Introduction
The external morphology of the glans and the baculum (os priapi) contains important characters for specific and sub-specific delimitation (Rocha-Barbosa et al. 2013), especially in rodents (Lidicker 1968; Bradley and Schmidly 1989; Sportono 1979; Simson et al. 1995; Calderón-Capote et al. 2016). However, there are few descriptive studies on the external genitalia morphology of hystricomorph rodents (Tullberg 1899; Pocock 1922; Dathe 1937; Hooper 1961, Atalar and Ceribasi 2006; Adebayo et al. 2011), a clade that includes the New World caviomorphs (e. g., New World porcupines, agoutis, acouchis, pacas and Guiñean pigs; sensu Upham and Patterson 2015). The New World porcupines of the genus Coendou are distributed on Central and South America (Voss 2011) and represents one of the less studied rodent groups in the Neotropics (Alberico et al. 2000). Due to the scarcity of specimens available for comparisons, the taxonomic status of many species within the genus is still under discussion (see Voss 2011; Voss et al. 2013; Ramírez-Chaves et al. 2016). Furthermore, information on Coendou genitalia is limited; indeed, there is only one brief published description of the male genitalia of an indeterminate species (reffered as Coendou novae-hispaniae) by Pocock (1922). Considering the scarcity of information about these structures in porcupines -and in general in caviomorph rodents- and the possibility to use this information to solve taxonomical problems, in this work we described the morphology of the glans and the baculum of the Quichua porcupine (Coendou quichua).
Materials and methods
The description is based on one adult specimen collected at the "Finca el Diviso", vereda La Colorada, municipality of San Vicente de Chucurí, Santander, Colombia (6° 47' 38.27'' N, -73° 28' 48.23'' W; 1,400 masl). The specimen was deposited in the mammalian collection of the Universidad Industrial de Santander (UIS-MHN-M-945). The penis was dissected and fixed in 5 % formaldehyde for one day and later preserved in 95 % ethanol. The baculum was extracted from the glans, and cleared in 5 % potassium hydroxide following Wassersug (1976). Both penis and baculum were photographed. Eight measurements were taken using a dial caliper to the nearest 0.001, following Hooper (1961) and Adebayo et al. (2011), and include: total glans length (TGL), glans head width (GHW), glans base width (GBW), total bacular length (TBL), baculum head width (BHW), baculum base width (BBW), sacculus urethralis spikes length (SusL), and sacculus urethralis length (SUL). In addition, the proportion of the total bacular length / total glans length (TBL / TGL), sacculus urethralis spikes length / sacculus urehtra-lis length (SusL / SUL) and sacculus urethralis length / total glans length (SUL / TGL), were calculated. For the glans, all measurements and photographs were taken before bacu-lum extraction. The nomenclature for the description of both glans and baculum follows previous works (Pocock 1922; Hooper 1961; Adebayo et al. 2011).
The glans and baculum were compared with the description of these structures in two others porcupines available in literature (Pocock 1922; Hooper 1961), the North American porcupine, Erethizon dorsatum, and the Malayan porcupine, Hystrix brachyura, and three additional Neotropical caviomorph rodents (Hooper 1961): Cuniculus paca, Dasy-procta punctata, and Cavia tschudii.
Results
The glans length of C. quichua is almost twice the glans head width (Table 1) and comprises the third part of the total penis length (41.2 mm). The glans is cylindrical, with the head wider than the base. The glans exhibits a yellow cream coloration, and as in others hystricomorphs with a notably tenuous dark coloration of the head (Figure 1A), observed pre and post formaldehyde fixation. Another remarkably characteristic of the glans is the presence of small tegumentary protuberances formed by 3-4 small spines (Figure 1D-E). The protuberances are densely distributed in the head of the glans, but scarcely in the base (Figure 1A). At the tip of the glans below to the urethra, there is an invagination called sacculus urethralis (SU). This SU has longitudinally corrugated walls and two developed spikes at the bottom, and as the rest of the glans, presents spiny tegumentary protuberances (Figure 1C). The baculum is as large as the C. quichua glans (Table 1), dorsally concave and ventrally convex, and have a distal end or head, a medial region or shaft and a proximal end or base. Both ends are wider than the shaft (Table 1), especially the base which is wider than head. Also, the thin shaft becomes wider and robust close to its base (Figure 1B).
Table 1 Glans and baculum measurements (in mm). TGL: Total glans length, GHW: Glans head width, GBW: Glans base width, TBL: Total bacular length, BHW: Baculum head width, BBW: Baculum base width, SUsL: Sacculus urethralis spikes length, SUL: Sacculus urethralis Length. There is no information of any measurement for H. brachyura.
