Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias forestales
versión impresa ISSN 2007-1132
Rev. mex. de cienc. forestales vol.9 no.50 México nov./dic. 2018
https://doi.org/10.29298/rmcf.v9i50.248
Articles
Germination of two Acacia species at elevated temperatures simulating climate change
1 Facultad de Ciencias Forestales. Universidad Autónoma de Nuevo León. México.
2 División de Ciencias Ambientales - IPICYT, México.
Temperature increase can affect germination in a positive or negative way. A temperature increment of 0.6 °C occurred during the last decades, and by 2099 an increment of 1.5 to 4.7 °C is expected. The aim in this study was to evaluate seed germination from certain provenances of Acacia farnesiana and A. schaffneri at different temperatures. The information generated thereby will make it possible to determine the ability of these two species to adapt to global warming. Fruits were collected from at least 10 mother plants per provenance per species; seeds were scarified and sown in soil in plastic trays, placed inside an incubator at actual soil temperature (Control (T0)), and subjected to 5 treatments with increased temperature: Control temperature +2 °C (T1), +5 °C (T2), +7 °C (T3), +10 °C (T4) and +15 °C (T5) for 15 days. A. farnesiana showed higher germination at T2 and T3, while A. schaffneri presented its higher germination at T2; there were not significant differences between the two species. A. farnesiana seeds had the fastest germination (t50) at T3, with significant differences between provenances (the fastest germination occurred at the School of Forest Sciences, UANL), and A. schaffneri seeds germinated fastest at T1, without significant differences between provenances. The results suggest that both species and their provenances can germinate if temperatures increase according to the current predictions.
Key words: Acacia farnesiana (Linn.) Willd.; Acacia schaffneri (L.) Willd.; global warming; germination rate; provenances; germination speed
El incremento en las temperaturas puede afectar de forma positiva o negativa la germinación. Se estima que la temperatura se incrementó en las últimas décadas 0.6 °C y se espera que para el año 2099 aumente entre 1.5 y 4.7 °C. El objetivo del presente trabajo fue evaluar la germinación de semillas de siete procedencias de Acacia farnesiana y tres de A. schaffneri a diferentes temperaturas. La información generada permitirá conocer la respuesta de ambas especies ante el incremento de las temperaturas. Se recolectaron frutos de al menos 10 plantas madre/por procedencia/taxón, se escarificaron las semillas, sembraron en charolas de plástico y se colocaron en una germinadora a temperatura de suelo real (Testigo (T0)), y cinco tratamientos con aumento de temperatura: testigo +2 °C (T1), +5 °C (T2), +7 °C (T3), +10 °C (T4) y +15 °C (T5), durante 15 días. A. farnesiana presentó un mayor porcentaje de germinación en el T2 y T3; mientras que A. schaffneri en el T2; no hubo diferencias significativas entre las procedencias de cada una de las especies. A. farnesiana germinó con mayor rapidez (t50) en el T3, con diferencias significativas entre procedencias (mayor velocidad FCF) y A. schaffneri en el T1, sin diferencias significativas entre sus procedencias. Los resultados sugieren que ambos taxa y sus procedencias tienen la capacidad de germinar si las temperaturas aumentan conforme a los pronósticos.
Palabras clave: Acacia farnesiana (Linn.) Willd.; Acacia schaffneri (L.) Willd.; calentamiento global; porcentajes de germinación; procedencias; velocidad de germinación
Introduction
The increase in temperature may affect the germination processes of seeds (Mann et al., 1998; Hughes, 2000). It is worth noting it may have a positive effect in temperate areas, but not necessarily in warmer areas (Fenner and Thompson, 2005). The mean surface temperature of the earth increased by an average of 0.6 °C in the XXth century, and by the year 2099 it is expected to increase by 1.5 to 4.7 °C (Mann et al., 1998; Solomon et al., 2007; Field et al., 2014). According to certain climate change related scenarios, temperatures are moving from the equator toward the poles and from lower to higher altitudes (Jurado et al., 2011; Loarie et al., 2009; Solomon et al., 2007); for this reason, a displacement of species in the same direction is expected to occur (Root et al., 2003; Parmesan and Yohe, 2003; Peñuelas et al., 2007).
Seed germination in areas outside the distribution boundaries of each taxon is the main promoting factor of the displacement of taxa (Holtmeier and Broll, 2005; Fenner and Thompson, 2005). Other authors point out the presence of displacement of the vegetation toward higher altitudes (Danby and Hik, 2007; Pepin et al., 2015). The main factors that exert a direct influence on germination are water availability, adequate temperature, and soil airing (Baskin and Baskin, 2001; Benech-Arnold and Sánchez, 2004).
