Introduction
The genus Eptesicus Rafinesque, 1820 in South America comprises 19 species. These 19 species are grouped into two subgenera. The subgenus Eptesicus includes nine species and several subspecies (Miranda et al. 2006; Davis and Gardner 2008; Díaz et al. 2016; Sánchez et al. 2019): Eptesicus andinus Allen, 1914 (monotypic), Eptesicus brasiliensis (Desmarest, 1819; with four subspecies), Eptesicus chiriquinus Thomas, 1920 (monotypic), Eptesicus diminutus Osgood, 1915 (with two subspecies), Eptesicus furinalis (d’Orbigny and Gervais, 1847; with two subspecies), Eptesicus fuscus (Palisot de Beauvois, 1796; one subspecies), Eptesicus innoxius (P. Gervais, 1841; monotypic), Eptesicus taddeii Miranda, Bernardi and Passos 2006 (monotypic), and Eptesicus ulapesensis Sánchez, Montani, Tomasco, Díaz and Barquez 2019 (monotypic). The subgenus Histiotus endemic to South America includes the other 10 species (Rodríguez-Posada et al. 2021). Histiotus has been considered by some authors as a subgenus of Eptesicus (Hoofer and Van Den Bussche 2003; Giménez et al. 2019; Simmons and Cirranello 2020), but this suggestion has not been followed by other authors (see Burgin et al. 2018; Moratelli et al. 2019; Barquez and Díaz 2020). Historically, Neotropical bats of the subgenus Eptesicus have been defined as species complexes based on fur variability. For example, Davis (1966) identified three long-haired species: E. andinus with two subspecies (E. inca and E. chiriquinus), E. montosus with two subspecies (E. montosus and E. chiralensis), and E. fuscus (monotypic). However, Simmons and Voss (1998) reviewed the holotypes of E. andinus and E. chiralensis and topotypic specimens of E. inca and E. chiriquinus, concluding that E. chiralensis and E. andinus are conspecifics and similarly, E. inca and E. chiriquinus are also conspecific with E. chiriquinus having priority over E. inca. Simmons and Voss (1998) suggested that E. andinus and E. chiriquinus can be differentiated by skull shape and the arrangement of the sagittal and nuchal ridges, as well as differences in size (E. chiriquinus tends to be larger than E. andinus).
Five species of the subgenus Eptesicus currently occur in Bolivia: E. andinus, E. brasiliensis, E. chiriquinus, E. diminutus, and E. furinalis (Anderson 1997; Siles 2007; Vargas-Espinoza 2007; Aguirre et al. 2010, 2019; Poma-Urey et al. 2019); being one of the countries with the highest diversity of the subgenus in South America. Despite that, there are several, information gaps related to the distribution and richness of the genus remain (Poma-Urey et al. 2019). Here, we describe a new species of the subgenus Eptesicus based on 19 specimens collected in the sub-Andean Bolivian-Tucumanian forest of Santa Cruz, and by comparisons with specimens from other localities of South America. The new species is morphologically similar to E. andinus but can be differentiated from E. andinus and its congeners by genetic distances, discrete morphological and morphometric traits.
Material and Methods
A total of 19 specimens of bats of subgenus Eptesicus were collected during field trips between 2007 and 2013 in two localities in the Province of Florida, Santa Cruz Department, Bolivia (Figure 1). To assess their specific identity, 131 specimens belonging to five species of Eptesicus with confirmed presence in Bolivia were also examined: E. andinus (n = 39), E. brasiliensis (n = 5), E. chiriquinus (n = 21), E. diminutus (n = 10), and Eptesicus furinalis (n = 56). The specimens reviewed are housed at the following institutions: American Museum of Natural History, New York, USA (AMNH); British Museum Natural History, London, England (BMNH); Colección de Mamíferos Lillo, Tucumán, Argentina (CML); The Field Museum of Natural History, Chicago, USA (FMNH); the Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá, Colombia (ICN); Noel Kempff Mercado Natural History Museum, Santa Cruz, Bolivia (MHNNKM); Museo de Historia Natural Universidad del Cauca, Popayán, Colombia (MHNUC); Museo de Historia Natural, Universidad de Caldas, Manizales, Colombia (MHN-UCa); Museo Universidad Distrital Francisco José de Caldas, Bogotá, Colombia (MUD); Colección Teriológica Universidad de Antioquia, Medellín, Colombia (CTUA); Colección Mastozoológica Universidad del Valle, Cali, Colombia (UV), and the National Museum of Natural History, Washington, DC., USA (USNM; Appendix 1).
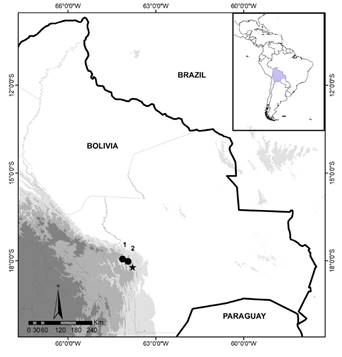
Figure 1 Collecting sites for Eptesicus langeri sp. nov. in South America. Municipalities of Samaipata and Pampagrande, Province of Florida, Department of Santa Cruz, Bolivia. Agua Rica Type locality (star), Reserve Municipal El Chape (circle 1) and Agua Clarita (circle 2).
