Introduction
Postnatal development and growth are life-history traits related to reproductive success, age at onset of adulthood, and longevity (Barclay and Harder 2003). Organisms with a faster development that reach a larger size in a shorter time have advantages over organisms with a slower growth rate (Kunz and Hood 2000; Dmitriew 2011). In bats, development and growth rate are affected by inheritance, maternal health, shelter conditions, and available food resources, among others (Kunz and Hood 2000). Bats undergo accelerated growth and development, lasting about one month in most Yangochiroptera (Kunz and Hood 2000). Likewise, weaning occurs at one month of age, although it is delayed to up to nine months in vampires (Kunz and Hood 2000; Delpietro and Russo 2002). Most studies on postnatal growth in bats focus on species with temperate distribution, rather than on tropical species (Kunz and Hood 2000; Richardson and Kunz 2000; Lin et al. 2010; Chaverri and Vonhoff 2011). For this reason, it is valuable to estimate the parameters related to postnatal development and growth in tropical and subtropical bat species, to understand the evolution of reproductive strategies of these organisms in different environments.
Natalus mexicanus is a bat species ranges from México to Panama and in Pacific and Caribbean islands; its habitat comprises from xeric shrubland to tropical rainforest, from sea level to 2,300 masl (Tejedor 2011, 2019). This species occupies mainly caves as daytime shelters and has also been found in mines and tree holes with high temperature and relative humidity (RH), from 22 to 26 °C and 74 to 99 %, respectively (Torres-Flores and López-Wilchis 2010; Tejedor 2011). Its diet includes a variety of arthropods, mainly arachnids (Torres-Flores and López-Wilchis 2019). Reproductive data from a population of Sonora indicate that the species is monoestrous, mating in summer and giving birth to a single offspring (Mitchell 1965). Since the species is widespread in México and thrives in different environments, some characteristics of its life history are considered to vary, as there is no reproductive information available for populations inhabiting southern México. The present study investigated the growth and flight development in N. mexicanus based on a population inhabiting “Los Laguitos”cave, Tuxtla Gutiérrez, Chiapas.
Materials and Methods
Study Area. The “Los Laguitos” cave is located 4 km to the NW of Tuxtla Gutiérrez (16º 46’ N, -93º 8’ W; altitude 781 masl), Chiapas, México. The cave has a horizontal structure consisting of three galleries with a single entrance. Inside the cave, the mean annual temperature is 32.3 °C and RH is 95.4 %; these values are higher than outdoor conditions (Martínez-Coronel et al. 2010). The local climate is warm subhumid, with a mean annual temperature of 24.7 °C (Cardoso 1979); the rainy season spans from May to October, with a well-marked summer drought or canicula in July and August and a dry season the rest of the year. The local vegetation is a tropical deciduous forest (Miranda 1998).
Growth and Development. To describe and quantify the growth and development of Natalus mexicanus, numbered plastic rings were attached to specimens at birth. The rings measured 4 mm in diameter by 4 mm width, with a mean weight of 0.04 g. The ring was attached around the right forearm in females and on the left forearm in males. Newborn specimens were determined based on the presence of fresh placenta (Kunz 1973), in addition to having a naked body, pink skin, folded ears, auditory meatus, closed eyes, and for being unable to move away from the perch site (Martínez-Coronel et al. 2014).
Individuals were recaptured at five-day intervals until their flight capacity made it impossible to locate and catch them. The age categories included were as follows: 1, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50 and 55 days post-birth, corresponding to the chronological age. After day 55, the capture of individuals was no longer possible due to the high number of individuals in the cave. Each specimen was individually manipulated next to its perch; on each occasion, the site, recapture date, ring number, and sex were recorded.
