Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.9 no.3 Texcoco abr./may. 2018
https://doi.org/10.29312/remexca.v9i3.1219
Articles
Identification of opaque black bean recombinant lines resistant to BCMV, BCMNV and BGYMV using molecular markers
1Campo Experimental Bajío-INIFAP. Carretera Celaya-San Miguel de Allende km 6.5, Celaya, Guanajuato, México. CP. 38110.
2Campo Experimental Centro de Chiapas-INIFAP. Carretera Ocozocoautla-Cintalapa km 3.0, Ocozocoautla, Chiapas. CP. 29140.
3Instituto Tecnológico de Celaya-Departamento de Ingeniería Bioquímica. Celaya, Guanajuato, México. CP. 38010.
4Campo Experimental Cotaxtla-INIFAP. Carretera Federal Veracruz-Córdoba km 34.5, Medellín de Bravo, Veracruz. CP. 94270.
The opaque black bean from the Mesoamerican collection is consumed and produced in Mexico. To identify recombinant lines of opaque black bean resistant to bean common mosaic virus (BCMV), mosaic virus and common bean necrosis (BCMNV) and bean golden yellow mosaic virus (BGYMV), during 2015 and 2016 a collection of 70 genotypes that included 20 varieties and 50 recombinant lines (LR) was evaluated. The LRs derived from three different crosses: ‛Negro Papaloapan’/SEN-46, ‛Negro Citlali’/XRAV-187-3 and ‛Jamapa Plus’/XRAV-187-3. All genotypes were inoculated with the strains BCMNV NL-3 and BGYMV-MX, and were genotyped with the molecular markers SW13, ENM, SBD5 and SR2 linked to the resistance genes I, bc-3, bc1 2 and bgm-1. The 21 LR were identified with plants that had the genes bgm-1, I, or the combination I + bc-3 and broad-spectrum or specific resistance to BCMV, BCMNV and BGYMV. All the LRs had a high proportion of plants with the I gene. Six LRs derived from the cross ‛Negro Papaloapan’/SEN-46 and two from each of the crosses ‛Negro Citlali’/XRAV-187-3 and ‛Jamapa Plus’/XRAV-187-3 had between 8% and 92% of plants with the genetic combination I + bc-3 that confers resistance to BCMV and BCMNV. While the genes I and bgm-1, which confers resistance to BCMV and BGYMV, were detected in three LRs of the ‛Negro Papaloapan’/SEN-46 and four of ‛Negro Citlali’/XRAV-187-3 crosses. Four LR derived from the cross ‛Negro Papaloapan’/SEN-46: ‛Negro Papaloapan’/SEN 46-7-8, ‛Negro Papaloapan’/SEN-46-7-11, ‛Negro Papaloapan’/SEN-46-7 -12 and ‛Negro Papaloapan’/SEN-46-7-13 had plants with the I, bc-3 and bgm-1 genes that confers broad-spectrum resistance to BCMV, BCMNV and BGYMV. The heterogeneity of the LR in the proportion of plants with the resistance genes, highlights the need to implement selection strategies assisted by molecular markers to homogenize them, and develop opaque black bean varieties with resistance to three of the viruses that most affect bean production in Latin America.
Keywords: Phaseolus vulgaris L., bc-3, bgm-1; eIF4E 2; SAMM
El frijol negro opaco de acervo mesoamericano se consume y produce en México. Con el objetivo de identificar líneas recombinantes de frijol negro opaco resistentes al virus del mosaico (BCMV), al virus del mosaico y la necrosis (BCMNV) y al virus del mosaico amarillo dorado del frijol (BGYMV), durante 2015 y 2016 se evaluó una colección de 70 genotipos que incluyeron 20 variedades y 50 líneas recombinantes (LR). Las LR derivaron de tres diferentes cruzas: ‘Negro Papaloapan’/SEN-46, ‘Negro Citlali’/XRAV-187-3 y ‘Jamapa Plus’/XRAV-187-3. Los genotipos se inocularon con las cepas BCMNV NL-3 y BGYMV-MX, y se genotipificaron con marcadores moleculares SW13, ENM, SBD5 y SR2 ligados a genes de resistencia I, bc-3, bc1 2 y bgm-1. Se identificaron 21 LR que tuvieron los genes bgm-1, I, o la combinación I + bc-3 y resistencia de amplio espectro a BCMV, BCMNV y BGYMV. Las LR tuvieron alta proporción de plantas con el gen I. Seis LR que derivaron de la cruza ‘Negro Papaloapan’/SEN-46 y dos de cada una de las cruzas ‘Negro Citlali’/XRAV-187-3 y ‘Jamapa Plus’/XRAV-187-3 tuvieron entre 8% y 92% de plantas con combinación genética I + bc-3 que confiere resistencia a BCMV y BCMNV. Mientras que los genes I y bgm-1, dan resistencia a BCMV y BGYMV, se detectaron en tres LR de la cruza ‘Negro Papaloapan’/SEN-46 y cuatro de ‘Negro Citlali’/XRAV-187-3. Cuatro LR derivadas de la cruza ‘Negro Papaloapan’/SEN-46: ‘Negro Papaloapan’/SEN 46-7-8, ‘Negro Papaloapan’/SEN-46-7-11, ‘Negro Papaloapan’/SEN-46-7-12 y ‘Negro Papaloapan’/SEN-46-7-13 tuvieron plantas con genes I, bc-3 y bgm-1 con resistencia de amplio espectro a BCMV, BCMNV y BGYMV. La heterogeneidad de LR en proporción de plantas con genes de resistencia, surge la estrategia de selección asistida por marcadores moleculares para homogenizarlas y desarrollar variedades de frijol negro opaco con resistencia a tres virus que afectan la producción de frijol en América Latina.