Several external, and cranial characters described in the literature (Simmons and Voss 1998; Davis and Gardner 2008; Díaz et al. 2016) were analysed in all the specimens reviewed, including the development of the sagittal and lambdoidal ridges, and the length and colour of the coat hairs (according to Ridgway 1912), and compared with the information provided in the literature (e. g., Davis 1966).
Morphometric analysis. Nineteen cranio-dental measurements from 149 specimens were explored. These measurements included: greatest length of the skull (GLS), condylo-canine length (CCL), basicraneal length (BL), palatal length (PAL), postorbital constriction (POC), braincase height (BCH), braincase breadth (BCB), mastoid breadth (MB), zygomatic breadth (ZB), interorbital breadth (IOB), breadth across canines (C-C), breadth across upper molars (M3-M3), maxillary toothrow length (C-M3), incisive length-M3 (I-M3), upper molariforms length (P-M3), lower canine-m3 length (c-m3), lower molariforms length (p-m3), mandibular length (MAL), mandibular height (MH; Simmons and Voss 1998; Ramírez-Chaves 2008). We also took seven external measurements (the first two from labels) including: total length (LT), length of tail (TL), length of hind foot (LF), length of ear (EL), length of forearm (FA), and two from the hairs: length of dorsal hair (PD) and length of ventral hair (PV). The cranio-dental measurements were taken with a 0.01 precision digital calliper, and then Log transformed for further normalization and analyses. To show the main measurements that separate the species into different groups, the data of the first two principal main components (PCA) were selected. To define the variability between the groups provided by the first two main components, a Discriminant Function Analysis (DFA; Brown and Wicker 2000) was performed, considering the first two DFAs. For both the PCA and DFA analyses, due to completeness only 14 cranio-dental measurements were used (GLS, BCH, BL, PAL, P-M3, MB, M3-M3, C-C, I-M3, C-M3, CCL, IOB, POC, BCB), and the analyses were calculated using the software PAST version 2.17c for Windows platform (Hammer et al. 2001). The PCA and DFA included four similar-sized species of Eptesicus (E. andinus, E. brasiliensis, E. chiriquinus, and E. furinalis), the holotypes of E. montosus Thomas, 1920, and E. chiralensis Anthony, 1926 (considered both junior synonyms of E. andinus), one smaller species (E. diminutus), and the 19 specimens of the Province of Florida in Bolivia. We also calculated the cranial index (CRI = (((POC + BCB) × GLS)/2) and the maxillary index (MXI = (((C-C + M3-M3) × C-M3)/2) (Baud and Menu 1993).
Molecular analyses. We extracted genomic DNA from muscle tissues preserved in 96 % ethanol from five specimens of Eptesicus collected in the sub-Andean Bolivian-Tucumanian forest of Bolivia, and two E. andinus, one E. chiriquinus, and one E. furinalis from Colombia. DNA were extracted using Wizard® Genomic DNA Purification kit (Promega Corporation) following the manufacturer´s protocol. Amplification of three mitochondrial genes was done as follows: the amplification of cytochrome-b (cyt-b) gene was performed using two pairs of primers. First pair of primers L14816: 5′-CCATCCAACATCTCAGCATGATGAAA-3 ′ and H15173: 5 ′ -CCCCTCAGAATGATATTTGTCCTCA-3 ′ which amplifies a ≈358 bp fragment (Parson et al. 2000), and the other pair of primers LGL765F: 5´-GAAAAACCAYCGTTGTWATTCAACT-3´ (Bickham et al. 1995) and LGL766R 5´-GTTTAATTAGAATYTYAGCTTTGGG-3´, targeting a ≈1140 bp fragment (Bickham et al. 2004). Amplification of nicotinamide adenine dinucleotide dehydrogenase (ND1) gene using primers ND1-Forward 5´-CGCCATTATATGATCAGGATGAGCC-3´ and ND1-Reverse 5´-GTWGAGATRAATCATATTAT-3´ which amplifies a ≈293-295 bp fragment (Hamilton et al. 2015). The cytochrome c oxidase subunit I (COI) gene was amplified using the primer pair MCOIF 5′-CTGTACTTAGATTTACAGTCTAATGCC-3′ and MCOIR 5′-CCAAAGCCAGGCAAAATTAAAATATA-3′, which amplify a fragment of approximately 657 bp (Sánchez et al. 2019). The final amplification reaction volume was 30 µL, which contained 16.84 µL ultrapure water, 6 µL 5X buffer, 1.8 µL MgCl2 (25 mM), 2.4 µL dNTP mix (10 mM), 0.36 µL of each primer (25 µM), 1.2 U of GoTaq Flexi DNA Polymerase (Promega), and 2 µL DNA (approximately 100-150 ng of DNA).