The separation of the ears, opening of the eyes, and emergence of the fur are continuous phenomena; however, for descriptive purposes, these characteristics were classified into discrete categories, defined as follows. As regards ears, three categories were recognized: Folded ears - ears attached to the sides of the head, with the auditory meatus closed. Partially upright ears - auditory meatus open, ears separated from the head, but with the tip bent. Upright ears - ears completely upright, as in adults. For the opening of the eyes, we defined three categories: Closed eyes - eyes completely covered by the eyelids, which are fused; separation line not visible. Partially opened eyes - eyes do not open completely, because the eyelids remain fused at the lateral commissures. Opened eyes - eyes completely visible, as the eyelids are fully separated. Regarding pigmentation and fur, five categories were used: I) pink skin, body completely naked; vibrissae absent, with only red spots observed in sites where vibrissae will emerge. II) Light-pink body, off-white membranes; vibrissae present; hairs smaller than 2 mm in genitals and toes. III) Grayish dorsum, with darker crown, interscapular region, patagium, forearm, and legs; shiny patagium and pinkish abdomen. IV) Pigmented abdomen; body covered with short grayish hairs measuring less than 3 mm between legs and hips. V) Fur darker, with brown hair on the dorsum and whitish hair in the abdomen; fur longer on legs and dorsum (> 3 mm) than on the abdomen.
Also, the forearm length and the length of the epiphyseal gap of the fourth metacarpal-phalangeal joint of the right wing were measured to the nearest 0.05 mm with a Mitutoyo digital caliper (Kunz and Anthony 1982); body mass was measured to the nearest 0.1 g with a spring balance. Temperature and RH were also recorded in each visit using a Taylor psychrometer. The readings were taken at 21:00 and 2:30 h at the center of the roost and at 1.20 m height.
Growth was quantified through the changes in forearm length, length of the cartilaginous epiphyseal gap of the fourth metacarpal-phalangeal joint of the right-wing, and body mass (Kunz and Anthony 1982). Flight development was described using the three categories proposed by Stern et al. (1997), namely: non-volant, individuals who hang from walls and are unable to fly, remaining still in the perching site or moving only through walls; semi-volant, individuals who attempt to fly but fail in staying in the air, colliding with walls and failing to perch; volant, individuals capable of flying in a straight line, gaining height and succeeding to perch.
Statistical analysis. The basic statistics, i. e., mean, and standard deviation, were calculated for all variables. Differences between sexes in individuals of the same age were evaluated with a Student’s-t test. This method was used for being robust to deviations from normality, as the sample sizes analyzed were small for the older age classes.
To determine the growth rate of each variable, the linear portion of each empirical curve was considered separately, and a linear regression was applied to estimate the slope. To obtain the growth model with the best fit for the relationship between age and forearm length, as well as between age and body mass, we used the growth equations of Gompertz, Von Bertalanffy and the logistic model (Zullinger et al. 1984). In the case of the length of the epiphyseal cartilage of the fourth metacarpal-phalangeal joint, different polynomial models were used (Brown and Rothery 1994). All statistical analyses were performed in NCSS (2020). A value of < 0.05 was used as a statistical significance criterion.
Results
We studied a total of 94 neonates (44 females and 50 males) of N. mexicanus specimens inhabiting “Los Laguitos” cave. All the individuals studied belonged to single-pup litters, as confirmed through the examination of pregnant females collected in previous months and at the beginning of the study. The parturition period lasted 25 days, from 25 May to 19 June 2009. During the day, pups slept hanging from their mothers, while at night, when mothers went out to forage, pups were transported to communal sites or nurseries, with mothers returning several times to breastfeed their pups along night. The mean temperature in the nursery was 32.8 ± 0.55 °C; this fluctuated over the first 30 days and tended to stabilize thereafter. RH was 96.8 ± 2.71 %, increasing from the beginning of the study to 45 days, tending to decrease thereafter.
Neonate condition. All neonates had umbilical cord and placenta; the abdominal skin was translucent, with visible viscera; ears folded and attached to the head; auditory meatus and eyes closed; pink body and skin completely devoid of hair. Red spots were observed in sites where vibrissae were to emerge. The patagium and uropatagium were whitish; the skull lacked the anterior fontanel but showed the posterior fontanel between the occipital and parietals; fat in shoulders and supra-scapular area (Figure 1a). Neonates emitted audible sounds and remained still at the site where they were left by their mothers, moving their heads upon hearing any sound; they attempted to suck milk when an object was brought closer.
Development of ears, fur, and eye opening. The ears began to separate from day one, and by day five of age, all recaptured specimens already had the auditory meatus open and the ears separated with the tip slightly bent. At day 10 of age, most individuals already had their ears fully upright (Table 1). The eyes remained closed over the first 15 days post-birth; in some 20-day individuals, the eyelids began to separate, disclosing the eyes in mid portion, as the lateral commissures remained attached. The last specimens with semi-closed eyes were 35 days old, although in other 25-day-old specimens the eyelids were already separated, and the eyes were fully open (Table 1).
