Introduction
The family Talpidae includes three subfamilies, Scalopinae, Talpinae, and Uropsilinae, with Scalopinae being restricted to America and containing four genera Condylura, Parascalops, Scalopus, and Scapanus (Shinohara et al. 2003; Hutterer 2005). Scapanus is the only genus including more than one species; S. latimanus, S. orarius, and S. townsendii (Hutterer 2005). A fourth species, S. anthonyi, has been considered, although it has undergone many taxonomic changes. S. anthonyi was described as a full species by Allen (1893), and later was considered to be a subspecies of S. latimanus (Palmer 1937). Palmer (1937) argued that morphometric characteristics of S. anthonyi, such as its smaller size and fewer number of upper premolars, also were present in S. l. occultus, and consequently, S. anthonyi should be considered a subspecies of S. latimanus (see: Palmer 1937; Hutchinson 1987).
In his review of American moles, Jackson (1915) recognized S. anthonyi as a species because S. anthonyi has a projection in the braincase between the interparietal and the mastoid, which was absent in S. l. occultus (Jackson 1915). However, Palmer (1937) did not acknowledge these characteristics in the specimens that he examined, and therefore did not consider S. anthonyi a valid species. Huey (1936)suggested an additional difference between S. l. occultus and S. anthonyi; specifically, the manus (part of the pentadactyl limb that includes the metacarpals and phalanges) in S. anthonyi is squarer and smaller, with broader and heavier phalanges and with tips of the pterygoids parallel. In an alternative view, Hutchinson (1987) suggested that S. anthonyi shared characteristics with S. orarius; however, he continued to recognize S. anthonyi as a subspecies of S. latimanus. Populations of S. anthonyi in San Pedro Mártir, Baja California and those of S. l. grinnelli and S. l. occultus in southern California and northern Baja California peninsula are smaller in size and the skull is wider in relation to all other subspecies of S. latimanus from central and northern California (Yates and Salazar-Bravo 2005). Differences in skull morphology also occur between these two groups. S. anthonyi has only two or three upper premolars, and the temporal fossae is larger (Allen 1893; Jackson 1915; Huey 1936; Yates and Salazar-Bravo 2005).
Previous morphological analyses of all subspecies of S. latimanus indicated that some subspecies should be junior synonyms (Yates and Salazar-Bravo 2005) of other subspecies. For example, S. l. grinnelli (Jackson 1914) of S. l. occultus (Grinnell and Storer 1916); S. dilatus (True 1894), S. alpinus (Merriam 1897), and S. l. caurinus (Palmer 1937) of S. l. latimanus (Bachman 1842); S. l. sericatus (Jackson 1914), S. l. campi (Grinnell and Storer 1916), and S. l. monoensis (Grinnell 1918) of S. l. minusculus (Bangs 1899). However, S. l. insularis (Palmer 1937) and S. l. parvus (Palmer 1937) were not subjected to taxonomic changes (Yates and Salazar-Bravo 2005).
Based on these previous studies, the taxonomic status of species within the S. latimanus group has revealed several inconsistencies. The goal of this study is to better define the phylogenetic relationships of populations within Scapanus and combine these relationships with known morphological characteristics to evaluate the potential number of species. To achieve this goal, three mitochondrial genes were sequenced: cytochrome b (Cytb; n = 23); cytochrome c oxidase subunit I (CoI, n = 29); and cytochrome c oxidase subunit III (Co3; n = 29).