The amplifications were performed on a Techne TCPLUS thermocycler, according to the following conditions for the cyt-b and ND1 genes: initial denaturation of 3 min at 94 °C, followed by 35 cycles of 30 s of denaturation at 94 °C, 30 s of annealing at variable temperature depending on markers (between 46 °C and 50 °C) and 30 s of extension at 72 °C, and a final extension of 5 min at 72 °C. Initial denaturation at 95 °C for 5 min, followed by 5 cycles at 94 °C for 5 min, 46 °C for 1 min 30 s, and 72 °C for 1 min 30 s, followed by 35 cycles at 94 °C for 1 min, 53 °C for 1 min, and 72 °C for 1 min, completing the reaction with a final extension cycle at 72 °C for 5 min, for the COI gene. The PCR products were quantified by fluorometry using a Quantus Fluorometer™ (Promega®) with the QuantiFluor® dsDNA System (Promega®), according to the manufacturer’s protocol. PCR products were sent to Macrogen Inc. (South Korea) for purification and DNA sequencing. The sequenced fragments were evaluated and edited using Geneious Trial v8.14 (Drummond et al. 2009). To further compare sequence divergence, we downloaded sequences of closely related taxa of the subgenera Eptesicus and Histiotus available in GenBank (Appendix 1 and 7). As outgroup, we used Myotis riparius and Neoromicia guineensis (Appendix 1 A and B).
The sequences for each gene were aligned using ClustalW (Thompson et al. 1997), included in the program MEGA X (Kumar et al. 2018). Intraspecific and interspecific nucleotide divergences were estimated with the program MEGA X, using the Kimura 2-Parameter distance model (K2P; Kimura 1980) and 1,000 bootstrap replications. For single and concatenated sets of mitochondrial genes we selected the best-fitting models of sequence evolution, using the Akaike Information Criterion (AIC) calculated with ModelFinder (Kalyaanamoorthy et al. 2017) in PhyloSuite (Zhang et al. 2020). For the concatenated analysis, and the cyt-b gene (Appendix 1) we selected the GTR+F+I+G4 substitution model. Bayesian Inference (BI) analysis was conducted with MrBayes 3.2.6 (Ronquist et al. 2012), with four parallel runs, 2,000,000 generations, in which the initial 25 % of sampled data were discarded as burn-in.
Phylogenetic analyses for the ND1 and COI genes were inferred by using the Maximum Likelihood (ML) method with different evolutionary models (GTR+F+G4 for ND1, and TVM+F+R2 for COI). The ML analysis was conducted with IQ‐TREE (Nguyen et al. 2015), 5,000 ultrafast bootstraps (Minh et al. 2013); as well as Shimodaira-Hasegawa-like approximate likelihood-ratio test (SH-like aLRT) for branches with 1,000 replicates (Guindon et al. 2010), all included in PhyloSuite platform (Zhang et al. 2020). Finally, we used the graphical viewer of phylogenetic trees FigTree v.1.4.3 (Rambaut 2007).
Results
Based on the review of 131 specimens of Eptesicus andinus, E. brasiliensis, E. chiriquinus, E. diminutus and E. furinalis, the 19 individuals from the Province of Florida, were assigned to the andinus group based on cranial morphology, E. andinus being the most morphological similar taxon. However, the specimens from Florida (Bolivia) showed a combination of discrete morphological characteristics that were not observed in any other Eptesicus known in South America. Among these, the presence of a well-developed frontal preorbital process allowed us to separate the specimens from Florida from specimens of E. andinus. The first two components of the PCA and DFA (Figure 2) account for 84.9 % and 84.7 % of the variation in the dataset, respectively (Table 1). PCA 1 accounted for 78.9 % of the variation, with all positive values, the highest values were given by the variables: GLS, CCL, I-M3 and C-M3. The second PCA explained 6.1 % of the variation; POC, BCB were among the variables with higher positive values, while the negative values were obtained for IOB, MB and PAL (Table 1).
Table 1 PCA and DFA results for 14 cranial variables of 138 specimens belonging to five Eptesicus species.
| Variable | Principal Component Analysis | Discriminant Function Analysis | ||
|---|---|---|---|---|
| PCA 1 | PCA 2 | DFA 1 | DFA 2 | |
| GLS | 0.966 | -0.094 | -28.789 | 47.078 |
| BCH | 0.824 | 0.081 | 3.874 | -33.086 |
| BL | 0.94 | -0.173 | -14.95 | 3.47 |
| PAL | 0.931 | -0.041 | 8.328 | 17.541 |
| P-M3 | 0.932 | -0.127 | 12.88 | 5.316 |
| MB | 0.896 | -0.001 | 8.694 | 60.88 |
| M3-M3 | 0.926 | -0.05 | 5.516 | -4.856 |
| C-C | 0.866 | -0.06 | -6.571 | -15.323 |
| I-M3 | 0.955 | -0.147 | 25.194 | 29.397 |
| C-M3 | 0.955 | -0.138 | 23.414 | -26.821 |
| CCL | 0.964 | -0.075 | 45.083 | -32.24 |
| IOB | 0.855 | -0.001 | 6.33 | -1.828 |
| POC | 0.608 | 0.711 | 8.475 | -24.852 |
| BCB | 0.74 | 0.479 | -1.94 | -26.743 |
| Eigenvalue | 11.039 | 0.85 | 9.33 | 11.49 |
| % variance | 78.9 | 6.1 | 73 | 11.7 |
| % variance accumulated | 78.9 | 84.9 | 73 | 84.7 |
The DFA shows that specimens from Province of Florida are part of the medium-sized Eptesicus along with E. furinalis and E. andinus (Figure 2). Although these species tend to overlap in cranial measurements, the variation observed in DFA 1 is positively influenced by the CCL, I-M3 and C-M3, while those that intervene negatively are BCB and C-C. For the DFA 2, the highest positive values are provided by MB, GLS, and I-M3, while the negative variables were IOB and M3-M3 (Table 1).