Anomalies in the climate, such as extraordinary or out-of-season rainfalls, or droughts, among others, may annul the vegetation dynamic processes, especially the germination of seeds (Savage et al., 1996). A relationship has been observed to exist between the increase in temperatures and the displacement of species that might cause great changes in the distribution and abundance of the latter (Root et al., 2003; Parmesan and Yohe, 2003).
The species richness grew in low areas of the Alps, perhaps due to the displacement of taxa of the vegetation type below the higher altitudes (Grabherr et al., 1994). Temperature changes which occur during the day reflect the natural conditions better and are more favorable to germination than constant temperatures (Silva and Aguiar, 2004).
Acacia farnesiana (L.) Willd and A. schaffneri (S. Watson) F. J. Herm. have economic and ecological importance, exhibit resistance to high temperatures and droughts, and are widely used as fuel, as fodder for livestock, and as a source of essential oils and tanning agents (García-Winder et al., 2009). A. farnesiana has a broad distribution interval; it grows in the American continent, from Argentina to the south of the United States of America, as well as in Africa, Australia and certain parts of Europe (Arévalo et al., 2010); while A. schaffneri has a less broad distribution; it occurs from northern Oaxaca to southern Texas, and does not show an invasive behavior (Valiente-Banuet et al., 2000). A. farnesiana is regarded as an invasive species (Arévalo et al., 2010; Cavalcante and Cox, 2016).
The distribution of species, which can be wide or scarce, depends, to a great extent, on their ability to germinate at a certain temperature interval (Fenner and Thompson, 2005). The climate conditions of different provenances of the seeds can influence the temperature requirements for germination of a particular species (Mondoni et al, 2008). Thus, in addition to the economic and ecological importance of A. farnesiana and A. shaffneri, the contrasting natural distribution suggests a differential adaptive ability, which renders them useful as study models.
Certain studies on germination, like that on A. polyphylla DC. seeds, have determined that temperature increases have no effect on the germination percentage but result in a higher germination speed (Neto et al., 2003), while A. shaffneri and other seeds from the desert of Chihuahua, like Prosopis laevigata (Humb. & Bonpl. ex Willd.) M.C. Johnst., Yucca decipiens Trel., and certain succulents had the highest germination percentage and the highest germination speed (Pérez-Sánchez et al., 2011). When a species exhibits a lower germination speed, the likelihood of the establishment of the seeds in the face of a climate change scenario diminishes, and this in turn affects the distribution of that species (Pérez-Sánchez et al., 2011).
It is essential to know what relationships there are between the increase in the temperatures and its consequences on seed germination, in order to determine the potential displacements of species (Smith et al., 2009) and, thus, develop regeneration or reforestation plans. This study assessed the germination of A. farnesiana seeds from seven provenances and A. schaffneri seeds from three provenances, at different temperatures. Higher germination percentages are expected at higher temperatures and from provenances located at higher altitudes.
Materials and Methods
Ripe fruits were harvested from 10 parent trees per species per provenance, in order to prevent bias due to genetic variation. The work was carried out in the summer of 2011, in nine localities in the country, in order to include the genetic diversity and determine whether or not there are differences between the provenances (Table 1).
Table 1 Altitude, temperature and precipitation of the provenances of Acacia farnesiana (L.) Willd. and Acacia schaffneri (L.) Willd. seeds, as well as of the vegetation type occurring in the harvesting area (SMN, 2011; Inegi, 2016).