Materials and Methods
Sample collection. The dataset included specimens of the genus Scapanus (n = 31) represented by the species S. orarius, S. townsendi, S. latimanus, and outgroup specimens of Condylura, Neurotrichus, and Scalopus (n = 6). Tissue samples were obtained from the Collection of Mammalian tissues at Centro de Investigaciones Biológicas del Noroeste (CIB), Field Museum of Natural History (FMNH), Museum of Southwestern Biology at the University of New Mexico (MBS), and Museum of Vertebrate Zoology at the University of California (MVZ). Information for localities and museum catalog numbers are provided in Table 1. All capture and handling methods followed the animal care and use guidelines of the American Society of Mammalogists (Sikes et al. 2016). For all analyses, we grouped specimens from these localities into three species, S. orarius, S. townsendi, and S. latimanus, with S. latimanus further subdivided into three geographic units: 1) central and northern California (Group A, localities 7-14); 2) southern California and northern Baja California peninsula (Group B, localities 15-17); and 3) Sierra de San Pedro Mártir (Group C, locality 18; Figure 1; Table 1). This resulted in 31 geographic samples of Scalopinae.
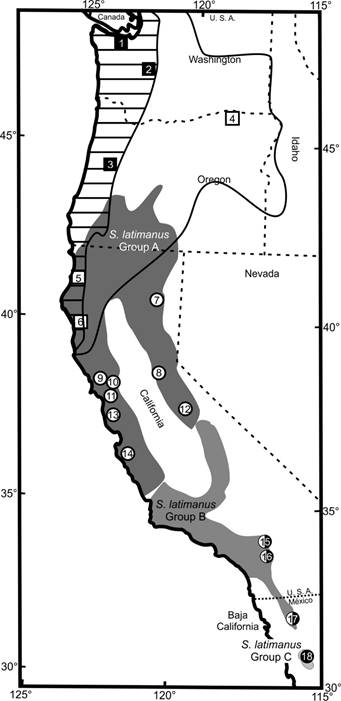
Figure 1 Distribution map of the species of the subfamily Scalopinae in North America. Scapanus townsendi (solid squares 1-3), S. orarius (open squares 4-6), and S. latimanus (circles). S. latimanus is split into three geographic groups: A) Central and northern California (localities 7-14, light gray circle); B) Southern California and northern Baja California peninsula (localities 15-17, half light/half dark circles); and C) Sierra de San Pedro Mártir (locality 18, dark gray circle).
DNA extraction and PCR conditions. Genomic DNA was extracted from muscle tissue preserved in 95 % ethanol (archived at -20 °C) or frozen (archived at -80 °C) using the DNAeasy Kit (QIAGEN Inc., Valencia, CA) protocols.
The following conditions were used for the initial double-strand amplification: 12.5 µl of (10 ng) template, 4.4 µl ddH2O, 2.5 µl of each primer pair (10 nM concentration), 0.474 µl (0.4 nM) dNTPs, 0.5 µl (3 mM) MgCl2, 0.125 µl Taq polymerase (platinum, Invitrogen, Carlsbad, CA), and 1× Taq buffer, to a final volume of 25 µl. The amplification conditions consisted of an initial denaturation at 94 °C for 3 min followed by 37 denaturation cycles at 94 °C for 45 s each; 60 s annealing at 50 °C (Cytb), 51 °C (Co1), 55 °C (Co3); and extension at 72 °C for 60 s; the products of the PCR amplification were verified in agarose gel, purified and sequenced both ways using the sequencing service of Macrogen Inc, Korea. The first part of the cytochrome b (Cytb, ~800 bp) gene was amplified using the primers MVZ05/MVZ16 (primer sequences given in Smith and Patton 1993; Smith 1998), the 658-bp fragment of cytochrome c oxidase subunit I (Co1) was amplified with the primers LCO1490/HCO2198 (Ivanova et al. 2007), and the 717-bp fragment of cytochrome c oxidase subunit III (Co3) was amplified with the primers L8618/H9323 (Riddle 1995). We aligned nucleotide sequences in Sequencher ver. 3.1 (Gene Codes Corp., Ann Arbor, Michigan), verified alignments visually, and translated them into amino acids for alignment confirmation. The haplotypes generated and used were deposited in GenBank (Table 1).
