Ateles geoffroyi). Valero and Byrne (2007) worked in the Otochma’ax Yetel Kooh reserve (Yucatán) and found that these monkeys are guided by spatial memory and are capable of planning routes. Smith-Aguilar et al. (2016) found a more concentrated use of space and higher rates of association (individuals brought together by resources of common interest) during periods of high fruit abundance. Campbell et al. (2005) investigated the terrestrial behavior of spider monkeys at five study sites in Perú, Ecuador, Panama, Costa Rica, and México (Punta Laguna, Yucatán) and concluded that this behavior occurred rarely, being more restricted in South America, where it occurred only in the context of eating soil or rotten wood and visiting salt licks. This contrasted with the behavior observed in Central and North America, where terrestrialism occurred more frequently while drinking water from streams during the dry season, when adult females escaped attacks by adult males, or as part of a chase game. Van-Belle et al. (2013) recorded, over 15 months, 691 movements of independent groups of another primate species, Alouatta pigra, at Palenque National Park, Chiapas, confirming that adult females showed leadership more frequently than males. Such female actions are beneficial for life as a group because they provide social cohesion by coordinating the timing and direction of travel, a behavior that has been observed in other mammals (Smith et al. 2015; Tokuyama and Furuichi 2017).
The home range has been determined in other terrestrial mammals for example the coyote (Canis latrans), in Durango (Servín and Huxley 1995) and Oaxaca (Marín-Sánchez et al. 2015), the gray fox (Urocyon cinereoargenteus), in Durango (Servín et al. 2014), the jaguar (Panthera onca) in Quintana Roo (González-Gallina et al. 2018), the Gaumer’s spiny pocket mouse (Heteromys gaumeri), in Yucatán (Cimé-Pool et al. 2002; Hernández-Betancourt et al. 2003), the Mexican spiny pocket mouse (Heteromys irroratus) in Oaxaca (Santos-Moreno and Santiago-Marcial 2012), the bobcat (Lynx rufus) in Durango and Chihuahua (Elizalde-Arellano et al. 2012) and Colima (Burton et al. 2003), the Tehuantepec Jackrabbit (Lepus flavigularis) in Oaxaca (Carrillo-Reyes et al. 2010), the white-lipped peccaries (Tayassu pecari) in Campeche (Reyna-Hurtado et al. 2009), and the white-tailed deer (Odocoileus virginianus) in Nuevo León (Bello et al. 2004).
A genetic structure study by Aguilera-Miller (2016) on the Cerralvo pocket mouse, Chaetodipus siccus, a species endemic to the Baja California Peninsula, revealed that the ethological interactions between individuals (e. g., dominant females more aggressive than subordinates) were responsible for females not dispersing and remaining in small geographic areas, pointing to the presence of female philopatry. In addition, Aguilera-Miller et al. (2018), based on the same molecular markers, found that the haplotypes considered to be ancestral were located at the periphery of the distribution area, while the derived haplotypes were located in the center of the distribution range, supporting again the hypothesis of strong philopatric behavior among females.
The 21 studies on nesting, rearing, and territoriality addressed primarily parental care and aggressive behavior. For example, observations on the endemic Mexican volcano mouse (Neotomodon alstoni), in captivity in México City confirmed that this species exhibits monogamous and biparental care behaviors (Luis et al. 2000). Males looked after the offspring when their testosterone levels were high (Luis et al. 2009, 2012, 2017), and the presence of males adversely affected maternal care but improved offspring survival (Luis et al. 2004).
The reproductive behavior of the Tehuantepec jackrabbit (Lepus flavigularis), an endemic species listed as endangered in the official Mexican laws (SEMARNAT 2010), was studied by Rioja-Paradela et al. (2011) in the State of Oaxaca. These authors found that the breeding season lasted 250 days per year; each female gave birth to two leverets that were weaned after 12 days and showed a higher survival rate relative to other leporids; predation by feral dogs (Canis familiaris) appeared to be the primary cause of mortality. On the other hand, Servín (1997) determined that, in a captive population of Mexican wolves (Canis lupus baileyi) from the La Michilía Biosphere Reserve, Durango, the mating occurred from January to April, the births occurred between April and May and the average number of offspring born per litter was four.