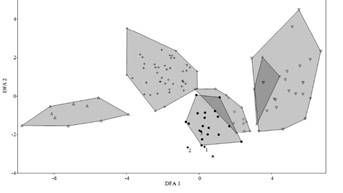

Figure 2 PCA (top) and DFA (bottom) graphs of 14 cranial and external measurements of six Eptesicus species: triangle E. diminutus (n = 10); cross, E. furinalis (n = 44); circle full, E. langeri (n = 19); diamond, E. andinus (n = 13); triangle invert, E. chiriquinus (n = 21); X, E. brasiliensis (n = 4). Holotype of E. langeri (asterisk); holotype of E. andinus (filled diamond 1); E. chiralensis (filled diamond 2); E. montosus (filled diamond 3).
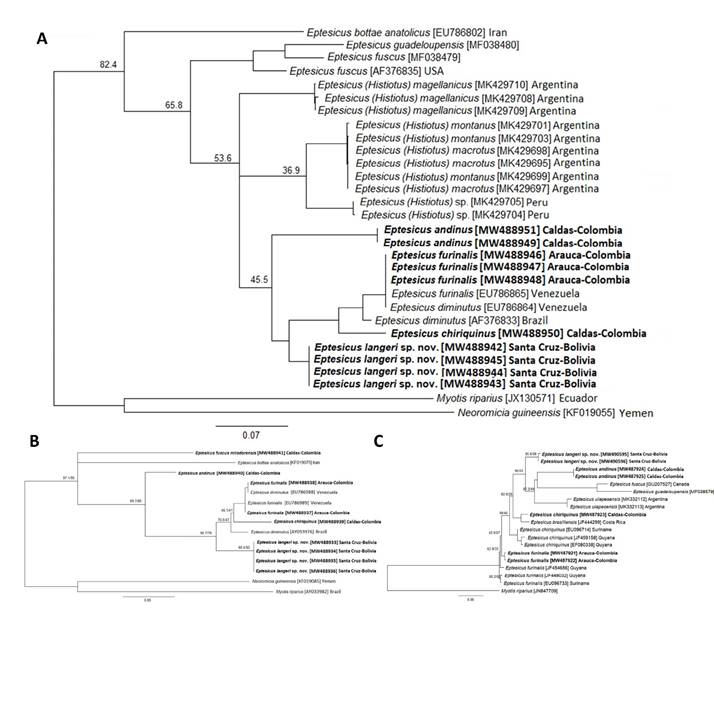
We obtained four cyt-b (GenBank accession numbers MW488942-MW488945), two COI (MW490595; MW490596], and four ND1 [MW488933-MW488936) individual sequences of specimens from the Bolivian-Tucumanian forest of Bolivia. A BLAST (Basic Local Alignment Search Tool) search of these cyt-b and ND1 sequences showed a range between 94.5 % and 92.9 % of identity with E. furinalis and E. diminutus. The specimens from Province of Florida, Bolivia, showed high cyt-b distances (Table 2) when compared with the cyt-b sequences of E. chiriquinus from Colombia (11 %), E. andinus (9.4 to 9.8 %), and E. furinalis (7.5 to 7.8 %). For the ND1 gene, the distances from Eptesicus from Bolivia were 8.7 to 8.8 % with E. chiriquinus (MHN-UCa 1951), 11.2 % with E. andinus, and 6.3 to 6.5 % with E. furinalis (Table 2). Similarly, the COI distances were between 9.7 to 12.7 % with E. andinus, and over 7.0 % with E. chiriquinus and E. furinalis (see Appendix 2). In addition, the Bayesian Inference and Maximum Likelihood consensus trees for the single and concatenated sets of mitochondrial genes show a monophyletic group conformed by the sequences of the Bolivian-Tucumanian forest of Bolivia specimens (Figure 3 and Appendix 1 to 3). Based on the results of the molecular and morphological analyses we described the Bolivian-Tucumanian forest specimens as a new species of Eptesicus.