Table 1 List of specimens examined, locations according to Figure 1. #Catalog = museum catalog number of the reference collection. GenBank accession number for mitochondrial marker. Group (Gr), Number of map (M), State (ST), * Zhao and Jian 2015. ** Mouchaty et al. 2000.
| Gr | M | Species | #Catalog | St | Locality | Lat | Long | Co1 | CO3 | Cytb |
|---|---|---|---|---|---|---|---|---|---|---|
| GenBank accession numbers | ||||||||||
| 1 | Scapanus t. olympicus | MSB 43550 | WA | 9.2 Mi S, 2.7 Mi W Port Angeles | 47.9851 | -123.4878 | MZ150455 | MZ217155 | MZ217129 | |
| 1 | Scapanus t. olympicus | MSB 43552 | WA | 9.2 Mi S, 2.7 Mi W Port Angeles | 47.9851 | -123.4878 | MZ150456 | MZ217156 | MZ217130 | |
| 2 | Scapanus t. towsendii | MVZ 220251 | WA | 24303 Se 468th Street, Enumclaw | 47.1809 | -122.0175 | MZ150457 | MZ217157 | ||
| 2 | Scapanus t. towsendii | MVZ 220252 | WA | 24303 Se 468th Street, Enumclaw | 47.1809 | -122.0175 | MZ150458 | MZ217158 | ||
| 4 | Scapanus t. townsendii | MSB 40780 | OR | 9 Mi E Alsea | 44.3817 | -123.4135 | MZ150459 | MZ217159 | MZ217131 | |
| 4 | Scapanus t. townsendii | MSB 40781 | OR | 9 Mi E Alsea | 44.3817 | -123.4135 | MZ150460 | MZ217160 | MZ217132 | |
| 3 | Scapanus o. schefferi | MSB 54620 | WA | 2 Mi W Walla Walla | 46.0647 | -118.3835 | MZ150461 | MZ217161 | MZ217133 | |
| 3 | Scapanus o. schefferi | MSB 54621 | WA | Country Club, Walla Walla | 46.0389 | -118.3503 | MZ150462 | MZ217162 | MZ217134 | |
| 5 | Scapanus o. orarius | MSB 43626 | CA | 3.8 Mi S, 2.7 Mi E Trinidad | 41.004 | -124.0916 | MZ217163 | MZ217135 | ||
| 5 | Scapanus o. orarius | MSB 43627 | CA | 3.8 Mi S, 2.7 Mi E Trinidad; T7n, R1e, Sec 8 | 41.004 | -124.0916 | MZ150463 | MZ217164 | MZ217136 | |
| 5 | Scapanus o. orarius | MSB 43628 | CA | 3.8 Mi S, 2.7 Mi E Trinidad; T7n, R1e, Sec 8 | 41.004 | -124.0916 | MZ150464 | |||
| 7 | Scapanus o. orarius | MVZ 224399 | CA | 11 Mi N Westport On Hwy 1. | 39.7506 | -123.819 | MZ150465 | MZ217165 | ||
| A | 6 | Scapanus l. dilatus | MVZ 217713 | CA | Eagle Lake Road (Lassen Co. A1), Eagle Lake. | 40.6235 | -120.8399 | MZ150466 | MZ217166 | MZ217137 |
| 8 | Scapanus l. dilatus | MSB 47919 | CA | 1 Mi S, 4.5 Mi E Somerset, 2850 | 38.6334 | -120.5984 | MZ150467 | MZ217167 | MZ217138 | |
| 9 | Scapanus l. caurinus | MVZ 216930 | CA | Easy Sweet Farm, Sebastapol | 38.472 | -122.8544 | MZ150468 | MZ217168 | ||
| 10 | Scapanus l. caurinus | MVZ 199506 | CA | 2930 Redwood Road, Napa | 38.3167 | -122.3385 | MZ150469 | MZ217169 | MZ217139 | |
| 11 | Scapanus l. latimanus | MVZ 218027 | CA | 103 Aldarado Rd., Berkeley | 37.8579 | -122.2396 | MZ150470 | |||
| 12 | Scapanus l. latimanus | MVZ 201320 | CA | Forest S of Chapel, Yosemite Valley | 37.7408 | -119.5907 | MZ217170 | |||
| 13 | Scapanus l. latimanus | MSB 48532 | CA | Palo Alto, Stanford University Campus | 37.429 | -122.1695 | MZ150471 | MZ217171 | MZ217140 | |
| 14 | Scapanus l. latimanus | MVZ 222251 | CA | Hastings Natural History Reservation | 36.3785 | -121.5568 | MZ150472 | MZ217172 | ||
| 14 | Scapanus l. latimanus | MVZ 228295 | CA | Haystack Hill, Hastings Natural History Reservation | 36.3847 | -121.5627 | MZ150473 | MZ217173 | MZ217141 | |