Table 1 Detachment of the ears and opening of the eyes in Natalus mexicanus inhabiting “Los Laguitos” cave, Chiapas, Mexico. For each age category, the number of individuals in each development stage of each character is given.
| Age/days | Ear detachment | Eye opening | ||||
|---|---|---|---|---|---|---|
| Folded | Semi-erect | Erect | Closed | Semi-open | Open | |
| 1 | 93 | 1 | 94 | |||
| 5 | 33 | 33 | ||||
| 10 | 3 | 20 | 23 | |||
| 15 | 24 | 24 | ||||
| 20 | 11 | 8 | 3 | |||
| 25 | 14 | 11 | 2 | 1 | ||
| 30 | 11 | 6 | 4 | 1 | ||
| 35 | 12 | 1 | 4 | 7 | ||
| 40 | 12 | 12 | ||||
| 45 | 3 | 3 | ||||
| 50 | 4 | 4 | ||||
| 55 | 3 | 3 | ||||
The growth of the fur was slowly; individuals remained pink and naked during the first five days post-birth (Figure 1a). Between 10 and 15 days of age, vibrissae had already grown, and hairs less than two mm long were observed in the genital area and toes (Table 2). At postnatal day 15, some individuals had the crown and dorsum darker than the rest of the body (Figure 1b), a condition that occurred up to day 35 in some individuals. However, in other 30-day-old individuals, the body was already covered with a grayish fur (< 2 mm), with the abdomen paler than the dorsum (Figure 1c). Also, in some 35-day-old individuals, the dorsum was covered with a dark brown fur more than three mm in length, being shorter and lighter-colored in the abdomen (Figure 1d).
| Age/days | Category | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| 1 | 94 | ||||
| 5 | 33 | ||||
| 10 | 5 | 18 | |||
| 15 | 21 | 3 | |||
| 20 | 8 | 3 | |||
| 25 | 8 | 6 | |||
| 30 | 3 | 7 | 1 | ||
| 35 | 2 | 6 | 4 | ||
| 40 | 3 | 9 | |||
| 45 | 1 | 2 | |||
| 50 | 1 | 3 | |||
| 55 | 3 | ||||

Figure 1 Specimens of Natalus mexicanus of different ages observed in “Los Laguitos” cave, Chiapas, Mexico, in 2009. a) Neonate with placenta, closed eyes, folded ears, pink, and naked body. b) 15-day-old individual with closed eyes; pigmentation appears in the dorsum and crown. c) 30-day individual with open eyes and covered with fine grayish fur; and d) 40-day individual covered with dark fur.
Neonates had a forearm length of 10.68 ± 0.54 mm in females and 10.72 ± 0.73 mm in males, representing 27.15 % and 27.26 %, respectively, of postpartum females. Body mass was 0.93 ± 0.21 g in females and 1.03 ± 0.22 g in males, representing 17.27 % and 19.20 % of postpartum females. The epiphyseal gap measured 2.3 ± 0.25 mm in females and 2.4 ± 0.27 mm in males.
Secondary sexual dimorphism. Of the three morphometric variables analyzed, only neonate body mass showed significant differences between sexes (T 93 = 2.37, p = 0.01), while sexes were statistically similar across the rest of the age categories. As regards forearm length and epiphyseal cartilage length, no differences between sexes were significant in any age category (P> 0.05); thus, data for both sexes were pooled together for subsequent analyses to increase the sample size.
Growth. Forearm length and body mass increased continuously over 55 days post-birth (Table 3). In the first 35 days, these variables increased linearly at a rate of 0.72 mm/day in length of and 0.07 g/day in body mass. After day 35, the growth rate of both variables decreased to 0.19 mm/day and 0.05 g/day, respectively (Figure 2a, b). The length of the epiphyseal gap increased over the first 25 days post-birth at 0.098 mm/day, and then closed at 0.075 mm/day (Table 3, Figure 2b).