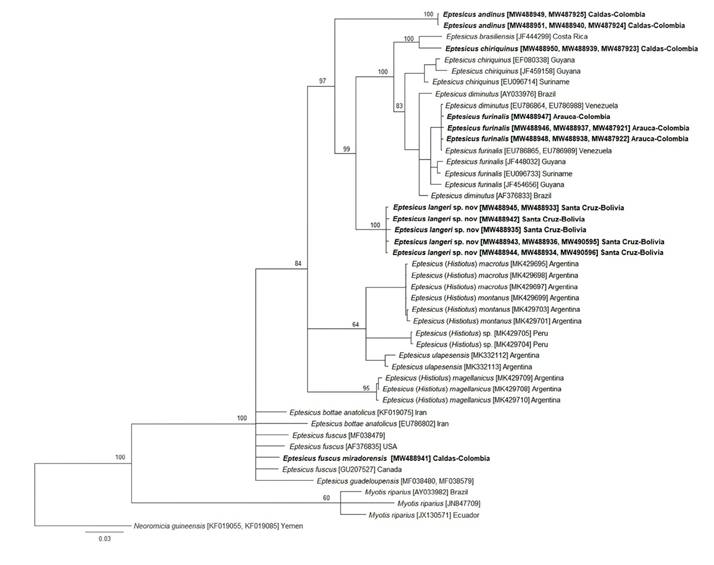
Figure 3 Phylogenetic tree inferred from the concatenation of cyt-b, ND1 and COI genes partial sequences of bats specimens collected in the present study (in bold) and sequences from GenBank accession numbers in brackets (cyt-b, ND1 and COI respectively) using Bayesian inference (BI) with the GTR+F+I+G4 evolutionary model, and Neoromicia guineensis and Myotis riparius were used as outgroup. Bayesian posterior probabilities (%) are indicated in the nodes.
Table 2 Distances based on Kimura two parameters for the mtDNA Cyt-b gene (intraspecific on the diagonal and interspecific below the diagonal), and for the mtDNA nicotinamide adenine dinucleotide dehydrogenase (ND1) gene (in bold, above the diagonal).
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Eptesicus sp. nov (Bolivia) | 0.000/0.000 | 0.112 | 0.068-0.072 | 0.082-0.084 | 0.205-0.213 | 0.167-0.170 | 0.061-0.072 | |||||
| 2 | E. andinus | 0.094-0.098 | 0 | 0.093 | 0.101 | 0.187 | 0.137 | 0.093-0.101 | |||||
| 3 | E. furinalis | 0.076-0.079 | 0.116-0.135 | 0 | 0.053-0.056 | 0.191-0.219 | 0.165-0.176 | 0.000-0.048 | |||||
| 4 | E. chiriquinus | 0.111-0.117 | 0.107-0.111 | 0.078-0.093 | - | 0.236 | 0.152 | 0.053-0.060 | |||||
| 5 | E. bottae anatolicus | 0.202-0.208 | 0.194-0.205 | 0.205-0.218 | 0.232 | - | 0.182 | 0.183-0.193 | |||||
| 6 | E. guadeloupensis | 0.118-0.140 | 0.191-0.203 | 0.135-0.176 | 0.18 | 0.202 | - | ||||||
| 7 | E. fuscus | 0.114-0.136 | 0.164-0.183 | 0.120-0.149 | 0.149-0.152 | 0.170-0.191 | 0.078-0.093 | 0.08 | 0.160-0.169 | ||||
| 8 | E. diminutus | 0.072-0.079 | 0.124-0.134 | 0.000-0.031 | 0.063-0.078 | 0.208-0.214 | 0.163-0.176 | 0.137-0.149 | 0.027 | ||||
| 9 | E. (Histiotus) montanus | 0.128-0.143 | 0.131-0.142 | 0.133-0.161 | 0.144-0.145 | 0.196-0.199 | 0.171-0.172 | 0.151.0.159 | 0.128-0.134 | 0.001-0.003 | |||
| 10 | E. (Histiotus) magellanicus | 0.093-0.103 | 0.124-0.136 | 0.120-0.132 | 0.139-0.143 | 0.181-0.183 | 0.159-0.160 | 0.134-0.154 | 0.132-0.145 | 0.132-0.137 | 0.002-0.004 | ||
| 11 | E. (Histiotus) sp. | 0.128-0.134 | 0.138-0.147 | 0.155-0.172 | 0.149-0.152 | 0.195-0.196 | 0.176-0.178 | 0.158-0.176 | 0.139-0.155 | 0.072-0.073 | 0.125-0.130 | 0.001 | |
| 12 | E. (Histiotus) macrotus | 0.128-0.138 | 0.131-0.143 | 0.133-0.156 | 0.144-0.145 | 0.196 | 0.171-0.172 | 0.151-0.159 | 0.127-0.136 | 0.000-0.004 | 0.132-0.135 | 0.072-0.074 | 0.000-0.001 |
Discussion
Our results show that, morphologically the Bolivian-Tucumanian forest specimens described here as Eptesicus langeri sp. nov. are part of the long-haired species of the subgenus Eptesicus that include high elevation Andean species such as E. andinus and E. chiriquinus, and cranially is similar to E. andinus rather to any other Eptesicus taxa. Most of the Neotropical Eptesicus have connected sagittal and nuchal ridges (in E. brasiliensis, E. chiriquinus, E. furinalis, E. innoxius, and E. taddeii), being E. andinus the exception, as this presents poorly developed ridges, creating a flattened and triangular space in dorsal view (Simmons and Voss 1998; Miranda et al. 2006; Tirira 2007; Ramírez-Chaves 2008). In addition, Neotropical Eptesicus have been grouped by hair length (Davis and Gardner 2008; Díaz et al. 2016; Sánchez et al. 2019), in short (less than 8.0 mm: E. brasiliensis, E. furinalis, E. taddeii and E. ulapesensis) and long-haired species (larger than 8.0 mm: E. andinus and E. chiriquinus). In contrast, some authors (e. g.,García-García et al. 2007; Gregorin and Loureiro 2011), provided longer hair measurements for E. brasiliensis (9 to 10 mm), overlapping mainly with E. chiriquinus (Simmons and Voss 1998). Despite the suggested morphological groups, our phylogenetic analyses show that these characters are not related to monophyletic clades, as shown by the position of E. langeri, E. chiriquinus and E. andinus in the phylogenetic trees (Figure 3 and Appendix 1 to 2).