| B | 15 | Scapanus l. occultus | MSB 47311 | CA | 10 Mi Se Big Bear City, Heart Bar campground | 34.1586 | -116.786 | MZ150474 | MZ217174 | MZ217142 |
| 16 | Scapanus l. occultus | MSB 47317 | CA | 3.6 Mi N, 9.8 Mi E Hemet, Lake Fulmor | 33.8052 | -116.7785 | MZ150475 | MZ217175 | MZ217143 | |
| 17 | Scapanus l. occultus | MSB 43120 | BC | Laguna Hanson | 32.0489 | -115.9056 | MZ150476 | MZ217176 | MZ217144 | |
| 17 | Scapanus l. occultus | MSB 40343 | BC | Laguna Hanson | 32.0489 | -115.9056 | MZ150477 | MZ217177 | MZ217145 | |
| 17 | Scapanus l. occultus | MSB 40344 | BC | Laguna Hanson | 32.0489 | -115.9056 | MZ150478 | MZ217178 | MZ217146 | |
| 17 | Scapanus l. occultus | MSB 40345 | BC | Laguna Hanson | 32.0489 | -115.9056 | MZ150479 | MZ217179 | MZ217147 | |
| 17 | Scapanus l. occultus | MSB 47308 | BC | Sierra Juárez, Laguna Hanson | 32.0489 | -115.9056 | MZ150480 | MZ217180 | MZ217148 | |
| C | 18 | Scapanus l. anthonyi | MSB 47306 | BC | Sierra San Pedro Mártir, 3.9 Mi by Road W Vallecitos | 31.0167 | -115.5333 | MZ150481 | MZ217181 | MZ217149 |
| 18 | Scapanus l. anthonyi | CIB 32000 | BC | Sierra San Pedro Mártir | 31.0167 | -115.5333 | MZ150482 | MZ217182 | MZ217150 | |
| 18 | Scapanus l. anthonyi | MSB 47307 | BC | Sierra San Pedro Mártir, 20 Mi S, 10.9 Mi E Vallecitos | 31.0167 | -115.5333 | MZ150483 | MZ217183 | MZ217151 | |
| Outgroup | Condylura cristata | KU144678 * | KU144678 | |||||||
| Condylura cristata | NC029762 * | NC_029762 | ||||||||
| Neurotrichus g. hyacinthinus | MVZ 200061 | CA | Headwaters of Big Austin Creek, N of Cazadero | 38.6138 | -123.1315 | MZ150484 | MZ217184 | MZ217152 | ||
| Scalopus a. machrinus | FMNH 167212 | MI | Fennville | 42.5939 | -86.1017 | MZ150485 | MZ217185 | MZ217153 | ||
| Scalopus a. machrinus | FMNH 167213 | MI | Fennville | 42.5939 | -86.1017 | MZ150486 | MZ217186 | MZ217154 | ||
| Talpa europaea | Y19192 ** | Y19192 |
Phylogenetics analysis. The methodology for phylogenetic analysis was similar to that used by Camargo and Álvarez-Castañeda (2020). The most appropriate substitution model for the dataset for each of the three gene regions, as well as for the concatenated series, was determined using the Akaike information criterion (AIC) as implemented in MrAIC (Nylander 2004). Four separate Bayesian inference and maximum-likelihood analyses were conducted on the three genes independently; the concatenated series had three partitions with one per gene (Cytb, Co1, and Co3). Bayesian analyses were implemented in (MrBayes ver. 3.0b4; Huelsenbeck and Ronquist 2001) with four separate runs with Markov-chain Monte Carlo simulations starting from a random tree. Each run was conducted for 20 million generations and sampled at intervals of 1,000 generations. Of the samples trees, the first 50 % were discarded as burn-in and all remaining trees were analyzed to find the posterior probability of resulting nodes. A consensus tree was generated with the 50 % majority-rule algorithm in PAUP 4.0b10 (Swofford 2002). The percentage of samples recovered in a particular clade was assumed to be the posterior probability of that clade in PAUP 4.0b10 using a heuristic search with 1,000 replicates and swapping with the TBR algorithm.