Palabras clave: Phaseolus vulgaris L., bc-3, bgm-1; eIF4E 2 , SAMM
Introduction
The black type bean, which includes the brilliant black of the Jalisco breed and the opaque black of the Mesoamerican breed, is the most produced in Mexico. In 2015, black bean production represented 37.9% of the national bean production (FIRA, 2016). Opaque black beans are produced in 15 states, mainly in conditions of residual moisture; Chiapas, Veracruz and Nayarit stand out for the volume of production (SIACON, 2015). Acute and mixed BCMV and BCMNV infections have been detected in all opaque black bean producing states (Flores-Esteves et al., 2003; Lepe-Soltero et al., 2012), while in Chiapas and Nayarit, in addition to these two species has been detected to the BGYMV (Garrido-Ramírez et al., 2000; Chiquito-Almanza et al., 2017), which together with the bean golden mosaic virus (BGMV) are considered one of the greatest limitations for the production of beans, not only in Mexico, but throughout Latin America (Beebe et al., 2011).
The high incidence of embryo infection by BCMV and BCMNV, with percentages that can reach up to 80%, makes the seed the main means of dissemination (Morales and Castaño, 1987). Subsequently, secondary transmission is mediated by several species of aphids, which transmit the virus in a non-persistent way inside and outside the crop, perpetuating the etiological agent and giving rise to a new cycle of the disease (Morales and Castaño, 2008).
In Mexican bean production systems, especially with small-scale and subsistence farmers, it is common for traditional varieties susceptible to these viruses to be planted, and for the harvested grain to be used as seed for the next production cycle, which allows the permanent existence of an inoculum source in all regions where beans are grown.
The BCMV and the BCMNV produce the diseases known as common mosaic and black bean root. In susceptible varieties, the BCMV can cause yield losses between 53% and 83% (Sastry, 2013b). Although the incorporation of monogenic dominant resistance conferred by the I gene prevents chronic systemic infection or common mosaic caused by some strains of BCMV, when these strains become infected with BCMNV or some strain inducing BCMV necrosis, a hypersensitive response known as apical necrosis or black root (Drijfhout, 1978).
That is why the simultaneous or staggered cultivation of varieties infected with BCMNV, and of varieties that have only monogenic dominant resistance, has contributed to creating outbreaks or pandemics of black root in the fields planted with varieties resistant to the common mosaic (Morales and Castaño, 2008). The best strategy to control common mosaic and black root is to cultivate resistant varieties that combine the dominant resistance conferred by gene I with the recessive genes bc1 2 , bc2 2 and bc-3, in order to eliminate the possibility of infection by strains of the BCMV or BCMNV (Morales and Castaño, 2008).
For its part, the BGYMV causes the disease known as yellow golden bean mosaic (Garrido-Ramírez et al., 2000). Susceptible bean genotypes, infected in the early stages of development, generally show intense systemic yellowing, and may have total losses due to the high incidence of flower abortion and pod deformation (Morales and Niessen, 1988). In bean genotypes of the Mesoamerican breed it is common that this virus cause’s deformation, dwarfism and flower abortion, particularly in high temperature conditions (Morales and Niessen, 1988).
The BGYMV is persistently transmitted by the insect vector Bemisia tabaci (Genn.) With a latency period of 4 to 48 h (Morales and Anderson, 2001). The incidence of the disease and the magnitude of the losses vary depending on the populations of the vector, the susceptibility of the variety, the cultural practices, and the environmental conditions, mainly precipitation, that affect the populations of B. tabaci (Morales and Anderson, 2001). Since the early 90’s, in the Tropic of Mexico have been reported performance losses attributed to BGYMV in susceptible genotypes such as ‛Negro Jamapa’ (87.6%) (Rodríguez and Yoshii, 1990), ‛Negro Tacaná’ (18%) and ‛Negro Husteco-81’ (40.5%) (López-Salinas et al., 1993).
One of the main sources of resistance to BGYMV is found in the Garrapato creole bean (G2402), which contains the recessive gene bgm-1 (Morales and Niessen, 1988). This gene reduces mosaic and systemic yellowing typical of the disease, and has been especially important and effective in Central America (Beebe et al., 1995) demonstrating that it is a stable and valuable gene to generate varieties tolerant to yellow gold mosaic (Blair et al., 2007).
The process of selecting resistant bean varieties can be accelerated by molecular marker assisted selection (SAMM). Currently MM is available linked to some resistance genes to BCMV and BCMNV, such as the dominant MM SW13, linked to gene I (Melotto et al., 1996) and SBD5 linked to the bcI 2 gene (Miklas et al., 2000a), or the MM co-dominate ENM linked to the bc-3 gene (Naderpour et al., 2010); while for BGYMV the co-dominant marker SR2 is available, linked to the bgm-1 gene (Miklas et al., 2005).
The objective of this study was to identify genotypes of black Mesoamerican black bean with resistance genes to BCMV, BCMNV and BGYMV by inoculation with these pathogens and genotyping with molecular markers linked to the resistance genes I, bc-3, bc1 2 and bgm-1. The genotypes identified will be used as a source of resistance to develop varieties with multiple resistance and with adaptation to the growing regions of this type of bean.
Materials and methods
Bean genotypes
It evaluated 70 genotypes of opaque black bean from the Mesoamerican collection, which included 20 commercial varieties, most developed by the National Institute of Forestry, Agriculture and Livestock Research (INIFAP), and 50 advanced recombinant lines (LR) derived from crosses ‛Black Papaloapan’/SEN-46, ‛Black Citlali’/XRAV-187-3 and ‛Jamapa plus’/XRAV-187-3 (Table 1).