Figure 4 Dorsal and ventral view of the coat colour pattern. From left to right: A. female (MNKM-5584 holotype) and B. male (MNKM- 5592 paratype) of E. langeri sp. nov. and C. E. andinus (MNKM-5598).
The lack of genetic data for E. andinus in previous phylogenetic analyses or species descriptions (Giménez et al. 2019; Sánchez et al. 2019), limited the assessment of the morphological and phylogenetic association within the Neotropical Eptesicus. In this way, our work is also filling these gaps by including and analysing the phylogenetic position of E. andinus for first time. In addition, our work showed that the diversity of Neotropical Eptesicus has been underestimated as suggested by newly proposed species (Sánchez et al. 2019). Finally, the systematics of Eptesicus and Histiotus is not deeply understood, and integral revisions of both taxa are needed (Giménez et al. 2019; Sánchez et al. 2019), therefore, the information that we provide here can be useful for additional integrative analyses at continental scale.
Taxonomy
Family Vespertilionidae Gray 1821
Genus Eptesicus Rafinesque, 1820
Subgenus Eptesicus Rafinesque, 1820
Eptesicus langeri sp. nov.
Holotype: The holotype is an adult lactating female, preserved as skin and skull, and deposited in the mammal collection of the Museo de Historia Natural Noel Kempff Mercado, Universidad Autónoma Gabriel René Moreno, Santa Cruz, Bolivia, with catalogue number MNKM-5584. Collected on December 01, 2013 by Luis H. Acosta, field number L. Acosta 732, at an elevation of 2,020 masl, in a fern grove of the Bolivian-Tucumanian forest surveyed during a biological diagnosis of the locality El Cedral-Agua Rica.
Type locality: El Cedral-Agua Rica, 15 km from the Municipality of Samaipata, Province of Florida, Department of Santa Cruz, Bolivia (-18° 13’ 10.55” S, -63° 47’ 49.74” W; 2,020 masl (Figure 1).
Paratypes: 18 individuals, six females (MNKM 4436, 5088, 5117, 5585, 5587, 5588) and seven males (MNKM 4678, 4679, 5126, 5590, 5591, 5592, 5692) from the type locality; four females (MNKM 5586, 5589, 5636, 5676) from Agua Clarita (-17° 56’ 47.71”S, -64° 08’ 0.28” W, 1,578 masl); and one female (MNKM 5697) from Reserva Municipal El Chape (-18° 01’ 21.46” S, -63° 56’ 50.23” W, 2,054 masl). External and cranial measurements are provided in Appendix 3 4 , 5.
Distribution: Eptesicus langeri sp. nov. is known from three localities: i) El Cedral-Agua Rica, ii) Agua Clarita, and iii) the Reserva Municipal El Chape, all three in the Province of Florida, Department of Santa Cruz, Bolivia (Figure 1).
Nomenclatural statement: A life science identifier (LSID) number was obtained for new species described herein: urn:lsid:zoobank.org:act:3723D032-6800-401F-ACEE-0326F1AE72B1.
Etymology: The epithet langeri is in honour of Fray Andrés Ma Langer o.p., a Dominican parish priest who made important contributions to the mammalogy of the inter-Andean valleys of Bolivia, especially in the Province of Florida of the Department of Santa Cruz. Several specimens collected by Fray Andrés Ma Langer are deposited and catalogued at the Museo de Historia Natural Noel Kempff Mercado.
Diagnosis: Eptesicus langeri is a medium-sized bat (forearm length 40 to 44 mm), with dorsal fur that is long (~ 8 mm), dark brown or orange-brown in females and dark brown in males (Figure 4). The skull has developed sagittal and lambdoidal ridges, that do not reach the posterior region of the skull (Figure 5). A developed preorbital process between the smallest width of the interorbital and postorbital (Figure 6). Coronoid process tall (Figure 5).

Figure 5 Dorsal (A), ventral (B) and lateral (C) views of the skull of the holotype of Eptesicus langeri sp. nov (MNKM-5584).