Maximum-likelihood (ML) analyses were performed in PAUP ver. 4.0b10 (Swofford 2002) algorithm (Felsenstein 1981) using a heuristic search with 1,000 replicates and swapping with the TBR algorithm. Reliability was assessed using each of the three codon positions individually while applying equal weights and nodal support using nonparametric bootstrapping. Members of each genus were used because although some phylogenetic analyses were done using allozymes (Yates and Greenbaum 1982; Moore 1986) the phylogenetic relationships among moles of North America were not previously examined using gene sequencing. Trees were rooted with Scalopini (Scalopus aquaticus), Urotrichini (Neurotrichus gibbsii), and Condylurini (Condylura cristata; Motokawa 2004).
Results
Phylogenetic analyses. AIC tests revealed that the best evolution model was a GTR model: Cytb (GTR + I + G), Co1 (GTR + I), Co3 (GTR + G), and the concatenated genes (GTR + I + G). BI and ML trees for Cytb, Co1, and Co3, and the concatenated data with four partitions converged on an essentially identical topology (Figure 2).
Analyses of the three genes within Scapanus resolved five haplogroups with strong bootstrap support (>95 %), as follows. Haplogroup 1: only specimens from San Pedro Mártir, Group C of S. latimanus; Haplogroup 2: specimens from southern California and northern Baja California peninsula, Group B of S. latimanus; Haplogroup 3: all S. latimanus specimens from Group A of central and northern California; Haplogroup 4: specimens of the two subspecies of S. townsendi with a very low percentage of differences between them; and Haplogroup 5: containing two groups, each with specimens of different subspecies of S. orarius (Figure 2).
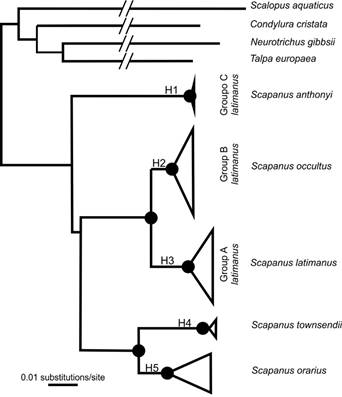
Figure 2 Bayesian tree constructed from three mitochondrial DNA genes (cytochrome b, cytochrome oxidase subunit I, and cytochrome oxidase subunit III) from members of the subfamily Scalopinae in North America and one of Talpa from Europe. Haplogroup 1 contains a single specimen from San Pedro Mártir, Group C of S. latimanus (S. anthonyi). Haplogroup 2 contains specimens from southern California and northern the Baja California peninsula, Group B of S. latimanus (S. occultus). Haplogroup 3 contains specimens from central and northern California, Group A of S. latimanus (S. latimanus). Haplogroup 4 is represented by two subspecies of S. townsendi. Haplogroup 5 is represented by two subspecies of S. orarius.
Scapanus latimanus Group C (Haplogroup 1) is separated from the other S. latimanus Groups A and B by two different species, S. townsendi (Haplogroup 4) and S. orarius (Haplogroup 5). The percentage of pairwise genetic differences (p-distance) for the three genes between Group C (San Pedro Mártir) and each of Group A (northern California) and Group B (southern California and northern Baja California) ranged from 7.22 to 10.50 %. The genetic differences between Group A and Group B ranged from 2.49 to 5.75 % (Table 2).