Table 1 Advanced recombinant lines and opaque black bean varieties evaluated.
| Recombinant lines | Recombinant lines | Varieties | |||||
| 1 | ‘Negro Papaloapan’/SEN-46-1-7 | 26 | ‘Negro Papaloapan’/SEN-46-7-1 | 51 | ‘Jamapa’ a | ||
| 2 | ‘Negro Papaloapan’/SEN-46-1-8 | 27 | ‘Negro Papaloapan’/SEN-46-7-6 | 52 | ‘Negro Veracruz’ a | ||
| 3 | ‘Negro Papaloapan’/SEN-46-1-10 | 28 | ‘Negro Papaloapan’/SEN-46-7-7 | 53 | ‘Negro Huasteco-81’ a | ||
| 4 | ‘Negro Papaloapan’/SEN-46-2-1 | 29 | ‘Negro Papaloapan’/SEN-46-7-8 | 54 | ‘Negro Cotaxlta-91’ a | ||
| 5 | ‘Negro Papaloapan’/SEN-46-2-2 | 30 | ‘Negro Papaloapan’/SEN-46-7-9 | 55 | ‘Negro INIFAP’ a | ||
| 6 | ‘Negro Papaloapan’/SEN-46-2-3 | 31 | ‘Negro Papaloapan’/SEN-46-7-10 | 56 | ‘Negro Tacana’ a | ||
| 7 | ‘Negro Papaloapan’/SEN-46-2-4 | 32 | ‘Negro Papaloapan’/SEN-46-7-11 | 57 | ‘Negro Tropical’ a | ||
| 8 | ‘Negro Papaloapan’/SEN-46-2-5 | 33 | ‘Negro Papaloapan’/SEN-46-7-12 | 58 | ‘Negro Medellín’ a | ||
| 9 | ‘Negro Papaloapan’/SEN-46-2-6 | 34 | ‘Negro Papaloapan’/SEN-46-7-13 | 59 | ‘Negro Papaloapan’ a | ||
| 10 | ‘Negro Papaloapan’/SEN-46-2-7 | 35 | ‘Negro Citlali’/XRAV-187-3-1-2 | 60 | ‘Negro Comapa’ a | ||
| 11 | ‘Negro Papaloapan’/SEN-46-2-11 | 36 | ‘Negro Citlali’/XRAV-187-3-1-5 | 61 | ‘Verdín’ a | ||
| 12 | ‘Negro Papaloapan’/SEN-46-3-2 | 37 | ‘Negro Citlali’/XRAV-187-3-1-6 | 62 | ‘Negro Grijalva’ a | ||
| 13 | ‘Negro Papaloapan’/SEN-46-3-5 | 38 | ‘Negro Citlali’/XRAV-187-3-1-8 | 63 | ‘T-39’ c | ||
| 14 | ‘Negro Papaloapan’/SEN-46-3-7 | 39 | ‘Negro Citlali’/XRAV-187-3-1-9 | 64 | ‘Negro 8025’ a | ||
| 15 | ‘Negro Papaloapan’/SEN-46-4-5 | 40 | ‘Negro Citlali’/XRAV-187-3-2-2 | 65 | ‘Negro Nayarit’ a | ||
| 16 | ‘Negro Papaloapan’/SEN-46-4-8 | 41 | ‘Negro Citlali’/XRAV-187-3-2-4 | 66 | DOR 448 b | ||
| 17 | ‘Negro Papaloapan’/SEN-46-4-9 | 42 | ‘Negro Citlali’/XRAV-187-3-2-5 | 67 | UCR 55 b | ||
| 18 | ‘Negro Papaloapan’/SEN-46-4-10 | 43 | ‘Negro Citlali’/XRAV-187-3-7-2 | 68 | ‘Jamapa Plus’ a | ||
| 19 | ‘Negro Papaloapan’/SEN-46-5-5 | 44 | ‘Negro Citlali’/XRAV-187-3-14-6 | 69 | Negro 17-99 a | ||
| 20 | ‘Negro Papaloapan’/SEN-46-6-1 | 45 | ‘Negro Citlali’/XRAV-187-3-14-7 | 70 | SCN-2 b | ||
| 21 | ‘Negro Papaloapan’/SEN-46-6-2 | 46 | ‘Negro Citlali’/XRAV-187-3-16-7 | ||||
| 22 | ‘Negro Papaloapan’/SEN-46-6-3 | 47 | ‘Jamapa Plus’/XRAV-187-3-1-2 | ||||
| 23 | ‘Negro Papaloapan’/SEN-46-6-4 | 48 | ‘Jamapa Plus’/XRAV-187-3-1-8 | ||||
| 24 | ‘Negro Papaloapan’/SEN-46-6-5 | 49 | ‘Jamapa Plus’/XRAV-187-3-4-1 | ||||
| 25 | ‘Negro Papaloapan’/SEN-46-6-6 | 50 | ‘Jamapa Plus’/XRAV-187-3-4-4 |
a= varieties generated by the INIFAP; b= varieties generated by CIAT; c= variety generated by the University of California, Davis, USA.
Viral strains
The phenotyping of resistance to BCMV and BCMNV was carried out with strain BCMNV NL-3, preserved in bean seeds of the variety Michelite 62, and provided by the Plant-Virus Interactions Laboratory of the Center for Research and Advanced Studies of the Polytechnic Institute National (CINVESTAV), in Irapuato, Mexico; to phenotyping resistance to BGYMV, components A and B (accession number to GenBank AF173555 and AF173556) of strain BGYMV-MX, provided by Dr. Robert L. Gilbertson of the Department of Plant Pathology of the University of California, Davis, USA.
Phenotyping resistance to BCMV, BCMNV and BGYMV
All the resistance evaluations were carried out under confined greenhouse conditions in the INIFAP facilities. The phenotyping of resistance to BCMV and BCMNV was performed in the Bajio Experimental Field located at 20° 34’ 48.75’’ north latitude 100° 49’ 16.490’’ west longitude, at an altitude of 1767 m, and that of BGYMV in the Campo Experimental Center of Chiapas located at 16° 46’ 48’’ north latitude 93° 24’ 21’’ west longitude, at an altitude of 800 m. To phenotyping the resistance to BCMV and BCMNV, 12 plants of each genotype were inoculated mechanically.