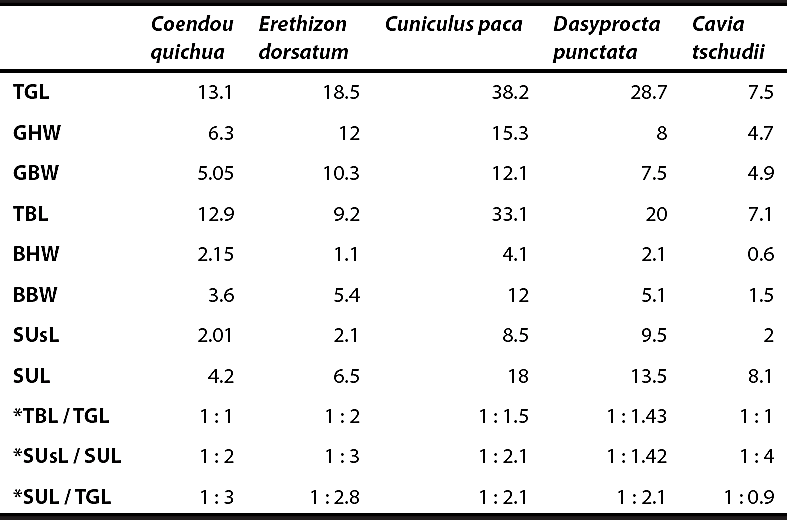
* Proportion (e. g. *TBL/ TGL: Proportion of the total bacular length / total glans length). Measurements of additional species taken from Hooper (1961).
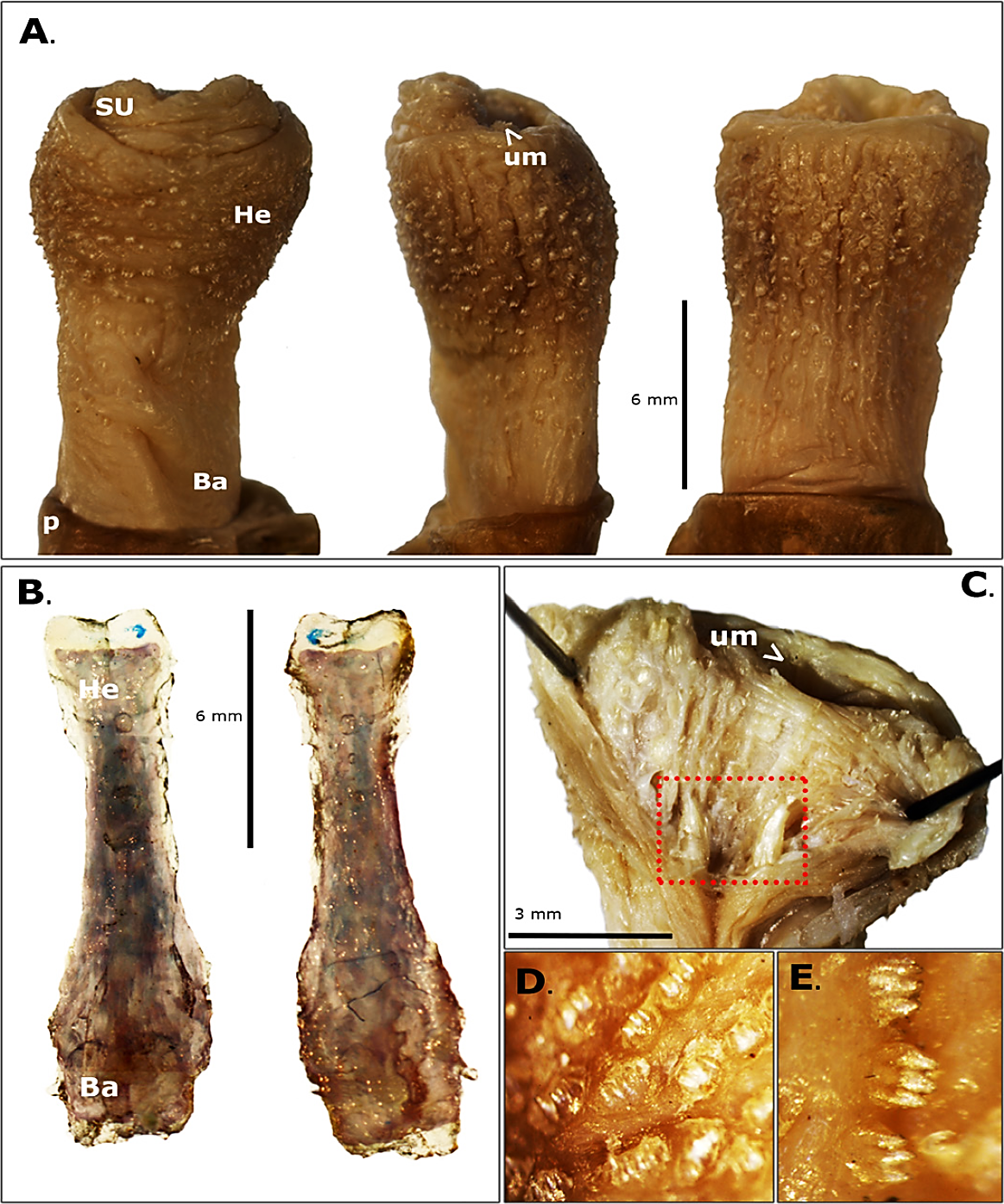
Figure 1 External view of the (A): penis glans, dorsal (left), lateral (middle) and ventral (right) views. SU: sacculus urethralis. He: head. Ba: base. p: prepuce. um: urinary meatus. (B): External view of the baculum, dorsal (left) and ventral (right) views. (C): Ventral internal view of the sacculus urethrlis and the two spikes (red rectangle). (D-E): Close up of the external tegumentary spiny protuberances.
Discussion
The glans shape (cylindrical with a round tip and exhibiting tegumentary protuberances) in C. quichua is similar to three other Neotropical caviomorphs; however, differences are found in the tegumentary protuberances. In C. quichua, E. dorsatum, and Cuniculus paca, the glans exhibit tegumentary protuberances compound by a single spine (Figure 2A), with variable size in the latter. Furthermore, Cuniculus paca presents a notably pair of large dorsolateral multi-toothed rasp (Figure 2D) which are absent in the other species. In D. punctata and Cavia tschudii, the protuberances are more complex, transversally oriented, and with a blade-like shape, forming a continuous serrate