Table 2 Genetic distances (pairwise distance, p) among samples of the three geographical groups of Scapanus latimanus, S. orarius, and S. townsendi for the mitochondrial cytochrome b (Cytb), cytochrome oxidase subunit I (Co1), and cytochrome oxidase subunit III (Co3).
| Group A | Group B | Group C | |
|---|---|---|---|
| S. latimanus Group A (central and northern California) | |||
| Cytb | 0.13-1.96 | 4.13-5.75 | 8.00-9.67 |
| Co1 | 0.00-1.22 | 2.59-3.65 | 9.59-10.50 |
| Co3 | 0.15-0.61 | 2.49-3.60 | 7.89-8.58 |
| Concatenated | 0.24-1.23 | 3.27-4.17 | 8.53-8.95 |
| S. latimanus Group B (southern California and northern Baja California) | |||
| Cytb | 4.13-5.75 | 0.0-1.50 | 7.88-9.52 |
| Co1 | 2.59-3.65 | 0.00-0.90 | 8.68-9.98 |
| Co3 | 2.49-3.60 | 0.0-0.92 | 7.22-7.55 |
| Concatenated | 3.27-4.17 | 0.00-0.99 | 7.82-8.01 |
| S. latimanus Group C (San Pedro Mártir) | |||
| Cytb | 8.00-9.67 | 7.88-9.52 | 0.00-1.75 |
| Co1 | 9.59-10.50 | 8.68-8.98 | 0.00-0.15 |
| Co3 | 7.89-8.58 | 7.22-7.55 | 0.00-0.15 |
| Concatenated | 8.53-8.95 | 7.82-8.01 | 0.05-0.09 |
| S. orarius | |||
| Cytb | 6.75-8.66 | 7.50-9.50 | 8.00-9.89 |
| Co1 | 8.98-10.05 | 9.52-9.89 | 9.44-10.20 |
| Co3 | 8.06-10.14 | 6.88-9.09 | 8.40-9.61 |
| Concatenated | 8.05-9.00 | 7.82-8.43 | 8.72-9.00 |
| S. townsendi | |||
| Cytb | 8.00-9.39 | 8.00-9.50 | 9.75-11.14 |
| Co1 | 8.98-8.98 | 7.91-8.98 | 9.59-10.20 |
| Co3 | 8.92-9.79 | 7.72-8.75 | 9.09-9.61 |
| Concatenated | 8.67-9.19 | 8.40-8.72 | 9.43-9.62 |
Discussion
Genetic data revealed that S. townsendi and S. orarius are monophyletic and sibling taxa, as reported by Shinohara et al. (2003), and are substantially different from S. latimanus, as previously reported by Moore (1986). However, the geographic groups of S. latimanus do not exhibit a north-south phylogenetic relationship. The S. latimanus Group C from San Pedro Mártir formed an inconsistent relationship with the other two S. latimanus haplogroups from California and the northern Baja California peninsula (Haplogroups 2 and 3). Genes Co3 (boot = 56) and Cytb (boot = 68) show S. latimanus Group C (San Pedro Mártir) as a sister group to specimens from southern California and Baja California. However, analyses of Co1 (boot = 95) and the concatenated group (boot = 95) show S. latimanus Group C basal to all Scapanus clades (boot = 95), including S. townsendi and S. orarius (Figure 2). Each of the topologies show that S. latimanus Group C differs from Groups A and B. Although Hutchinson (1987) reported that S. anthonyi shared characteristics with S. orarius, this was not supported by the sequence data.