The inoculum was obtained by macerating 1 g of the foliar tissue of the Michelite 62 variety infected with BCMNV NL-3 in 0.01 M phosphate buffer pH 7.5 (1:10 w/v); a sterile cotton gauze was moistened in the macerated solution, and the primary leaves stretched well in stage V2 were rubbed. The inoculation was performed between 7:00 and 9:00 am. The incidence of common mosaic and black root symptoms was determined visually seven days after inoculation (DDI), according to the range of typical symptoms developed in beans inoculated with BCMNV (Drijfhout, 1978). Those plants that did not present symptoms were re-inoculated with the same method in the two primary leaves and the first trifoil, and the incidence of 21 DDI symptoms was determined. Genotypes that showed no symptoms after re-inoculation were considered resistant.
To phenotypeize the resistance to BGYMV, 10 plants of each genotype were agro-inoculated in stage V2 by means of syringe puncture in the first internode (Hou et al., 1988). As inoculum, a 1:1 mixture of cell suspensions of the clones of BGYMV in agrobacterium pBGMXAbin (DNA-A) and pBGMXBbin (DNA-B) (Garrido-Ramírez et al., 2000), previously cultivated in liquid medium LB + Kanamycin (90 mg L-1) was used, in shaking for 48 h and analyzed by PCR to verify the permanence of both components.
The first symptoms of yellowing of ribs, golden mosaic and distortion of leaves were evaluated 15 DDI. The plants that showed some of these symptoms were conserved up to stage R8 (60 DDI) to register the development of the symptoms that included shortening of internodes, flower abortion and deformation of pods. Confirmation of infection by BGYMV was performed by PCR with the degenerate primers PAL1v1978/PAR1c496 for component A and PBLv2040/PCRc2 for component B following the procedure described by Rojas et al. (1993).
Genotyping with molecular markers
To genotype the resistance to BCMV and BCMNV, the total DNA extraction was performed with the extraction method based on CTAB (Abarshi et al., 2010), while for the BGYMV the Dellaporta method was used (Gilbertson et al., 1991). The genotyping of resistance to BCMV and BCMNV was carried out with the codominant MM of CAPS ENM type, linked to the bc-3 gene, and the MMs of type SCAR SW13, linked to gene I, and SBD5, linked to the bcI 2 gene, according to Naderpour et al. (2010); Melotto et al. (1996); Miklas et al. (2000a), respectively. The expected amplicon size for MM SW13 and SBD5 was 650 bp and 1 400 bp, respectively.
The amplification of a 541 bp fragment with the MM ENM was confirmed by electrophoresis, and the restriction of 1 μL of the PCR product in a final volume of 10 μL was performed with 1 U of the Fau I enzyme in 1x buffer according to the manufacturer's instructions (New England Biolabs). Resistant homozygotes (eIF4E 2 /eIF4E 2 ) presented a fragment of 418 bp and another of 123 bp. On the other hand, the genotyping of resistance to BGYMV was carried out with the codominant MM SR2 linked to the bgm-1 gene according to Miklas et al. (2005); resistant homozygotes (bgm-1/bgm-1) amplified a 530 bp fragment and susceptible ones a 570 bp fragment. All PCR products were resolved by electrophoresis in 1% agarose and visualized with biotin (GelRed™) under UV light.
For each of the genotypes, the percentage of plants with the MM linked to the resistance genes I, bc-3 and bgm-1 was determined by dividing the number of plants with the MM between the number of plants genotyped and multiplied by one hundred. Plants that did not show symptoms when inoculated with BCMNV NL-3 and had both MM linked to genes I and bc-3 were considered resistant to BCMNV and BCMV; while those that did not show symptoms when inoculated with BGYMV-MX and had MM linked to the bgm-1 gene were considered resistant to BGYMV.
Results and discussion
The 69 opaque black bean genotypes were phenotyped and genotyped, including advanced 50 LRs obtained from the crosses ‛Negro Papaloapan’/SEN-46 (34 LR), ‛Negro Citlali’/XRAV-187-3 (12 LR) and ‛Jamapa Plus’/XRAV-187-3 (4 LR) (Table 1). The progenitors ‛Negro Papaloapan’ and XRAV-187-3 possess genes for resistance to BCMV, BCMNV and BGYMV. ‛Negro Papaloapan’ derived from line DOR-454 generated by the International Center for Tropical Agriculture (CIAT) (López-Salinas et al., 2007), this line originated from the cross (DOR-364 x G-18521) x (DOR-365 x LM-30630), it has quantitative resistance to BGYMV (higher QTL) (Miklas et al., 1996), gene I resistance to BCMV and probably also genes bc-3 and Ur-5, resistance to BCMNV and rust, respectively (Miklas et al., 2000b).
For its part, XRAV-187-3 is a line derived from crosses PR0003-124/‛Raven’ (Beaver et al., 2014). PR0003-124 was derived from the cross ‛DOR483/BelNeb RR-2//MUS83/DOR483’, which was selected in Puerto Rico for its resistance to BGYMV, root rot and tolerance to high temperatures; has the bgm-1 gene and the QTL SW12, both of resistance to BGYMV, as well as the gene I (Beaver et al., 2014), on the other hand ‛Raven’ was developed and released by the Michigan Agricultural Experiment Station, has adaptation to the tropics, genes I and bc-3, as well as the Ur-3 gene for rust resistance (Kelly et al., 1994).