| Species | Provenance | State | Altitude masl | Coordinates | Temperature in September (°C) | Precipitation in September (mm) | Vegetation type |
|---|---|---|---|---|---|---|---|
| Acacia farnesiana | School of Forest Sciences | Nuevo León | 350 | 24°57’33” N 99°32’30” O | Average: 22.8 Max: Sep 32.8 Min: Sep 20.6 | 174.6 | Tamaulipan Thornscrub |
| Linares-Iturbide road | Nuevo León | 450 | 24°46’51” N 99°40’03” O | Average: 21.3 Max: 30.4 Min: 18.1 | 260.8 | Submontane scrub | |
| Los Ángeles ejido | Nuevo León | 550 | 24°47’33” N 99°49’20” O | Average: 22.1 Max: 31.7 Mn: 20.2 | 211.6 | Submontane scrub: | |
| Agua de la Mula ejido | Coahuila | 1 250 | 25°29’33” N 101°23’49” O | Average: 27.0 Max: 28.7 Min: 15.1 | 59.2 | Rosetophile desert scrub | |
| Puebla ejido | Coahuila | 1 430 | 25°25’52” N 101°17’53” O | Average: 27.0 Max: 28.7 Min:15.1 | 59.2 | Rosetophile desert scrub and Microphile desert scrub | |
| 5 de Mayo ejido | Coahuila | 1 700 | 25°23’18” N 101°15’54” O | Promedio: 27.0 Máx: 28.7 Mín: 15.1 | 59.2 | Rosetophile desert scrub | |
| Chiapa de Corzo | Chiapas | 1 880 | 16°47’15” N 92°51’07” O | Average: 16.7 Max: 25.4 Min: 12.0 | 86.9 | Low deciduous forest | |
| Acacia schaffneri | Nazas | Durango | 1 735 | 25°03’33.9” N 104°13’16” O | Average: 17.8 Max: 29.6 Min: 10.6 | 80.8 | Submontane scrub and Rosetophile desert scrub |
| Capital | San Luis Potosí | 1 800 | 22°08’46” N 100°52’42” O | Average: 22.0 Max: 29.8 Min: 17.9 | 99.2 | Microphile desert scrub | |
| School of Forest Sciences | Nuevo León | 350 | 24°57’33” N 99°32’30” O | Average: 22.8 Max: Sep 32.8 Min: Sep 20.6 | 174.6 | Tamaulipan thornscrub |
Seeds were harvested from ripe, healthy pods free of damage or boreholes. The seeds were then manually scarified using a file to remove part of their tegument (Flores and Jurado, 1998; Rodrigues et al., 2016), as they exhibited physical latency (Martínez-Pérez et al., 2006). The seeds were germinated in a LumistellTM seed geminator, in polyethylene trays with 288 cavities, each with a capacity of 10.29 mL. The substrate consisted of earth from the Tamaulipan thornscrub (60 %), peat moss (30 %) and vermiculite (10 %). Two seeds were sown in each cavity of the trays, and the treatments were applied in six blocks, each with four repetitions per species/provenance, adding up to a total of 48 seeds per species/provenance.
Temperatures occurring during the day were calculated based on hourly data of soil temperatures present in September (the month with the most abundant precipitation) at the weather station closest to the work area (WCC, 2011). Soil temperatures per hour per day from 8:00 am to 5:00 pm were used to estimate the mean temperature, regarded as the control treatment (CT).
The increase in the temperatures was simulated with five treatments (T1 to T5), adding 2, 5, 7, 10 and 15 °C, respectively, to the temperatures of the control. The changes were made manually during the assay (15 days). The response variables were: I) germination percentage, and II) germination speed or t 50 , i.e. the time at which 50 % of the total number of seeds germinated (Coolbear et al., 1984).
They were analyzed using the free R-Project 2.11.0 statistical software (R-project, 2010). Kolmogorov-Smirnov tests were conducted for the normality of the data (abnormal data were transformed with of the arc sine), and Flinger’s tests were carried out to determine the homogeneity of variances. A Kruskal Wallis test was applied to those data did not exhibit normality. The design utilized was a nested variance analysis; the factors were provenance and temperature, and germination was the response variable. Furthermore, Tukey’s tests were conducted in order to determine differences between the provenances of the seeds, the treatments, and the interactions between these and the seed provenances.
Results and Discussion
Germination percentages
The germination percentage of A. farnesiana seeds was higher with treatments T2 and T3 (temperature of the control treatment+2 and +7 °C), i.e. of 93 to 100 %, while treatments T4 and T5 yielded lower values (d.f. =5, PChisq= < 0.001). The germination percentages were similar between provenances (d.f. = 6, PChisq=0.49), (Table 2), 72 and 99 %.
Table 2 Comparison between the germination percentages of the different treatments for each of the provenances of Acacia farnesiana (Linn.) Willd.