Table 3 Postnatal growth of Natalus mexicanus inhabiting “Los Laguitos” cave, Chiapas, Mexico. For each age category, changes in body mass (grams), forearm length and length of the cartilaginous epiphyseal gap of the fourth metacarpal-phalangeal joint (millimeters) of the right wing are shown. For each measurement, the sample size (n) and mean ± standard deviation are shown.
| Age/days | n | Weight | Forearm | Epiphysis |
|---|---|---|---|---|
| 1 | 94 | 0.98 ± 0.02 | 10.71 ± 0.64 | 2.36 ± 0.02 |
| 5 | 33 | 1.63 ± 0.06 | 12.70 ± 0.15 | 2.96 ± 0.08 |
| 10 | 23 | 2.43 ± 0.13 | 16.22 ± 1.67 | 3.53 ± 0.08 |
| 15 | 24 | 2.85 ± 0.08 | 20.05 ± 2.02 | 4.07 ± 0.08 |
| 20 | 11 | 3.69 ± 0.19 | 24.60 ± 2.73 | 4.10 ± 0.17 |
| 25 | 14 | 4.20 ± 0.21 | 28.74 ± 3.05 | 4.44 ± 0.10 |
| 30 | 11 | 4.55 ± 0.19 | 31.22 ± 3.58 | 4.40 ± 0.13 |
| 35 | 12 | 5.08 ± 0.17 | 34.41 ± 1.23 | 4.05 ± 0.16 |
| 40 | 12 | 5.45 ± 0.20 | 36.90 ± 1.28 | 3.52 ± 0.12 |
| 45 | 3 | 5.40 ± 0.40 | 38.00 ± 0.42 | 3.60 ± 0.20 |
| 50 | 4 | 5.97 ± 0.25 | 38.30 ± 2.67 | 2.72 ± 0.30 |
| 55 | 3 | 6.16 ± 0.28 | 39.20 ± 0.30 | 1.93 ± 0.23 |
Growth models. Of the three models used to describe the forearm and body mass growth patterns, the logistic model yielded the best fit; therefore, the only equations shown below correspond to this model: forearm = 42.44 / (1 + 3.29-0.07 (age)), R 2 = 0.96; mass = 5.67 / (1 + 4.630.10 (age)), R 2 = 0.91. For its part, the growth pattern of the length of the epiphyseal gap fitted a second-order polynomial model (epiphyseal gap length = -0.0032x2 + 0.1678x + 2.2084, R 2 = 0.80; Figure 2a, b), which predicts the complete closure or calcification of the cartilaginous epiphysis between 63 and 64 days of age.
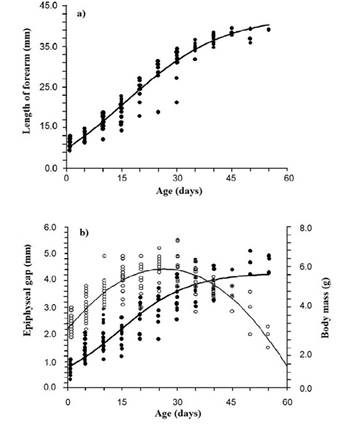
Figure 2 Growth trends of three morphometric variables evaluated in Natalus mexicanus from “Los Laguitos”, Chiapas, Mexico, in 2009. a) forearm length. b) length of the cartilaginous ephiphyseal gap of the metacarpal-phalangeal joint of the fourth finger and body mass. The length of forearm and epiphysis gap curves correspond to those generated by the logistic equation model, while the one for body mass corresponds to a polynomial model.
Flight development. At birth, 100 % of neonates were classified as non-volant. The first individual classified as a semi-volant was 20 days old, and the first to achieve sustained flight was 35 days old. At 50 and 55 days of age, all recaptured specimens were volant (Table 4). The number of specimens recaptured after day 1 post-birth decreased with time, because after marking, some pups were moved from the original perch site by the mother. Besides, as pups developed, they began moving through the walls and mingling with the rest of the population; finally, when they started flying, they flew away from the nursery as soon as they noticed our presence, so recapturing them became impossible.