Phenotyping resistance to BCMV, BCMNV and BGYMV
With the exception of the Negro 17-99 genotype, from which no seed germinated, the resistance to BCMV and BCMNV of 12 plants of 50 genotypes was phenotyped, in the remaining 19 genotypes between 8 and 11 plants were evaluated. The 797 plants were inoculated corresponding to 69 genotypes with strain BCMNV-NL-3 and seven days later, 631 plants showed localized necrotic lesions (LNL) in the primary leaves inoculated (Figure 1F), which indicated the presence of gene I and absence of some recessive gene bc (Drijfhout, 1978). Nine days after the re-inoculation some plants developed LNL, and 21 DDI, common mosaic symptoms were observed in 120 plants (Figure 1I and 1J) and no symptoms in 46 plants (Figure 1H). The plants that presented LNL at the first inoculation developed black roots and died (Figure 1G).
The symptoms of LNL usually manifest from 6 to 10 DDI, while those of common mosaic take between 15 and 20 DDI (Drijfhout, 1978). The development of common mosaic symptoms in plants inoculated with BCMNV NL-3 indicated the absence of gene I, while the absence of symptoms suggested the presence of a recessive gene such as bc1 2 , bc2 2 , bc-3 alone or in combination with the gene I (Drijfhout, 1978).
To phenotyping resistance to BGYMV, between two and nine plants were used with the exception of the genotype Negro 17-99, and the LR 'Negro Citlali'/XRAV-187-3-1-8 in which there was no germination. The inoculated plants showed diverse symptoms that included the absence of symptoms (Figure 1C), yellowing of ribs (Figure 1A), yellow gold mosaic (Figure 1B), leaf deformation (Figure 1E), shortening of ribs (Figure 1D) and in cases where the infected plants were allowed to reach stage R8, flower abortion and pod deformation were observed.
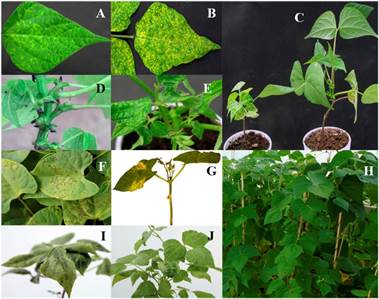
Figure 1 Phenotyping resistance to BCMV, BCMNV and BGYMV in the genotypes of the assay. A, B, C, D and E: symptoms induced by the inoculation of BGYMV-MX. F, G, H, I and J: symptoms induced by BCMNV NL-3. A) yellowing of ribs; B) golden mosaic; C) dwarfism in a susceptible plant (left) compared to a resistant plant with the bgm-1 gene (right); D) shortening of internodes and abortion of flowers and pods; E) leaf deformation; F) localized necrotic lesions in the genotypes that only possess the I gene; G) black root; H) resistant plants with the genetic combination I + bc-3; I) mosaic in ribs; and J) common mosaic.
By PCR, the presence of BGYMV was confirmed in the plants that developed symptoms, while in the asymptomatic plants no amplification was obtained, which confirmed the absence of the virus. In bean agroinoculation works with the BGYMV-MX strain, Garrido-Ramírez et al. (2000) observed differences according to the origin of the genotypes. It is probable that the variety of symptoms expressed in the inoculated plants was due to the fact that the LRs have DOR-454 and PR0003-124 in their pedigree, so that in addition to the bgm-1 gene, some could also have the QTL of greater resistance (SW12), which would explain the heterogeneity in the development of symptoms.
Genotyping with molecular markers linked to genes I, bc-3 and bc1 2
With the intention of reducing the cost of detection of the MM linked to the resistance genes to BCMV and BCMNV, a sample composed of all the plants of each genotype was genotyped. The electrophoretic pattern of MM SW13, ENM and SR2 is shown in Figure 2. In all composite samples, MM SW13 linked to gene I was detected. MM SBD5 was detected only in the LR ‛Negro Papaloapan’/SEN-46 -6-5, which suggested that at least one plant in this LR possessed the bc1 2 gene, since 10 of the plants developed black root and two necrosis in the stem; these last plants were genotyped individually, and 45 DDI the only plant that had the combination of genes I + bc1 2 died due to the development of black root. The combination of the I + bc1 2 genes confers resistance to BCMNV NL-3 between 17 and 26 °C, but breaks at 30 °C (Drijfhout, 1978); The development of necrosis in 10 DDI veins has also been observed with strain BCMNV NL-3, although it is not indicated at what temperature (Bello et al., 2014). In this work, the temperature oscillated between 30 and 36 °C in the hottest hours of the day.
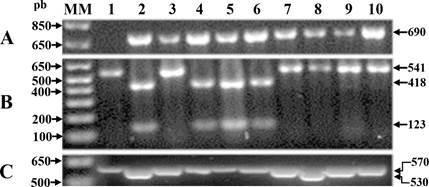
Figure 2 Representative figure of genotyping with molecular markers linked to resistance genes to BCMV, BCMNV and BGYMV in recombinant black bean lines. Lanes 1 and 2: negative and positive control, respectively. Lanes 3 to 10: problem samples; A) molecular marker (MM) SW13. A 690 bp fragment indicates the presence of MM linked to gene I; B) MM ENM. The presence of two fragments (418 bp and 123 bp) after digestion with the enzyme Fau I indicates the presence of MM linked to the bc-3 gene and C) molecular marker SR2; a fragment of 530 bp indicates the presence of MM linked to the bgm-1 gene.
In 55 of the genotypes, codominant MM type CAPS ENM was not detected, which indicated that none of them had the bc-3 gene; only 10 of the LR and elite genotype SCN-2 showed the typical banding pattern of a heterozygote eIF4E 2 /eIF4E 3 and four that of a homozygote eIF4E 2 / eIF4E 2 indicating that some LR are still heterogeneous in relation to the presence of this gene.