Description: Eptesicus langeri is a medium-sized bat, similar in size to E. furinalis and E. andinus (Table 3). The dorsal and ventral fur is bicoloured with dark bases and light tips (dark brown to dark orange, near one quarter hair length; Figure 4). Both sexes present the dorsal fur longer between the shoulders (7 to 9 mm) and shorter in the middle part of the body (~ 6 mm), the ventral region hairs differ from the back by being lighter. Males have a longer dark brown dorsal fur between the shoulders (~ 9 mm) and shorter in the middle of the back (~ 7 mm). Pregnant and lactating females have dorsal fur coloured “orange-brown”.
The skull is long, with developed sagittal and lambdoidal ridges. The sagittal crest gives the skull an elevated appearance that is more evident in the middle part of the cranial vault, while the lambdoidal or nuchal crest is developed at the edges of the occipitoparietal suture. Both ridges do not come into contact in the posterior region of the skull, leaving a gap between them (Figure 5).
Eptesicus langeri presents a well-developed frontal preorbital processes (Figure 6). In adult females these processes are more developed than in adult males. The upper internal incisors are larger than the external incisors. There is a small diastema between the upper external incisors and canine. The canine is attached to the premolar. First and second upper molars are similar, and the occlusal surfaces are W-shaped. The third upper molar is smaller than the rest of the molars and posteriorly extends beyond the anterior insertion of the zygomatic arches. The lower external incisors are in contact with the canines. The lower canine is slightly inclined towards the back, and in contact with first lower premolar which is approximately half the height of the canine; second lower premolar is higher than the first and in contact with first lower molar.
The mandible has a triangular and slightly curved coronoid process. The anterior part of the dentary has a straight oblique mandibular line; the mandibular process is slightly curved with a rounded end. A semi-circular mandibular incisure is located between the coronoid process and the anterior part of the dentary; the angular process is dorsally curved, and the mandibular ramus has a central depression (Figure 5).
Comparisons: Eptesicus langeri is a medium-sized bat (FA: 40.09 to 44.1 mm; GLS: 14.75 to 16.15 mm) with a dorsal and ventral fur length between 6.0 to 10.0 mm. It can be easily distinguished of other South American Eptesicus based on the forearm length, E. diminutus < 37.0 mm, E. innoxius < 39.0 mm, E. furinalis < 41.0 mm, E. taddeii > 44.1 mm and E. fuscus miradorensis > 49.0 mm. E. langeri is smaller than E. brasiliensis, E. chiriquinus, E. taddeii and E. ulapesensis, especially in the GLS (Table 3), and discrete characters of the skull and skin (Appendix 9). Eptesicus langeri is characterized by a highly developed preorbital process, which is poorly developed in E. andinus, E. furinalis (Figure 6), E. brasiliensis, E. fuscus miradorensis, E. innoxius, and E. taddeii, whereas in E. chiriquinus this process is evident but not well-developed. Based on dorsal fur length, E. langeri can be differentiated of E. furinalis (< 7.0 mm) and E. ulapesensis (~ 6.0 mm). In E. taddeii the coat is reddish and 7.0 mm long (Miranda et al. 2006).

Figure 6 Top: Frontal-dorsal view of the crania of the frontal pre-orbital process: A, B. Eptesicus langeri (MNKM-5564, Holotype female, MNKM-5592, Paratype male). C. E. andinus (AMNH 33807, Holotype). D. E. montosus (BMNH 2.1.1.1, Holotype). E. E. chiralensis (AMNH 47219, Holotype). Bottom: Space between sagittal and lambdoidal crest; notch and base of lambdoidal crest (red arrows); space between lambdoidal ridges and the parietal (white line).
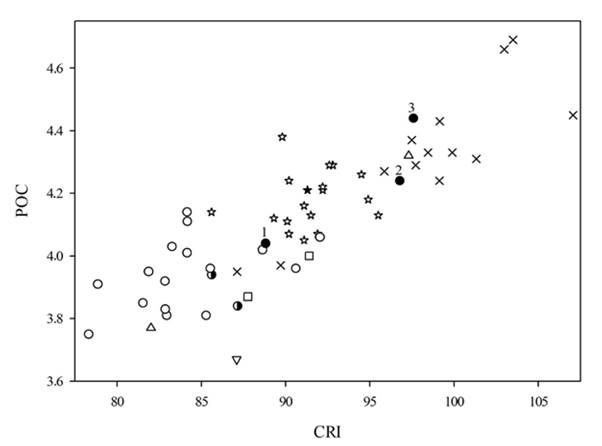
Comparison with holotypes of Eptesicus andinus (AMNH 33807), E. chiralensis (AMNH 47219), and E. montosus (BMNH 2.1.1.1): Eptesicus langeri can be differentiated from E. andinus and two of its junior synonyms (E. chiralensis and E. montosus) by cranial characteristics including: a) lambdoidal crest developed in the lateral region of the interparietal in E. langeri, vs. poorly developed in E. andinus, E. chiralensis, and E. montosus (Figures 6 to 8). B) base of the lambdoidal crest, broad in posterior view and with a smooth notch in the middle region in E. langeri, vs. lacking a notch and almost straight in E. andinus, E. chiralensis, and E. montosus (Figure 6). C) Frontal preorbital process present in E. langeri vs. poorly developed in E. andinus, E. chiralensis, and E. montosus (Figures 6 to 7). D) circular appearance in the nasal-premaxillary region in E. langeri, vs. rectangular in E. andinus, E. chiralensis and E. montosus (Figure 8). E) angular process of the mandible with a robust tip in E. langeri, vs. delicate in E. andinus, E. chiralensis and E. montosus (Figure 8). F) ascending ramus of the coronoid process lacking a steep slope in E. langeri (31º), E. andinus (30º) and E. chiralensis (35º), vs. with steep slope in E. montosus (40º; Figure 8). G) ramus of ventral mandible curved in E. langeri, vs. smoothly curve in E. andinus and E. montosus, and straight in E. chiralensis (Figure 8). Eptesicus langeri overlaps in some external and cranial measurements with Eptesicus andinus, E. chiralensis, and E. montosus although E. langeri can be separated when the postorbital width and cranial index are plotted (Appendix 6).