Table 4 Flight development in Natalus mexicanus inhabiting “Los Laguitos” cave, Chiapas, Mexico. For each age category, the number of individuals in each flight development stage is given.
| Age (days) | Category | ||
|---|---|---|---|
| Non-volant | Semi-volant | Volant | |
| 1 | 94 | ||
| 5 | 33 | ||
| 10 | 23 | ||
| 15 | 24 | ||
| 20 | 10 | 1 | |
| 25 | 9 | 5 | |
| 30 | 5 | 6 | |
| 35 | 1 | 8 | 3 |
| 40 | 3 | 9 | |
| 45 | 1 | 2 | |
| 50 | 4 | ||
| 55 | 3 | ||
Discussion
Natalus mexicanus uses daytime shelters with temperatures ranging from 25.0 to 36.6 °C and RH above 74 % (Mitchell 1965; Torres-Flores and López-Wilchis 2010). These conditions occur all year round in “Los Laguitos”, maintaining a mean temperature of 32.3 °C and RH of 95.4 % (Martínez-Coronel et al. 2010) and were above average during the study period. The conditions inside the cave were favorable for the growth and development of pups because they are born naked and lose moisture easily (Mitchell 1965; Kunz and Hood 2000; Ruczynski 2006; Pretzlaff et al. 2010).
In “Los Laguitos” cave, the period of births of N. mexicanus lasted 25 days, from late May to late June; in Alamos, Sonora, it spans from late July to early August (Mitchell 1965), one month after it ended in the Chiapas population. These results differ from the findings by Tejedor (2019), who reported similar patterns in northern and southern populations. However, for a species with a wide geographic distribution such as N. mexicanus, with populations that do not migrate latitudinally, differences are likely to exist because of local adaptations to different conditions (Kunz and Hood 2000; Barclay and Harder 2003). Geographic differences in birth season, growth, and postnatal development have also been reported for Leptonycteris yerbabuenae and Miniopterus schreibersii (Gould 1975; Richardson 1977; Martínez-Coronel et al. 2014), species with wide distributions.
Bats give birth to altricial pups, although there are differences in the degree of development between species (Kunz and Hood 2000). Examples of neonates that are born with the body covered with hair and open eyes and auditory meatus include Rosettus leschenaulti and R. amplexicaudatus (Elangovan et al. 2002; Giannini et al. 2006), Artibeus sp., Carollia perspicillata, Desmodus rotundus, Diphylla ecaudata, Glossophaga sp., and Uroderma bilobatum (Kleiman and Davis 1979; Kunz and Hood 2000; Delpietro and Russo 2002; Cretekos et al. 2005; Reyes-Amaya and Jerez 2013; Kohles et al. 2018), Myotis albescens and Eumops patagonicus (Rodríguez et al. 2018). Most of these species thrive in tropical areas, use outdoor shelters, and neonates are carried around by their mothers during foraging from day one post-birth (Kleiman and Davis 1979; Hernández-Mijangos et al. 2009). However, other species such as Hipposideros speoris, Leptonycteris yerbabuenae, Myotis emarginatus, Rhinolophus mehelyi, and Tadarida brasiliensis (McCracken and Gustin 1991; Sharifi 2004; Martínez-Coronel et al. 2014; Doss et al. 2018; Eghbali and Sharifi 2018), give birth to completely naked pups that remain with the mother during the day, presumably because of their poor thermoregulation (Studier and O’Farrel 1972). Pups are left in nurseries during the night and are recovered by mothers upon returning from foraging to be breastfed (Martínez-Coronel et al. 2014). These data contrast with studies on a population of N. mexicanus in Sonora and on Pipistrellus pipistrellus (Mitchell 1965; Hughes et al. 1995). These studies reported that pups remained separate from adults during the day and were only recovered at night to be breastfed; the authors provided no explanation for this behavior.