The plants of each genotype that did not develop symptoms of common mosaic or black root after the second inoculation were genotyped individually with MMs linked to genes I, bc-3 and bc1 2 . In the LR ‛Negro Papaloapan’/SEN-46-4-9, ‛Negro Papaloapan’/SEN-46-7-6, ‛Negro Papaloapan’/SEN-46-7-7, ‛Negro Citlali’/XRAV-187 -3-14-7, ‛Jamapa Plus’/XRAV-187-3-4-1, ‛Negro Papaloapan’/SEN-46-5-5 and ‛Negro Citlali’/XRAV-187-3-1-9 detected between one and three asymptomatic plants inoculation with BCMNV NL-3 that had MM SW13 bound to gene I, but not MM SBD5 bound to bc1 2 gene or MM ENM bound to bc-3 gene, so it was not it was able to determine if the observed resistance was due to some other resistance gene not yet described, to an error in the process of detecting MM or the inoculation of these plants in particular.
Genotypes where plants were detected only with MM SW13 were ‛Negro Papaloapan’/SEN-46-4-5 (one plant), ‛Negro Papaloapan’/XRAV-187-3-1-8 (one plant), SCN- 2 (one plant) ‛Jamapa Plus’/XRAV-187-3-4-1 (one plant) and T-39 (four plants), so they were expected to develop symptoms of necrosis by inoculating them with strain BCMNV NL-3. However, they only presented mosaics in the trifoil. This situation could be due to the fact that MM SW13 can identify false negatives, as reported by Bello et al. (2014) who developed a MM of more codominant type that theoretically solves this situation.
A plant from each of the genotypes ‛Negro Papaloapan’/SEN-46-7-6, ‛Negro Papaloapan’/SEN 46-7-13 and ‛Jamapa Plus’/XRAV-187-3-4-4 had only the MM ENM in a homozygous state (eIF4E 2 /eIF4E 2 ), while 15 genotypes had between one and 11 individuals with the combination of genes I + bc-3 (Table 2), of which six stood out for their greater proportion of plants with this combination genetics: ‛Negro Papaloapan’/SEN-46-7-10 (92%); ‛Jamapa Plus’/XRAV-187-3-4-4 (88%); ‛Negro Papaloapan’/SEN 46-5-5 (82%); ‛Negro Papaloapan’/SEN-46-7-7, ‛Negro Citlali'/XRAV-187-3-1-9, and SCN-2 (75%); the other nine genotypes had between 8% and 67% of plants with both genes (Table 2). The varieties and LR with the highest proportion of plants with the genetic combination I + bc-3 represent sources of resistance to BCMNV and BCMV in the bean stock of black opaque type. It is important to note that in some plants with the combination I + bc-3 weak symptoms of what appear to be mosaics were observed. However, it could be due to some nutritional deficiency.
Table 2 Percentage of genotypes with molecular markers linked to genes I, bc-3 and bgm-1 of resistance to BCMV, BCMNV and BGYMV.
| Recombinant line or variety | Resistance genes a | |||
| bgm-1 | I | bc-3 | I + bc-3 | |
| ‘Negro Papaloapan’/SEN-46-1-8 | 50 | 100 | - | - |
| ‘Negro Papaloapan’/SEN-46-4-5 | - | 92 | - | 8 |
| ‘Negro Papaloapan’/SEN-46-5-5 | - | 10 | - | 82 |
| ‘Negro Papaloapan’/SEN-46-6-2 | 100 | 100 | - | - |
| ‘Negro Papaloapan’/SEN-46-6-4 | 75 | 100 | - | - |
| ‘Negro Papaloapan’/SEN-46-7-6 | - | 30 | 10 | 60 |
| ‘Negro Papaloapan’/SEN-46-7-7 | - | 17 | - | 75 |
| ‘Negro Papaloapan’/SEN-46-7-8 | 100 | 33 | - | 67 |
| ‘Negro Papaloapan’/SEN-46-7-9 | - | 90 | - | 10 |
| ‘Negro Papaloapan’/SEN-46-7-10 | - | 8 | - | 92 |
| ‘Negro Papaloapan’/SEN-46-7-11 | 67 | 83 | - | 17 |
| ‘Negro Papaloapan’/SEN-46-7-12 | 67 | 75 | - | 25 |
| ‘Negro Papaloapan’/SEN-46-7-13 | 100 | 25 | 8 | 67 |
| ‘Negro Citlali’/XRAV-187-3-1-5 | 100 | 100 | - | - |
| ‘Negro Citlali’/XRAV-187-3-1-6 | 100 | 100 | - | - |
| ‘Negro Citlali’/XRAV-187 3-1-8 | - | 38 | - | 63 |
| ‘Negro Citlali’/XRAV-187 3-1-9 | - | 25 | - | 75 |
| ‘Negro Citlali’/XRAV-187-3-2-2 | 100 | 100 | - | - |
| ‘Negro Citlali’/XRAV-187-3-16-7 | 25 | 100 | - | - |
| ‘Jamapa Plus’/XRAV-187-3-4-1 | - | 44 | - | 56 |
| ‘Jamapa Plus’/XRAV-187-3-4-4 | - | 0 | 12 | 88 |
| ‘Negro Papaloapan’ | 100 | 92 | - | - |
| T-39 | 67 | 82 | - | - |
| ‘Verdín’ | 67 | 100 | - | - |
| SCN-2 | - | 25 | - | 75 |
a= (%) of plants with the resistance genes bgm-1, I and bc-3 determined with MM SR2, SW13 and ENM, respectively; - = absence of MM linked to the resistance gene.
Although the breakage of resistance of the combination of the I + bc-3 genes by the effect of some necrotic strain such as BCMNV NL-3 has not been reported so far and the studies in which the resistance to “high temperatures” have been evaluated were carried out at 32 °C (Pasev et al., 2013), if there are records that the resistance conferred by gene I to strain BCMV NY-15 in the Black Turtle differential variety of GR 8 (Drifjhout, 1978) is dependent on the dose of the gene (II, Ii or ii) and breaks at temperatures above 34 °C (Witmer-Collmer et al., 2000). Drijfhout, (1978) also reported the breakdown of the resistance of the varieties Black Tourtle Soup (I), Widusa (I), Jubila (I + bc1), Impr. Tendergr. (I + bc1), Top Crop (I +bc1) and Amanda (I + bc1 2 ) when inoculated with strains NL-3, NL-5, and NL-6 at 30 °C, and some of them also developed necrosis systemic with strains NL-2 and NL-8.