Studies on primates showed that the Guatemalan black howler, Alouatta pigra, in the State of Campeche lengthens its foraging periods during lactation (Duarte-Dias et al. 2011); maternal care by the mantled howler monkey, A. palliata, in the State of Veracruz, was directly related to lactation stage and food availability (Duarte-Dias et al. 2018). Duarte-Dias (2005) provided a detailed description of labor and birth in A. palliata under semi-captivity conditions at Agaltepec island in Catemaco, Veracruz. He described the labor and birth stages, behavioral events, and their timing and duration; the patterns described are representative of the birth process in this species.
Other studies addressed aggressive patterns in spider monkeys (Ateles geoffroyi) from protected areas in Yucatán. Valero et al. (2006) recorded collective aggressions by sexually mature males towards a single younger individual, with a fatal outcome. Aureli et al. (2006) reported, for the first time, instances of assault groups advancing on the ground in unusual silence, similar to the behavior of chimpanzees (Kelly 2000; Watts et al. 2006). Although no fatal outcomes were observed, these behaviors may be related to factors such as reduction in mating opportunities, number of males relative to the neighboring community, or strengthening of intra-group ties. These findings suggest that this behavior might have evolved primarily through mutualism where participants gain direct benefits from their physical fitness. Intergroup aggression in defense of the cooperative group was observed in the Guatemalan black howler (A. pigra), at Palenque, Chiapas (Van-Belle et al. 2014); intragroup aggression, even including infanticide, was observed at Balancán, Tabasco (García-Feria et al. 2016).
The 20 studies on social behavior, cooperation, and kinship focused mostly on primates. Pastor-Nieto (2001) studied spider monkeys, Ateles geoffroyi, at the Zoológico Centenario zoo in Yucatán, finding that social relationships such as food sharing were influenced by affiliative behavior (e. g., mutual grooming) rather than by kinship. Social relationships were observed to improve the physical fitness of wild spider monkeys in the Yucatán Peninsula. For example, there was a closer proximity between family dyads, as well as between male-male relationships (Rebecchini et al. 2011); affiliative behaviors between males were found to be more common when they were young (Schaffner et al. 2012) and when new members entered the group and acted as bond initiators (Aureli and Schaffner 2007). Slater et al. (2009) found that competition for resources and the need for cooperation affected social interaction patterns, particularly the social relationships between females as they spent much time on feeding and showed greater aggressiveness. However, the presence of small infants influenced the social behavior of females as, according to Slater et al. (2007), mothers with infants received significantly more approaches and hugs from other females.
The fifteen behavioral studies on homeostasis and time budgets included a radiotelemetry study of activity patterns of the white-tailed deer (Odoicoileus virginianus), in a xerophilous scrub in the State of Coahuila (Gallina and Bello Gutiérrez 2014). A study of locomotor activity of the Mexican wolf (Canis lupus baileyi), in relation to lunar phases, which recorded the most intense activity during the waxing moon, decreasing activity during the full and last quarter moons, and the lowest locomotive activity during new moon (Sánchez-Ferrer et al. 2016). Other studies found interspecific differences in the way primates allocate time to different activities: the mantled howler monkey, A. palliata, spent a considerable amount of time resting, feeding, and moving around (Muñoz et al. 2001); Guatemalan black howlers (A. pigra), rested more frequently in high-quality habitats (Rangel-Negrín et al. 2018); separately, the periods of rest and activity of spider monkeys (A. geoffroyi), were mainly driven by light-darkness periods and environmental factors such as temperature, precipitation, and humidity (Muñoz-Delgado et al. 2004). In this last species, in Catemaco, Veracruz, Muñoz-Delgado et al. (2014) studied the impact of housing conditions and season on the daily timing and pattern, and Muñoz-Delgado et al. (2018), recorded that these primates respond to visitor (tourist) activity since it modified their normally pronounced bimodal diel activity pattern and developed a superimposed infradian activity rhythm peaking on Saturday and Sunday.