In the population of N. mexicanus inhabiting “Los Laguitos”, Chiapas, pups are born with eyes closed and remain so for the first 15 days post-birth; eyes are completely open by day 25. On the other hand, in a population of the same species living in Alamos, Sonora (Mitchell 1965), eyes were fully open by day 17. The results of this study contrast with findings for some species of insectivorous bats, such as Myotis capaccinii, M. emarginatus, Pipistrellus mimus, Rhinolophus mehelyi, and Vespertilio sinensis, in which eyes open between four and eight days of age (Isaac and Marimuthu 1996; Jin et al. 2012 a, b; Eghbali and Sharifi 2018; Mehdizadeh et al. 2018), whereas in Hipposideros pomona eyes open completely until day 13 post-birth (Lin et al. 2011). Eye opening in the population of N. mexicanus studied occurs later, possibly because pups are born in a less advanced development stage compared to the other species. Likewise, the development of the fur of N. mexicanus in “Los Laguitos” follows a pattern like that of the eyes. The body remains apparently naked during the first 15 days post-birth, with long hairs evident only in the genital area and rostrum. A short, grayish fur is visible until 30 days of age, while a long, dark fur appears between 35 and 40 days old. In contrast, Mitchell (1965) reported that in the population of Sonora, the body of pups was already covered with a short fur by day 17 post-birth. In Thyroptera tricolor and V. sinensis, a grayish fur appears at 21 and 16 days of age, respectively (Chaverri and Vonhoff 2011; Jin et al. 2012 b), and in M. emarginatus between 25 to 30 days (Eghbali and Sharifi 2018). These data indicate that N. mexicanus is a species in which eye opening and fur development take longer.
Bats give birth to relatively large neonates compared to terrestrial mammals of the same size. This reduces the risk of dying from sudden changes in environmental temperature, relative humidity, and predation, as bat pups require less investment in parental care before reaching independence (Racey and Entwistle 2000). In N. mexicanus, the body mass of neonates is 18.23 % relative to adults, a value that is below the average of 23.0 ± 8.0 % reported for Chiroptera (Barclay and Harder 2003). In other bat species, neonate body mass is equivalent to 20.7 % of adult biomass in Phyllostomus hastatus (Stern and Kunz 1998), 25 % in Tadarida brasiliensis (Kunz and Robson 1995); 26 % in T. tricolor (Chaverri and Vonhoff 2011), 28 % in Carollia perspicillata (Kleiman and Davis 1979) and M. lucifugus (O´Farrell and Studier 1973), 39 % in H. pomona, 40 % in M. capaccini (Mehdizadeh et al. 2018), and 56 % in Artibeus watsoni (Chaverri and Kunz 2006).
The secondary sexual dimorphism in bats is variable; in most species, females tend to be larger than males (Ralls 1977; Hurtado et al. 2015; Ruelas 2019). In the case of small-sized species, when females are larger than males, this is a consequence of their reproductive role. During nursing, females take care of pups from conception to weaning; therefore, they must store energy during the first weeks of gestation, since by the end of pregnancy the amount of food consumed decreases because of the space occupied by the fetus (De Camargo and Oliveira 2013; Barclay and Harder 2003). Besides, carrying the fetus or pup during foraging implies a greater winged load; consequently, wings tend to be larger to improve flight performance (De Camargo and Oliveira 2013; Hurtado et al. 2015; Stevens and Platt 2015). When males are larger than females, these differences are explained as related to sexual competition (Ralls 1977). In N. mexicanus living in “Los Laguitos” cave, of the three morphometric variables analyzed, we only found significant differences in neonate body mass, with males being significantly larger than females. However, no evidence of secondary sexual dimorphism was found in the other variables at any age categories. These results are consistent with those of Tejedor (2011), who found that in adults of N. mexicanus the sexes are similar in forearm length and body mass.
The pattern of forearm growth, body mass, and epiphyseal gap in N. mexicanus was like that of Hipposideros cineraceus, M. capaccinii, T. brasiliensis, and T. tricolor (Kunz and Robson 1995; Jin et al. 2010; Chaverri and Vonhoff 2011; Mehdizadeh et al. 2018). In the first two variables, growth was linear in the period prior to sustained flight, which started at 35 days of age, and the growth rate decreased thereafter. Similar results were observed in the population of Alamos, Sonora (Mitchell 1965). During flight, wing fingers are subjected to stress; thus, phalanges must be sufficiently resistant, and the wing membrane must have a minimum area, for flapping to produce the aerodynamic force necessary to sustain the body and move it through air (Hughes et al. 1995; Stern et al. 1997; Elangovan et al. 2002). Therefore, phalanges were expected to experience accelerated growth before the onset of sustained flight, while the cartilaginous areas of the epiphyses undergo increasing calcification to avoid dislocations. In N. mexicanus, the epiphyseal gap reached its maximum size at 25 days of age, closing in the following days before sustained flight was achieved, as in other species (Stern and Kunz 1998; Lin et al. 2010; Jin et al. 2012a, b; Mehdizadeh et al. 2018). The growth model obtained predicted that the epiphysis gap should close completely at day 64. However, this prediction could not be corroborated in the present study, since specimens could no longer be recaptured after 55 days post-birth because they mingled with the rest of the population, which included several thousands of individuals. The epiphyseal gap closure time was intermediate relative to other insectivorous species such as Pipistrelus subflavus (6.6 g), with closure at 45 days (Hoying and Kunz 1998); R. mehelyi (14 g), at 55 days (Sharifi 2004); and R. hipposideros (8 g), at 80 days (Reiter 2004).