Genotyping with the molecular marker SR2 linked to the bgm-1 gene
In some plants of a group of eleven LR: seven of the cross ‛Negro Papaloapan’/SEN-46 and four of ‛Negro Citlali’/XRAV-187-3, and in the varieties DOR 448, ‛Negro Papaloapan’, ‘Verdín’ and T-39 no symptoms were observed, nor was BGYMV detected by PCR. In these plants a single amplicon of 530 bp was obtained, indicating that they were resistant homozygotes (bgm-1/bgm-1). However, in another group of five LR and the varieties ‛Negro 8025’, ‛Negro Veracruz’, ‛Negro Huasteco-81’ and ‛Negro Comapa’, symptoms of BGYMV infection were observed despite having the MM SR2 bound to the bgm-1 gene.
Although in practical terms the bgm-1 gene is the most important for conferring resistance to BGYMV (Beebe et al., 1995; Singh et al., 2000), MM SR2 linked to this gene is associated with resistance to general symptoms of the disease as yellowing or chlorosis, but not with symptoms such as dwarfism, which is related to other resistance factors such as the Dwf gene (Blair et al., 2007). Other resistance genes such as Bgp1, linked to the normal development of the sheaths during infection (Acevedo-Román et al., 2004), or the W12 locus of resistance to BGYMV (Miklas et al., 1996, 2000b) are more effective when they are present with the bgm-1 gene.
The DOR-364 line, with the QTL of resistance to BGYMV, significantly increased the resistance to this virus with the incorporation of the bgm-1 gene recovered from the Mexican accession G2402 (‛Garrapato’). Subsequent lines such as DOR-482 had a disease index of 2, on a scale of 1= immune to 9= totally susceptible, while DOR-364 received a rating of 4 (Miles and Pandey, 2004). Because in the present study only MM SR2 was used, it is likely that the absence of symptoms in the LR and resistant varieties is due to the joint action of the bgm-1 gene with some other genes, such as the QTL greater resistance to BGYMV present in the pedigree of ‛Negro Papaloapan’ and XRAV-187-3.
All LRs had a high proportion of plants with gene I, which confers resistance to the non-inducing strains of BCMV necrosis; six LR derived from the cross ‘Negro Papaloapan’/SEN-46 and two LR from each of the crosses ‘Negro Citlali’/XRAV-187-3 and ‛Jamapa Plus’/XRAV-187-3-4-1 had between 8% and 92% of plants with the genetic combination I + bc-3,, which confers resistance to BCMV and BCMNV; whereas the genes I and bgm-1, which confers resistance to BCMV and BGYMV, were detected in seven LR, three of the cross ‘Negro Papaloapan’/SEN-46 and four of the cross ‛Negro Citlali’/XRAV-187- 3, some of which were homogeneous in the percentage of plants with both genes (Table 2).
Four LR derived from the cross ‘Negro Papaloapan’/SEN-46: ‘Negro Papaloapan’/SEN-46-7-8, ‘Negro Papaloapan’/SEN-46-7-11, ‘Negro Papaloapan’/SEN-46- 7-12 and ‘Negro Papaloapan’/SEN-46-7-13 stood out for the percentage of plants with the I, bc-3 and bgm-1 genes (Table 2) that confers resistance to BCMV, BCMNV and BGYMV.
The heterogeneity of the LR in the proportion of plants with each of the resistance genes highlights the need to homogenize them by using MM to develop opaque black bean varieties with general or specific resistance to three of the viruses that most affect bean production in Latin America. The use of varieties with specific resistance to the predominant viruses in each region will contribute to reduce the losses and spread of these viruses within and between producing regions.
Conclusions
In the collection of 70 opaque black bean materials from INIFAP, 21 lines recombinant were identified with different percentages of plants with the I, bc-3 and bgm-1, genes, and general or specific resistance to BCMV, BCMNV and BGYMV. The recombinant lines ‘Negro Papaloapan’/SEN-46-7-8, ‘Negro Papaloapan’/SEN-46-7-11, ‘Negro Papaloapan’/SEN-46-7-12 and ‘Negro Papaloapan’/SEN-46 -7-13 stood out for the percentage of plants with the combination I + bc-3 and the bgm-1 gene, as well as for their broad-spectrum resistance to the three viruses. The heterogeneity of the LR in the proportion of plants with each of the resistance genes highlights the need to implement selection strategies assisted by molecular markers to homogenize them according to their resistance genes, and to develop opaque black bean varieties with adaptation to the growing regions of this type of bean and general or specific resistance to three of the viruses that most affect bean production in Latin America.
Acknowledgments
To INIFAP for financing the project: improvement of black beans for tolerance to drought, acid soils and angular spot and use of biotechnological tools in the identification of viruses and genotypes resistant to these in the southeast of Mexico, num. SIGI: 1046532968. Al PhD Jorge A. Acosta-Gallegos for performing the crosses of the recombinant lines evaluated in this study.