| Provenances | Kruskal Wallis | ||||||
|---|---|---|---|---|---|---|---|
| Treatments | |||||||
| T0 | T1 | T2 | T3 | T4 | T5 | Total | |
| FCF | 95.8 ±2.88 ab | 100 ± 0 a | 100 ± 0 a | 95.8 ± 2.88ab | 66.6 ± 5.51c | 75.0± 4.63 bc | 88.8±4.41 a |
| Los Ángeles | 95.8 ± 2.88 ab | 75.0 ± 5.64ab | 100 ± 0 a | 91.6 ± 4.0 abc | 75.0± 4.6 ab | 70.8 ± 3.9 c | 84.7±4.49a |
| Linares road | 91.6 ± 3.1ab | 54.1 ± 6.6c | 100 ± 0 a | 100 ± 0 a | 50.0± 3.6 c | 79.1 ± 2.88bc | 79.1±5.19a |
| Agua ejido | 79.1 ± 4.99ab | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 62.5 ± 5.33bc | 50.0 ± 3.69 c | 81.9±5.0 a |
| 5 de Mayo ejido | 79.1 ± 4.99ab | 66.6 ± 4.85bc | 100 ± 0 a | 95.8 ± 2.88a | 54.1 ± 3.9 c | 66.6 ± 4.85bc | 77.0±4.84 a |
| Puebla ejido | 79.1 ± 3.99 b | 91.6 ± 3.1 ab | 100 ± 0 a | 87.5 ± 3.9 ab | 70.8±3.5bc | 50.0 ± 4.6 c | 79.8±4.56a |
| Chiapa de C. | 75.0 ± 3.09c | 95.8 ± 2.8 ab | 100 ± 0 a | 83.3 ± 3.6 abc | 83.3 ± 4.8abc | 70.8 ± 5.3bc | 84.7±4.28a |
| Total | 85.1±4.06 c | 83.3±5.14 bc | 100 ± 0a | 93.4±3.37b | 66.0±4.78 c | 66.0±4.47 c | |
FCF = School of Forest Sciences. The mean of the germination percentage is accompanied by the standard error. Different letters indicate differences (p<0.05) between treatments and provenances.
The comparison between treatments for all provenances was P < 0.05 (Kruskal-Walis), with the highest germination percentages for treatment T2 (temperature of the control treatment +5 °C); treatments T4 and T5 exhibited lower figures, except for the Chiapa de Corzo provenance with T4.
The treatment with the highest germination percentage for A. schaffneri was T2 (temperature of the control treatment +5 °C), with a mean of 89 %, and the treatment with the lowest percentage was T5, with 38 % (F= 18.43, d.f. = 5, p= <0.001) (Table 3). The seed germination was similar in the comparison between the provenances, with averages of 61 and 57 % (F= 1.15, d.f. = 2, p= 0.323). The interactions between provenances and treatments were significant, with different results for the Nazas provenance, with respect to the other two provenances for most of the treatments (Figure 1). Differences were also estimated for the FCF provenance, which had the highest germination percentage with treatment T4 (F= 0.09, d.f. = 10, p= <0.001).
Table 3 Comparison between the germination percentages of the different treatments for each of the provenances of Acacia schaffneri (L.) Willd.
| Provenances | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| Treatments | |||||||
| T0 | T1 | T2 | T3 | T4 | T5 | Totals | |
| FCF | 33.3 ± 6.8cd | 70.8 ± 7.9bc | 100 ± 0.0a | 33.3 ± 6.8cd | 58.3 ± 8.3bcd | 50. ± 6.8bcd | 57.6±5.4a |
| Capital | 58.3 ±14.4bc | 79.1 ±10.4 ab | 100 ± 0.0a | 66.6 ± 6.8bcd | 50 ± 13.6bcd | 20.8 ± 7.9 d | 62.4±6.2a |
| Nazas | 75.0 ± 4.8 bc | 45.8 ± 10.4bcd | 75.0 ± 4.8 abc | 79.1 ± 4.1abc | 45.8 ± 12.5bcd | 33.3 ± 11. cd | 59.0±4.9a |
| Total | 55.5±7.2bc | 65.2±6.6b | 91.6±3.8a | 59.7±6.6bc | 51.3±6.3bc | 34.7±5.9c | |
FCF = School of Forest Sciences. The mean of the germination percentage is accompanied by the standard error. Different letters indicate differences (p<0.05) between treatments and provenances.
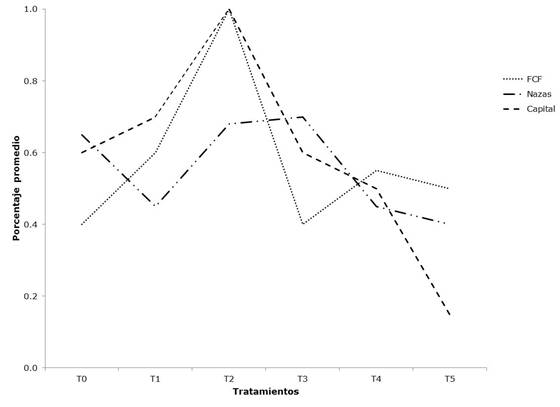
Tratamiento = Treatment; Porcentaje promedio = Mean percentage.
X axis shows the control treatment T0 and the five treatments; axis Y shows the seed germination percentage.