We did not perform a morphometric analysis of the craniodental measurements because this previously was reported by Yates and Salazar-Bravo (2005). Yates and Salazar-Bravo (2005) reported statistical differentiation in morphological characters between S. l. occultus and S. anthonyi separated by a distance > 50 km. In addition, they found significant differences between S. l. occultus and S. l. latimanus sensus (Yates and Salazar-Bravo 2005), with S. l. occultus being smaller overall. No specimens of S. l. occultus and S. l. latimanus have been collected in sympatry and in the two areas where S. l. occultus and S. l. latimanus occur, namely the southern part of Sierra Nevada and northern portion of Santa Barbara, it appears that S. l. occultus occurs in lower altitudes, and S. l. latimanus at higher altitudes.
Based on the genetic distance values between Groups A, B, and C, coupled with the morphological differences between them (Yates and Salazar-Bravo 2005), these groups can be considered as different species. The main morphological variations in the specimens of these groups are a smaller size in relation to the northern populations of S. latimanus and the variation in the number of upper premolars (Palmer 1937; Yates and Salazar-Bravo 2005).
Further, based on the sequence data, Scapanus latimanus from northern and southern California form two haplogroups. Haplogroup 3 includes all the specimens assigned to S. latimanus Group A (northern California) and Haplogroup 2 includes Group B (southern California and north Baja California peninsula). The population from San Pedro Mártir previously was considered as a distinct species, S. anthonyi (Allen 1893; Jackson 1915; Huey 1936; Yates and Salazar-Bravo 2005) and later subsumed into S. latimanus based on morphological characters (although a large series of specimens was never reviewed, which may have biased the interpretation), based primarily on the smaller size and number of upper premolars (Palmer 1937; Hutterer 2005). The morphological analyses (Yates and Salazar-Bravo 2005) and genetic analyses performed in this study support the consideration of S. anthonyi as a distinct species and indicates that S. anthonyi is restricted in distribution to the San Pedro Mártir mountain range.
Based on our phylogenetic analysis and its morphological characteristics (Allen 1893; Jackson 1915; Huey 1936; Yates and Salazar-Bravo 2005), we support that S. anthonyi is a different species from S. latimanus. Additionally, we propose that specimens known as S. latimanus occultus (including S. l. grinnelli) from southern California and northern Baja California peninsula should be considered as a distinct species (S. occultus) different from S. latimanus from central and north California and from S. anthonyi inhabiting San Pedro Mártir. Therefore, we consider that the genus Scapanus contains five species that should be recognized as S. anthonyi, S. latimanus, S. occultus, S. orarius, and S. townsendi.
Scapanus anthonyiAllen 1893
1893. Scapanus anthonyi Allen, Bull Amer. Mus. Nat. Hist., 5:200, August. Type locality: “Sierra San Pedro Martir, 7000 ft, Baja California [México]”. Adult male, skin and skull, American Museum of Natural History number 6313, collected by A. W. Anthony.
1937. Scapanus latimanus anthonyi Palmer, J. Mamm. 18:312, August. Name combination.
Geographic range. Restricted to the highlands of Sierra San Pedro Mártir, Baja California, México.
Diagnosis and comparison. Scapanus anthonyi can be differentiated from the other species of Scapanus in having fewer than seven unicuspid teeth behind the incisors in the mandible and maxilla and total skull length <32.5 mm. Projection present in the braincase between the interparietal and the mastoid (Jackson 1915). Manus more square and smaller, with broader and heavier phalanges, tips of the pterygoids bones of the upper palate parallel (Huey 1936). Smaller in all craniodental and somatic measurements relative to all other subspecies of S. latimanus, and teeth larger and crowded (Yates and Salazar-Bravo 2005:494 in table 3). Differing from S. orarius and S. townsendii in a smaller in size; dorsal coloration darker, almost black; no spaces between all unicuspid teeth, usually crowded; and rostrum short and broad.