line (Figure 2B-C). In addition, in Cavia tschudii those serrate lines are larger than in D. punctata, and are distributed only in specific places of the glans (Figure 2B). As in other hystricomorph rodents (Tullberg 1899; Pocock 1922; Dathe 1937; Hooper 1961; Atalar and Ceribasi 2006; Adebayo et al. 2011), C. quichua presents a sacculus urethralis (SU; Figure 1A-C), an invagination at the tip of the glans below to the urethra, that is everted during erection (Layne 1960; Contreras et al. 1993). When comparing E. dorsatum with C. quichua, the SU has smooth and not corrugated walls in the former, lacking of spiny protuberances (Figure 2A). Although the SUL / TGL proportion remains the same for both species, C. quichua has longer spikes compared with the length of the SU (Table 1). For the three additional Neotropical caviomorphs compared, the SU is larger when contrasted with the length of the glans, especially for Cavia tschudii (Table 1), which differentiate it from C. quichua. Also, C. quichua and Cuniculus paca have similar-sized spikes (compared with the SU length) which are longer than in Cavia tschudii, but are shorter than in D. punctata (Table 1; Figure 2). Finally, as in C. quichua, the walls of the SU in all three Neotropical caviomorph are corrugated with the presence of tegumentary protuberances (Figure 2B-C).