Figure 1 Interaction graphs of the germination percentage between treatments and provenances of Acacia schaffneri (L.) Willd.
The results for both species are similar to those documented for Erythrina verna Vell. and Albizia lebbeck (L.) Benth. (Demuner et al., 2008; Dutra et al., 2008), in which the increase in temperature causes an increase in the germination percentages, as was the case with treatments T2 and T3 of this study. Whereas extreme temperatures result in a reduction of the germination percentages, as documented by Silva and Aguiar (2004) for Cnidosculus phyullacanthus Pohl. Other studies show no differences in the germination percentages due to an increase in the temperatures for Colubrina glandulosa Perkins, Stryphnodendron adstringens (Mart.) Coville and Acacia polyphylla DC. (Albuquerque et al., 1998; Neto et al., 2003; Martins et al., 2008).
According to Pérez-Sánchez et al. (2011), A. schaffneri seeds subjected to high temperatures registered even higher germination percentages than the control treatments; however, their germination was slower, conversely to the results documented in this work, which show an increase in the germination speed with the increased temperature. Despite the varied environmental conditions of the considered provenances, no differences were found between them.
Germination speed (t 50 )
A. farnesiana seeds germinated sooner with treatments T3 and T1, while treatments T4 and T5 resulted in a lower speed; the highest germination speed was registered with T3 (F= 366.91, d.f. = 5, p= < 0.001).
The provenance with the lowest germination speed was Ejido 5 de Mayo, while FCF had the highest (F = 2.84, d.f. = 6, p= 0.01) (Table 4). The interactions between provenances and treatments were not significant (F= 1.4, d.f. = 30, p= 0.1(; this shows that the provenances had a similar behavior (Figure 2).
Table 4 Germination speed of Acacia farnesiana (L.) Willd.
| Provenances | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| Treatments °C | |||||||
| T0 | T1 | T2 | T3 | T4 | T5 | Total | |
| FCF | 4.5 ± 0.22bcd | 3.1 ± 0.19 bcd | 4.6 ± 0.23 bcd | 2.7 ± 0.25bcd | 8.1 ± 0.38 a | 8.8 ± 0.3 a | 5.3±0.49 b |
| Los Ángeles | 5.7 ± 0.34 b | 4.0 ± 0.67bcd | 4.1 ± 0.14 bcd | 2.8 ± 0.12 bcd | 9.0 ± 0.18 a | 9.5 ± 0.25 a | 5.8±0.54 ab |
| Linares road | 4.6 ± 0.21 bcd | 3.5 ± 0.3 bcd | 3.8 ± 0.24bcd | 2.5 ± 0.03bcd | 8.8± 0.27 a | 9.6 ± 0.47 a | 5.5±0.57 ab |
| Agua ejido | 4.3 ± 0.11 bcd | 3.4 ± 0.26 cd | 3.7 ± 0.09 bcd | 2.7 ± 0.03 cd | 8.4 ± 0.38 a | 9.8 ± 0.81 a | 5.4±0.58 ab |
| 5 de Mayo ejido | 4.8 ± 0.2 bcd | 5.2 ± 0.52 bc | 3.8 ± 0.09 bcd | 3.4 ± 0.37 bcd | 8.8 ± 0.44 a | 10.1 ± 1.0a | 6.0±0.55 a |
| Puebla ejido | 5.1 ± 0.42 bc | 3.7 ± 0.27 bcd | 3.9 ± 0.21bcd | 2.7 ± 0.09 bcd | 8.9 ± 0.15 a | 9.2 ± 0.47 a | 5.6±0.54 ab |
| Chiapa de C. | 5.4 ± 0.52 bc | 3.5 ± 0.51bcd | 4.1 ± 0.16bcd | 3.1 ± 0.43 bcd | 8.0 ± 0.38 a | 8.4 ± 0.43 a | 5.4±0.46 ab |
| Total | 4.9±0.13 c | 3.8±0.18 d | 4.0±0.08 d | 2.8±0.1 e | 8.6±0.13 b | 9.3±0.22 a | |
FCF = School of Forest Sciences. Mean and standard error for the comparisons between treatments and provenances. Different letters indicate differences (p<0.05) between treatments and provenances.