Comments. Scapanus anthonyi has a distribution restricted to the upper portions of the Sierra San Pedro Mártir, within the pine and oak-pine forest. Collecting moles in the region is complex for several reasons. First, the gopher Thomomys nigricans is very abundant in the same area, so it is common to find gophers galleries that impinge upon and destroy mole galleries. Second, both species share a sympatric distribution throughout the mountain range. Third, although this region is a protected area, large numbers of cattle graze in the area and destroy the mole galleries. Fourth, specimens of S. anthonyi are very small in size, so their galleries also are small and the soil relief (molehill) that results from gallery construction is very difficult to determine. Fifth, galleries have a simple structure, and raise just 3 cm above the ground, and any leaf litter makes these molehills invisible (Cortés-Calva pers. obs.).
In the area, both Scapanus and Thomomys are named “topos” (moles) with no distinction between them, and only old ranchers give different names to them. Scapanus are called “topos de manoplas” (baseball-gloved moles) in reference to its forefoot size. Thomomys are known only as “topos”.
Scapanus occultusGrinnell and Swarth 1912
1912. Scapanus latimanus occultus Grinnel and Swarth, Univ. California, Publ. Zool., 10:131, April. Type locality: “Santa Ana canyon, 400 ft (12 mi NE Santa Ana), Orange County California”. Subadult female, skin and skull, Museum of Vertebrate Zoology, University of California, Berkeley, number 2369, collected by H. S. Swarth.
1914. Scapanus latimanus grinnelli Jackson, Proc. Biol. Soc. Washington, 27:56. Considered as junior synonym.
Geographic range. From Laguna Hanson (Sierra de Juárez) Baja California, México northwestward to Santa Barbara and northward to Yosemite Valley in Mariposa County, California.
Diagnosis and comparison. Scapanus occultus can be differentiated from S. latimanus in its smaller size and longer and wider skull (Yates and Salazar-Bravo 2005:494 in table 3). Some specimens have fewer than seven unicuspid teeth, but only on a single side of the mandible or maxilla. It differs from S. orarius and S. townsendii by the same characteristics mentioned in S. anthonyi.
Scapanus latimanus (Bachman 1842)
1842. Scapanus latimanus Bachman, Boston Jour. Nat. Hist., 4:34. Type locality “probably from Santa Clara, Santa Clara, California” Mounted specimen with imperfect skull, Berlin Museum, collected during October 1834.
1912. Scapanus latimanus latimanus Grinnell and Swarth, Univ. California, Publ. Zool., 10:131, April. First use of current name combination.
Geographic range. From Santa Barbara and Yosemite Valley, California, northward to southcentral Oregon.
Diagnosis and comparison. Scapanus latimanus can be differentiated from S. orarius and S. townsendii by the same characteristics mentioned in S. anthonyi.
Keys for the species of Scapanus
1. Dorsal coloration usually brown to gray. All unicuspid teeth with variable spacing between them and usually crowded; rostrum short and broad ............................................ 2
1a. Dorsal coloration almost black. All unicuspid teeth with regular spacing between them, and not crowded; rostrum long and narrow .................................................................... 4
2. Fewer than seven unicuspid teeth behind the incisors in the mandible and maxilla; total skull length less than 32.5 mm .............................................................. Scapanus anthonyi
2a. Seven unicuspid teeth behind the incisors in the mandible and maxilla; total skull length >32.5 mm ............. 3
3. Total length >161.0 mm. Skull length >34.0 mm in males and 33.4 mm in females. Ratio of mastoidal breadth to greatest skull length <49%, including the population of Alameda Island, California .......................... Scapanus latimanus
3a. Total length <161.0 mm. Skull length <34.0 mm in males and 33.4 mm in females. Ratio of mastoidal breadth to greatest skull length >49%, not including the population of Alameda Island, California ....................... Scapanus occultus
4. Total length >200.0 mm on average. Sublacrimal-maxillary ridge well developed; skull > 40.0 mm ............................................................................................ Scapanus townsendii
4a. Total length <200.0 mm on average. Sublacrimal-maxillary ridge little developed; skull <40.0 mm ..................................................................................................... Scapanus orarius











 nova página do texto(beta)
nova página do texto(beta)