Figure 7 Dorsal, and ventral, view of skulls (left to right) of A. Eptesicus langeri sp. nov (MNKM-5592, Paratype). B. E. andinus (AMNH 33807, Holotype). C. E. montosus (BMNH 2.1.1.1, Holotype). D. E. chiralensis (AMNH 47219, Holotype).
Ecology: The type locality is part of the Bolivian-Tucumanian forest, characterized by Chari (Parapiptadenia excelsa) and Tipa (Tipuana tipu) trees, and connected to the Andean vegetation of the Peruvian-Bolivian Yungas (Navarro 2011). Specimens of the type locality were collected from 19:24 to 23:30 h. Most specimens were collected using mist nets installed at a height of 5 to 8 m above ground in forest clearings (newly opened road) of a secondary road. Lactating females and males with testicles in scrotal position have been recorded from November to January. Specimens from Agua Clarita were collected inside a hollow of a standing tree. Other bat species reported at the type locality are Anoura geoffroyi, Sturnira lilium, S. oporaphilum, Chrotopterus auritus, Desmodus rotundus, Platyrrhinus masu, Myotis nigricans, and M. keaysi.
We suggest that discreet dental skull characters should be used together with some measurements for an accurate identification of E. langeri. Thus, we propose the following identification key for some South American Eptesicus (sensu Davis and Gardner 2008), with emphasis on some cranial variables:
Taxonomic key
1. Ears longer than 20.0 mm, extending well beyond muzzle ..........................................................................subgenus Histiotus
1a. Ears normal, less than 20.0 mm, not extending beyond muzzle ......................................................subgenus Eptesicus 2
2. Skull longer than 16.3 mm; upper toothrow > 6.3 mm ....3
2a. Skull less than 16.3 mm; upper toothrow less than 6.3 mm .............................................................................................7
3. Forearm greater than 49.0 mm ........................................................................................................Eptesicus fuscus miradorensis
3a. Forearm less than 49.0 mm .....................................................4
4. U-shaped nasal opening ..........................Eptesicus brasiliensis
4a. V-shaped nasal opening ............................................................5
5. Dorsal fur longer than 12.0 mm ..........Eptesicus chiriquinus
5a. Dorsal fur less than 12.0 mm ...................................................6
6. Skull length between 17.3 to 18.4 mm .....Eptesicus taddeii
6a. Skull length between 15.9 to 17.0 mm ...........................................................................................................Eptesicus ulapesensis
7. Skull less than 13.6 mm; upper toothrow less than 5.0 mm .........................................................................Eptesicus diminutus
7a. Skull longer than 13.6 mm; upper toothrow longer than 5.0 mm .............................................................................................8
8. Sagittal and lambdoidal crests connected ...........................9
8a. Skull with separate sagittal and lambdoidal ridges forming a triangular flat bone ..............................................10
9. Pale greyish brown hair colour .................Eptesicus innoxius
9a. Dark brown to blackish brown hair colour ..........................................................................................................Eptesicus furinalis
10. Skull with very poorly developed/absent sagittal and lambdoidal ridges, poorly developed preorbital process .............................................................................Eptesicus andinus
10a. Skull with evident/developed sagittal and lambdoidal crests, preorbital process present .............Eptesicus langeri


Figure 8 Lateral view of the skull, the white lines show the shape of the rostrum and skull: A. Eptesicus langeri sp. Nov (MNKM-5592, Paratype). B. E. andinus (AMNH 33807, Holotype). C. E. montosus (BMNH 2.1.1.1, Holotype). D. E. chiralensis (AMNH 47219, Holotype “inversed”), scale 10mm. Lateral view of the mandible of: E. Eptesicus langeri sp. nov (MNKM-5592, Paratype). F. E. andinus (AMNH 33807, Holotype). G. E. montosus (BMNH 2.1.1.1, Holotype). H. E. chiralensis (AMNH 47219, Holotype), scale 5mm.











 nova página do texto(beta)
nova página do texto(beta)