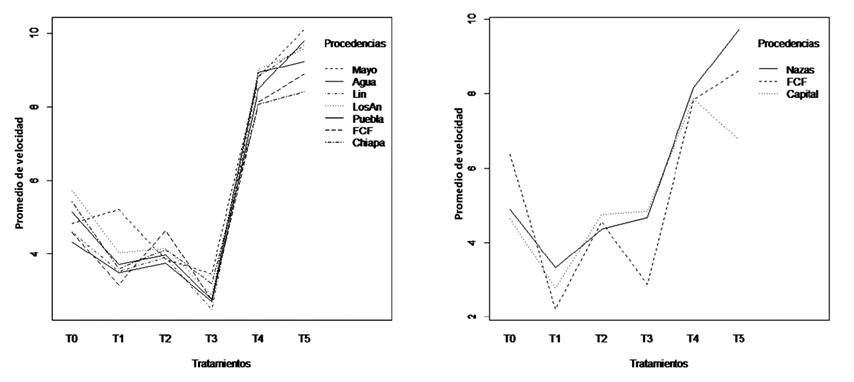
Tratamiento = Treatment; Promedio de velocidad = Mean speed; Procedencias = Provenances
X axis shows the control treatment T0 and the five treatments; axis Y shows the number of days that the seeds took to germinate. The names of the provenances of A. farnesiana were abbreviated.
Figure 2 Interaction graphs of the germination speed between treatments and provenances, A. farnesiana, (left) and A. schaffneri, (right).
The treatments that exhibited the lowest germination speed for A. schaffneri and A. farnesiana were T4 and T5, with means of 7.9 and 8.3 days, respectively. Treatment T1 registered the highest speed, with a mean of 2.7 days (F= 32.5, d.f. = 5, p= <0.001). There were no significant differences between the three provenances, with means ranging between 5.8 and 5.2 (F= 1.24, d.f. = 2, p= 0.29) (Table 5). The interactions between provenances and treatments evidenced no significant differences (F= 1.83, d.f. = 10, p= 0.07), given that the results of the various seed provenances were similar for the treatments, with parallel lines for the provenances (Figure 2).
Table 5 Germination speed of Acacia schaffneri (L.) Willd.
| Provenances | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| Treatments °C | |||||||
| T0 | T1 | T2 | T3 | T4 | T5 | Total | |
| FCF | 6.37 ± 0.62ab | 2.2 ± 0.29d | 4.56 ± 0.03 b | 2.87 ± 0.12 d | 7.83 ± 0.49 ab | 8.62 ± 0.37 a | 5.41±0.51 a |
| Capital | 4.62 ± 0.07 b | 2.76 ± 0.1 b | 4.75 ± 0.16 c | 4.83 ± 0.26 b | 7.87 ± 0.12 abc | 6.75 ± 2.8 ab | 5.26±0.48 a |
| Nazas | 4.89 ± 0.14 c | 3.31 ± 0.31 b | 4.35 ± 0.01 b | 4.66 ± 0.68 b | 8.16 ± 0.34 abc | 9.75 ± 1.1 a | 5.85±0.52 a |
| Total | 5.2±0.3 b | 2.7±0.19 c | 4.5±0.07 b | 4.1±0.34 bc | 7.9±0.19 a | 8.3±0.85 a | |
FCF = School of Forest Sciences. Mean and standard error for the comparisons between treatments and provenances. Different letters indicate differences (p<0.05) between treatments and provenances.
A three-day delay in the germination can reduce the chances of establishment of the seedlings in a climate change scenario (Pérez-Sánchez et al., 2011), due to the rapid evaporation of the soil water brought about by high temperatures. A factor that exerts a direct influence on seed germination is the availability of water in the soil, for both the germination and the survival of the seedlings (Baskin and Baskin, 2001; Benech-Arnold and Sánchez, 2004). In the two studied species, with treatments T4 and T5, the germination speed were considerably lower than with the rest of the treatments, so that the seeds germinated 5-6 days later. No differences in the germination speed were obtained for the provenances of A. schaffneri. A. farnesiana registered a lower speed for FCF, compared with Ejido 5 de Mayo; no differences were found in the remaining provenances.
The highest germination speed was different for the two species; in the case of A. farnesiana, it corresponded to T3, with 2.8 days, and in that of A. schaffneri, to treatment T1, of 2.7. Furthermore, the treatment with the highest germination percentage for A. schaffneri was not the one that yielded the highest germination speed.
Conclusions
The difference in germination ability between the different populations of the same species is interpreted as a potential adaptation to the characteristics of the environment (climate factors) of their provenance. In the present study, Acacia farnesiana and A. schaffneri seeds germinated with all five treatments. These results make it possible to prognosticate that neither species will have difficulties to germinate in case the temperature should increase according to the predicted scenarios.
Acknowledgements
This study was partially sponsored by PAICYT (UANL) and Conacyt (CB-2015-01 255453).