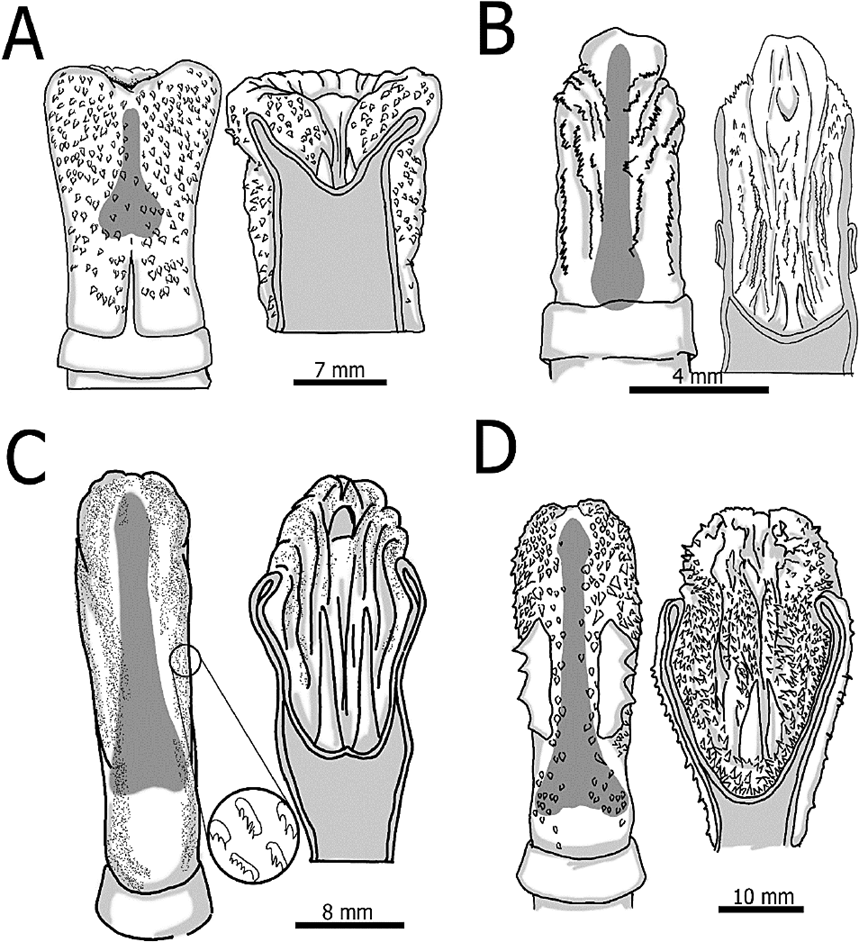
Figure 2 Glans (left), sacculus urethralis (right) and baculum (in gray) for the four species compared: Erethizon dorsatum (A), Cavia tschudii (B), Dasyproctapunctata (C) and Cuniculus paca (D), modified from Hooper (1961).
The baculum is one of the most varying structure among mammal species. The general bacular form in C. quichua (head and base wider than the shaft, and the base wider than the head; Figure 2C-D), is similar to the one presented in D. punctata and Cuniculus paca. In contrast, in E. dorsatum and Cavia tschudii, only the base of the baculum is wider, while the shaft and head are thinner and have the same width (Hooper 1961). The tip of the head is round in E. dorsatum and the Neotropical caviomorph (Figure 2), but in C. quichua it is flat (Figure 1B). This character seems to be unique for Coendou. In contrast, the base of the baculum in C. quichua is round (Figure 1B), similar to the one observed in Cavia tschudii, whereas for E. dorsatum, D. punctata and Cuniculus paca, the base is flattened with two remarkably lobes at both sides (Hooper 1961; Figure 2).
The comparison of the baculum between C. quichua and H. brachyura is limited because the brief description of this structure in the latter, which only includes the glans. The glans for both species are similar, being the only difference the bottom of the SU, near to the spikes, which in H. brachyura is smooth, without spiny protuberances (see Pocock 1922).
Although the family Erethizontidae is more closely related to the Cavioidea than to Hystricidae (Huchon and Douzery 2001; Blanga-Kanfi et al. 2009; Antoine et al. 2011), the glans of individuals of the family Erethizontidae are more similar to these of Hystricidae. Dathe (1937) suggested that glans with a heavy armature (tegumentary protuberances) and a deep sacculus urethralis were characteristics of primitive hystricomorphs; however, Erethizontidae is more basal than the Cavioidea, and Hystricidae is the basal group from the Hystricognathi (see Blanga-Kanfi et al. 2009). Due to the similarity in the glans between Erethizontidae and Hystricidae, glans with a slight armature (simply tegumentary protuberances) and a non-deep sacculus urethalis (compared with the total glans length) should be characteristics of primitive hystricomorphs. Whereas, for the baculum, this structure shows no general pattern for all species, and it seems to be characteristic for each genus, especially for C. quichua. In contrast, the baculum of the Hystricomorpha compared are clearly different from other rodents, such as Proechymis (see Hooper 1961), Marmota and Citellus (see Burt 1960), and several Sigmodontinae rodents (see Calderón-Capote et al. 2016). In addition, for these species, both glans and baculum exhibit important characters that might be used for taxonomic identification, such as the tegumentary protuberances, SU depth, or the baculum head shape. However, bacular differences within Coendou are still unknown, therefore it is necessary the description of the genitalia of additional species.











 nueva página del texto (beta)
nueva página del texto (beta)