The 14 studies on mating systems included a study of the breeding season and reproductive behavior of the Mexican prairie dog (Cynomys mexicanus), in Coahuila (Rioja-Paradela et al. 2008a); an early effort to determine the mating behavior of the Tehuantepec jackrabbit (Lepus flavigularis), in the State of Oaxaca (Rioja-Paradela et al. 2008b); and the first report on the sexual behavior of the Mexican wolf (Canis lupus baileyi) at the San Juan de Aragón zoo (México City) in summer (Soto et al. 2013). All these species are listed as endangered (SEMARNAT 2010; Álvarez-Castañeda et al. 2019; Lorenzo and Smith 2019).
The structure and social interactions of bats and primates were also studied. Ortega and Arita (1999) found that Mexican fruit bats (Artibeus jamaicensis), from the Yucatán Peninsula formed harems consisting of 4 to 18 females and one or two males, where males could play different roles: dominant (one in each harem), subordinate (present only in large harems), or satellite (not associated with a harem). Dominant males actively defended females, particularly during the breeding season when they displayed more agonistic responses towards male visitors (Ortega and Arita 2000). However, some subordinate males may have associated with harems as satellites, provided that they contributed some benefit to the dominant male (Ortega and Arita 2002). In addition, Ortega and Martínez-Rodríguez (2011) registered that broad tailed bat (Nyctinomops laticaudatus) in the archaeological zone of Uxmal, Yucatán, shows a promiscuous mating system, and the males display agonistic-type behavioral activities.
A study at the Palenque National Park (Tabasco, México) showed that the black howlers (Alouatta pigra) with high androgens levels and fecal glucocorticoids, had almost exclusive access to fertile females (Van-Belle et al. 2009a), in addition males rarely solicited sexual interactions, but instead monitored the females reproductive status by sniffing their genitals, and maintained significantly closer proximity to females during their periovulatory periods (Van-Belle et al. 2009b). In contrast, studies of spider monkeys (Ateles geoffroyi) from Yucatán found that males with fewer reproductive opportunities resorted to infanticide (Gibson et al. 2008) or attacked females (Slater et al. 2008), likely as a means of sexual coercion to increase their mating chances.
Studies on comparative social behavior (n = 11) addressed, for example, the role of agonistic behavior in explaining the relative abundance of the Mexican volcano mouse (Neotomodon alstoni), in a small mammal community at the Sierra del Ajusco mountain range in central México. Habitat partitioning by occupying different microhabitats or maintaining discrete central areas were mechanisms likely allowing the coexistence of N. alstoni and other species such as the black-eared mouse (Peromyscus melanotis), and the Mexican vole (Microtus mexicanus), which usually defended their territory in preferred microhabitats against N. alstoni (Fa et al. 1996). The first known cases of infanticide and forced copulation in spider monkeys (A. geoffroyi) from two communities in México and four in Perú (Gibson et al. 2008) were also described. Gibson et al. 2008 and Hernández-Saintmartín et al. (2013) studied activity patterns of jaguars, pumas, and their potential prey species in San Luis Potosí, where the activity peaks of both felids suggest that temporal segregation is a strategy which minimizes interspecific encounters allowing the coexistence of several individuals.
The ten studies that focused on conservation and behavior looked only at primates, addressing the close relationship between anthropogenic disturbance factors and behavioral changes or increased stress levels in animals. Rangel-Negrín et al. (2016) found that Guatemalan black howlers (A. pigra) from the State of Campeche exhibited a narrower behavioral repertoire and higher psychosocial stress levels when living in altered or disturbed habitats. The latter was also observed in Guatemalan black howlers from the Yucatán Peninsula (Rangel-Negrín et al. 2014), Balancán, Tabasco (Martínez-Mota et al. 2007), and El Zapotal Ecological and Recreational Center, State of Veracruz (Aguilar-Melo et al. 2013), as a response to tourism in the latter. However, stress has also been recorded in A. palliata by translocations in southern Veracruz (Aguilar‐Cucurachi et al. 2010).