As mentioned above, body mass increased in the period prior to sustained flight and no decrease was observed during the learning process, as reported for other bat species (Kunz 1973; Hughes et al. 1995; Hoying and Kunz 1998; Lin et al. 2010). Hoying and Kunz (1998) suggest that the decrease in body mass during this stage may be a consequence of the energy demand required by flight. However, Hughes et al. (1995) mention that it may be a strategy of the species, since when young bats start flying, their muscles still do not produce sufficient force and thus the wing load is greater; hence, reducing body mass facilitates learning to perform the necessary flight maneuvers. To note, these theories are not entirely satisfactory, as more than half of the species studied do not undergo a decrease in body mass during this stage; therefore, further research is needed (Hoying and Kunz 1998; Lin et al. 2010).
Of the three models used to describe the increase in body mass and forearm length in N. mexicanus, the logistic model best fitted the empirical data, as reported elsewhere (Hoying and Kunz 1998; Lin et al. 2010; Mehdizadeh et al. 2018). In N. mexicanus, the growth constants of body mass and forearm length were 0.10 and 0.07, respectively. These values indicate a lower growth rate relative to other tropical insectivorous species such as H. cineraceus, with weight and forearm values of 0.12 and 0.10, respectively, or H. larvatus, with 0.12 and 0.13, respectively (Jin et al. 2010; Lin et al. 2010). Also, Kunz and Hood (2000) and Lin et al. (2010) mention that although it is valid to compare growth rates between species, it should be noted that some variables affect growth rate, such as temperature, food availability, maternal size, and colony size, which cannot be controlled by the researcher, especially in field studies.
Regardless of the degree of development of the eyes and fur in neonates, bats are altricial before flight and depend entirely on the mother during the first postnatal days (Kunz and Hood 2000). In most species, sustained flight begins when pups attain a relative mass above 70 % and a forearm length of more than 90 % in relation to adults (Barclay 1994; Stern and Kunz 1998). There are exceptions, as the case of L. yerbabuenae, which attains sustained flight when body mass is 50 % and forearm length is 84 % relative to adults (Martínez-Coronel et al. 2014), while R. leschenautti attains it with 35 % body mass and 75 % forearm length (Elangovan et al. 2002). In the present study, we found that N. mexicanus displayed sustained flight at 35 days of age, when body mass was 86.38 % and forearm length was 95.35 % relative to adults. These values are above those recorded for other insectivorous species such as M. capaccinii, which achieves flight at day 28, with a weight of 82.60 % and forearm length of 90.31 % (Mehdizadeh et al. 2018); P. mimus at day 29, with a weight of about 80 % (Isaac and Marimuthu 1996); H. larvatus at day 24, with a weight of 61.4 % and forearm length of 92.4 % (Lin et al. 2010); R. mehelyi at day 28; with a weight of 92 % and forearm length of 87 % (Sharifi 2004). In contrast, in T. tricolor the onset of flight occurs up to day 60, with a weight of 77 % and forearm length of 95 % (Chaverri and Vonhoff 2011); and in Artibeus watsoni flight starts at day 35, when forearm length reaches 100 % and weight is 80 % relative to adults (Chaverri and Kunz 2006). Except for pteropodids, these results suggest that there is no trend regarding the age at onset of flight in the different species and families studied in temperate and tropical areas, nor between insectivorous and frugivorous bats (Kunz and Hood 2000; Lin et al. 2010).











 nova página do texto(beta)
nova página do texto(beta)