REFERENCES
Albuquerque, M. D. F., J. D. Rodríguez, T. L. Minohara, D. Tebaldi e D. M Silva L. 1998. Influência da temperatura e do substrato na germinação de sementes de saguaraji (Colubrina glandulosa Perk.-Rhamnaceae). Revista Brasileira de Sementes 20(2):346-349. [ Links ]
Arévalo J., R., L. Afonso, A. Naranjo and M. Salas. 2010. Invasion of the Gran Canaria ravines ecosystems (Canary Islands) by the exotic species Acacia farnesiana. Plant Ecology 206:185-193. [ Links ]
Baskin, C. C. and J. M. Baskin. 2001. Ecology, Biogeography and Evolution of Dormancy and Germination. Academic Press. San Diego, CA USA. 667 p. [ Links ]
Benech-Arnold, R. L. and R. A. Sánchez. 2004. Handbook of seed physiology: Applications to agriculture. Ed. Food Products, Haworth Reference Press. New York, N Y USA. 480 p. [ Links ]
Cavalcante, A. M. and R. D. Cox. 2016. Acacia farnesiana (L.) Willd. a potentially invasive alien species? International Journal of Ecology and Environmental Sciences 42(3):209-215. [ Links ]
Coolbear, P., A. Francis and D. Grierson. 1984. The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. Journal of Experimental Botany 35(11): 1609-1617. [ Links ]
Danby, R. K. and D. S. Hik. 2007. Variability, contingency and rapid change in recent subarctic alpine tree line dynamics. Journal of Ecology 95(2):352-363. [ Links ]
Demuner, V. G., C. Adami, J. Mauri, S. Dalcolmo e S. A. Hebling. 2008. Influência da luz e da temperatura na germinação de sementes de Erythrina verna (Leguminosae, Papilionoideae). Museu de Biologia Professor Mello Leitão 24: 101-110. [ Links ]
Dutra, A. S., S. M. Filho e F. O. Diniz, 2008. Dormência, substrato e temperatura para germinação de sementes de albízia (Albizia lebbeck (L.). Revista Ciência Agronômica 38(3):291-296. [ Links ]
Fenner, M. and K. Thompson. 2005. The Ecology of Seeds. Ed. Cambridge University. Cambridge, UK. 250 p. [ Links ]
Field, C. B., V. Barros, D. Dokken, K. Mach, M. Mastrandrea y T. Bilir. 2014. Cambio climático 2014 Impactos, adaptación y vulnerabilidad. Contribución del Grupo de trabajo II al Quinto Informe de Evaluación del Grupo Intergubernamental de Expertos sobre el Cambio Climático. Organización Meteorológica Mundial. Ginebra, Suiza. 1820 p. [ Links ]
Flores, J. and E. Jurado. 1998. Germination and early growth traits of 14 plants species native to northern Mexico. Southwestern Naturalist 43:40-46. [ Links ]
García-Winder, L. R., S. Goñi-Sedeño, P. A. Olguín-Lara, G. Díaz-Salgado and C. M. Arriaga-Jordán. 2009. Huizache (Acacia farnesiana) whole pods (flesh and seeds) as an alternative feed for sheep in México. Tropical Animal Health and Production 41:1615-1621. [ Links ]
Grabherr, G., M. Gotffried and H. Paull. 1994. Climate effects on mountain plants. Nature 369(6480):448. [ Links ]
Holtmeier, F. K. and G. Broll. 2005. Sensitivity and response of northern hemisphere altitudinal and polar treelines to environmental change at landscape and local scales. Global Ecology and Biogeography 14:395-410. [ Links ]
Hughes, L. 2000. Biological consequences of global warming: is the signal already apparent? Trends in Ecology and Evolution 15(2):56-61. [ Links ]
Instituto Nacional de Estadística y Geografía (Inegi). 2016. Mapa digital de México. http://gaia.inegi.org.mx/mdm6/viewer.html (4 de junio de 2013). [ Links ]
Jurado, E., J. F. García, J. Flores, E. Estrada and H. González. 2011. Abundance of seedlings in response to elevation and nurse species in Northeastern Mexico. Southwestern Naturalist 56(2):154-161. [ Links ]
Loarie, S. R., P. B. Duffy, H. Hamilton, G. P. Asner, C. B. Field and D. D. Ackerly. 2009. The velocity of climate change. Nature 462 (7276):1052-1055. [ Links ]
Mann, M. E., R. S. Bradley and M. K. Hughes. 1998. Global-Scale temperature patterns and climate forcing over the past six centuries. Nature 392 (6678):779-787. [ Links ]