However, these behaviors are not unique to howler monkeys. Spider monkeys (A. geoffroyi) from an island in Catemaco lake, Veracruz, showed increased agonistic behaviors and fewer vocalizations in the presence of tourists (Pérez-Galicia et al. 2017). An alternative to address stressful behaviors in captivity was suggested by Márquez-Arias et al. (2014). They showed that, at the Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz in México City, environmental enrichment ameliorated behavioral issues caused by confinement.
The nine articles that addressed communication behavior looked mostly at vocalization aspects. Servín (2000) found that Mexican wolves (Canis lupus baileyi) in captivity at La Michilía, State of Durango, howled more frequently and for longer periods during mating, and were heard more often at dawn and dusk. A comparison of two groups of spider monkeys (Ateles geoffroyi) from México and Costa Rica revealed that contact calls in each group showed differential variations between individuals (Santorelli et al. 2013). On the other hand, the studies by Briseño-Jaramillo et al. (2015, 2017, 2018) on Guatemalan black howlers (A. pigra) from Yucatán, provided the first description of a unique behavior associated with the call (placing a hand in front of the mouth while vocalizing), a study that opened up a new line of research on how non-human primates developed strategies to overcome limitations in acoustic plasticity. These authors identified a repertoire of vocal calls that included twelve distinct calls (three of which are emitted exclusively by females and two only by males) and confirmed the presence of non-random patterns through which individual calls can be differentiated, even those from members of other groups, which might represent potential “conversation rules” (Briseño-Jaramillo et al. 2018).
Self-defense (n = 4), learning (n = 2), and cognition (n = 2) were studied least frequently; altogether, they accounted for less than 5 % of the articles published during this period. Studies on self-defense described aspects such as the evasive behavior of A. palliata towards a group of the potential predator tayra, Eira barbara, in Playa Escondida, Veracruz (Asensio and Gómez-Marín 2002) and climbing more than 5 m as an escape mechanism of the American hog-nosed skunk (Conepatus leuconotus), when chased by humans in Colima (México) and Texas (USA; Brashear et al. 2010).
Finally, the articles on learning addressed the cultural transmission of behavior in primates. For example, Santorelli et al. (2011) compared variants of universal behaviors (defined as those used across all communities) of spider monkeys at three communities, two in Punta Laguna, Yucatán, México and one in Santa Rosa, Costa Rica. Six behaviors were identified that were likely maintained through social learning: 1) fruit extraction using the hand instead of the mouth; 2) drinking by licking instead of dribbling; 3) drinking using the left hand instead of the right hand; 4) contact greeting instead of non-contact greeting; 5) resting sitting upright; and 6) resting by leaning laterally. These results may have several implications for the study of spider monkey behavior: on the one hand, they suggest the possibility of a behavioral repertoire larger than the one reported by previous studies (e. g., McGrew 1998; Watson and Caldwell 2009); on the other, that the relative use of universal behavioral variants can reinforce community membership.
Briseño-Jaramillo et al. (2015) provided the first description of a unique behavior (placing a hand in front of the mouth while vocalizing) associated with the call of Guatemalan black howlers at Palenque National Park, Chiapas. They concluded that this behavior is transmitted culturally and plays a role in intergroup competition and intragroup cohesion.
In conclusion, ethological research on mammals in México - as represented by published articles in which this was the main study subject - reveals three distinct periods: the first (1900 to 1953) with no published papers, the second (1954 to 1995) was characterized by a low production of publications, while the third (1996 to 2018) shows a linear increase in the number of articles published, usually in foreign journals, with Mexican authors having an increasing participation. More than 90 % of all the studies focused on primates, rodents, bats, and carnivores and most studies were developed in the State of Veracruz and southeast México. Ethological studies have not explored other mammalian orders nor have focused on the northern part of the country. A diverse set of studies have been addressed, particularly over the last two decades; those such as foraging, movement, nesting, breeding, and territoriality, followed by social behavior, cooperation and kinship stand out in terms of the number of articles published. Despite its relatively recent development compared to disciplines such as paleontology, evolution, biogeography, and others, mammalian ethology in México has already made significant contributions given the growing number of mammalogists interested in this field and the increase in national and international collaborations, which therefore indicates that mammal ethology will surely continue its development and consolidation.











 nova página do texto(beta)
nova página do texto(beta)