Martínez-Pérez, G., A. Orozco-Segovia y C. Martorell. 2006. Efectividad de algunos tratamientos pre-germinativos para ocho especies leñosas de la Mixteca Alta Oaxaqueña con características relevantes para la restauración. Boletín de la Sociedad Botánica de México 9:9-20. [ Links ]
Martins, C. C., C. G. Machado and J. Nakagawa. 2008. Temperature and substrate for germination test of Stryphnodendron adstringens (Mart) Coville (Leguminosae). Revista Árvore 32(4):633-639. [ Links ]
Mondoni, A., R. Probert, G. Rossi, F. Hay and C. Bonomi. 2008. Habitat-correlated seed germination behaviour in populations of wood anemone (Anemone nemorosa L.) from northern Italy. Seed Science Research 18(4): 213-222. [ Links ]
Neto, J. C. A., I. B. Aguiar e V. M. Ferreira. 2003. Efeito da temperatura e da luz na germinação de sementes de Acacia polyphylla DC. Revista Brasileira de Botanica 26(2):249-256. [ Links ]
Pepin, N., R. S. Bradley, H. F. Diaz, M. Baraër, E. B. Caceres, N. Forsythe and J. R. Miller. 2015. Elevation-dependent warming in mountain regions of the world. Nature Climate Change 5(5):424. [ Links ]
Peñuelas, J., R. Ogaya, M. Boada and A. S. Jump. 2007. Migration, invasion and decline: changes in recruitment and forest structure in a warming-linked shift of European beech forest in Catalonia (NE Spain). Ecography 30(6): 830-838. [ Links ]
Parmesan, C. and G. Yohe. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421(6918):37-42. [ Links ]
Pérez-Sánchez R. M., E. Jurado, L. Chapa-Vargas y J. Flores. 2011. Seed germination of Southern Chihuahuan Desert plants in response to elevated temperatures. Journal of Arid Environment 75 (10):978-980. [ Links ]
R-Project. 2010. R: A language and environment for statistical computing. R Foundation for Statistical Computing. R Development Core Team. Vienna, Austria. http://www.R-project.org . (2 de junio de 2011). [ Links ]
Rodrigues M., P. A. F., E.U. Alves, A. P. dos Anjos Neto, R.L.S. de Medeiros, D.D.C F. Junior, J. M. Mondego and A.K.D. Bezerra. 2016. Temperature and pre-germinative treatments for overcoming Acacia farnesiana (L.) Willd. (Fabaceae) seeds dormancy. African Journal of Agricultural Research 11(37):3548-3553. [ Links ]
Root, T. L., J. T. Price, K. R. Hall, S. H. Schneider, C. Rosenzweig and J. A. Pounds. 2003. Fingerprints of global warming on wild animals and plants. Nature 421 (6918):57-60. [ Links ]
Savage, M., P. M. Brown and J. Feddema. 1996. The role of climate in a pine forest regeneration pulse in the southwestern United States. Ecoscience 3 (3):310-318. [ Links ]
Silva, L. M. M. e I. B. Aguiar. 2004. Efeito dos substratos e temperaturas na germinação de sementes de Cnidosculus phyllacanthus Pax & K. Hoffm. (Faveleira). Revista Brasileira de Sementes 26(1):9-14. [ Links ]
Smith, K. W., M. J. Germino, D. M. Johnson and K. Reinhardt. 2009. The altitude of alpine treeline: a bellwether of climatic change effects. Botanical Review 75(2):163-190. [ Links ]
Servicio meteorológico Nacional (SMN). 2011. Información Climatológica por Estado Comisión Nacional del Agua. http:\\smn.cna.bob.mx/climatologia/normales/estacion/catalogos.html (3 de junio de 2011). [ Links ]
Solomon, S., D. Quin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor and H. L. Miller. 2007. Climate Change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. Cambridge, UK., New York, NY USA. 996 p. [ Links ]
Valiente-Banuet, A., A. Casas, A. Alcántara, P. Dávila, N. Flores-Hernández, J. L. Villaseñor, M. Coro y J. Ortega. 2000. La vegetación del Valle de Tehuacán-Cuicatlán. Botanical Sciencies 67:25-74. [ Links ]
National Water and Climate Center (WCC). 2011. Soil and Air temperature (1996). Natural Resources Conservation Service. http://www.wcc.nrcs.usda.gov/nwcc/site?sitenum=2016&state=tx (2 de junio de 2011). [ Links ]
Received: March 16, 2018; Accepted: September 25, 2018











 texto en
texto en 