REFERENCES
Abarshi, M. M.; Mohammed, I. U.; Wasswa, P.; Hillocks, R. J.; Holt, J.; Legg, J. P.; Seal, S. E. and Maruthi, M. N. 2010. Optimization of diagnostic RT-PCR protocols and sampling procedures for the reliable and cost-effective detection of Cassava brown streak virus. J. Virol. Methods. 163(1):353-359. [ Links ]
Acevedo, R. M.; Molina, C. A.; Angel, S. J. C.; Germán, M. C. and Beaver, J. S. 2004. Inheritance of normal pod development in bean golden yellow mosaic resistant common bean. J. Am. Soc. Hortic. Sci. 129(4):549-552. [ Links ]
Beaver, J. S.; Prophete, E. H.; Rosas, J. C.; Godoy, L. G.; Steadman, J. R. and Porch, T. G. 2014. Release of ‛XRAV-40-4’ black bean (Phaseolus vulgaris L.) cultivar. J. Agric. Univ. P. R. 98(1):83-87. [ Links ]
Beebe, S.; Ramirez, J.; Jarvis, A.; Rao, E. M.; Mosquera, G.; Bueno, J. M. and Blair, M. W. 2011. Genetic improvement of common beans and the challenges of climate change. In: crop adaptation to climate change. Yadav, S. S.; Redden, R.; Hatfield, J. L.; Lotze-Campen, H. and Hall, A. J. W. (Eds). 1s t (Ed.). Wiley-Blackwell: United Kingdom. 356-369 pp. [ Links ]
Beebe, S. E.; Ochoa, I.; Skroch, P.; Nienhuis, J. and Tivang, J. 1995. Genetic diversity among common bean breeding lines developed for Central America. Crop Sci. 35(4):1178-1183. [ Links ]
Bello, M. H.; Moghaddam, S. M.; Massoudi, M.; McClean, P. E.; Cregan, P. B. and Miklas, P. N. 2014. Application of in silico bulked segregant analysis for rapid development of markers linked to Bean common mosaic virus resistance in common bean. BMC Genomics 15(903):1-13. [ Links ]
Blair, M. W.; Rodríguez, L. M.; Pedraza, F.; Morales, F. and Beebe, S. 2007. Genetic mapping of the bean golden yellow mosaic geminivirus resistance gene bgm-1 and linkage with potyvirus resistance in common bean (Phaseolus vulgaris L.). Theoretical Appl. Gen. 114(2):261-271. [ Links ]
Chiquito, A. E.; Acosta, G. J. A.; García, A. N. C.; Garrido, R. E. R.; Montero, T. V.; Guevara, O. L. and Anaya, L. J. L. 2017. Simultaneous detection of both RNA and DNA viruses infecting dry bean and occurrence of mixed infections by BGYMV, BCMV and BCMNV in the Central-west region of Mexico. Viruses. 9(63):1-13. [ Links ]
Drijfhout, E. 1978. Genetic interaction between Phaseolus vulgaris and bean common mosaic virus with implications for strain identification and breeding for resistance. Agricultural Research Reports, Vol. 872. Centre for Agriculture Publishing and Documentation. Wageningen, The Netherlands. 1-98 pp. [ Links ]
FIRA (Fideicomisos Instituidos en Relación con la Agricultura). 2016. Panorama agroalimentario. frijol 2016. https://www.gob.mx/cms/uploads/attachment/file/200638/ panorama-agroalimentario-frijol-2016.pdf. [ Links ]
Flores, E. N.; Acosta, G. J. A. and Silva, R. L. 2003. Bean common mosaic virus and Bean common mosaic necrosis virus in Mexico. Plant Dis. 87(1):21-5. [ Links ]
Garrido, R. E. R.; Sudarshana, M. R. and Gilbertson, R. L. 2000. Bean golden yellow mosaic virus from Chiapas, Mexico: characterization, pseudorecombination with other bean-infecting geminiviruses and germ plasm screening. Phytopathology. 90(11):1224-1232. [ Links ]
Gilbertson, R. L.; Faria, J. C.; Hanson, S. F.; Morales, F. J.; Ahlquist, P.; Maxwell, D. P. and Russell, D. R. 1991. Cloning of the complete DNA genome of four bean-infecting geminivirus and determining their infectivity by electric-discharge particle-acceleration. Phytopathology . 81(9):980-985. [ Links ]
Hou, Y. M.; Paplomatas, E. J. and Gilbertson R. L. 1998. Host adaptation and replication properties of two bipartite geminiviruses and their pseudorecombinants. Mol. Plant-Microbe Interact. 11(3):208-217. [ Links ]
Kelly, J. D.; Hosfield, G. L.; Varner, G. V.; Uebersax, M. A.; Haley, S. D. and Taylor, J. 1994. Registration of ‘Raven’ black bean. Crop sci. 34(5):1406-1407. [ Links ]
Lepe, S. D.; Sánchez, G. B. M.; Jiménez, H. Y.; Salinas, P. R. A.; García, N. M. A.; González, de L. D.; Becerra, L. E. N.; Acosta, G. J. A. and Silva, R. L. 2012. Presence of BCMV and BCMNV in five dry bean-producing states in Mexico. Trop. Subtrop. Agroecosyst. 15(2):313-321. [ Links ]
López, S. E.; Becerra. L. E. N.; Acosta, G. J. A. y Villar, S. B. 1993. Variedades de frijol tolerantes al virus del mosaico dorado para el trópico de México. Agric. Téc. Méx. 19(2):99-109. [ Links ]
López, S. E.; Tosquy, V. O. H.; Villar, S. B.; Cumpián, G. J.; Ugalde, A. F. J. y Becerra, L. E. N. 2007. Negro Papaloapan, nuevo cultivar de frijol para las áreas tropicales de México. Agric. Téc. Méx. 33(3):259-269. [ Links ]
Melotto, M.; Afanador, L. and Kelly, J. D. 1996. Development of a SCAR marker linked to the I gene in common bean. Genome. 39(6):1216-1219. [ Links ]
Miklas, P. N.; Delorme, R.; Stone, V.; Daly, M. J.; Stavely, J. R.; Basset, M. J. and Beaver, J. 2000b. Bacterial, fungal and viral disease resistance loci mapped in a recombinant inbred common bean population (“Dorado”/XAN 176). J. Am. Soc. Hortic. Sci . 125(4):476-481. [ Links ]
Miklas, P. N.; Johnson, E.; Stone, V.; Beaver, J.; Montoya, C. and Zapata, M. 1996. Selective mapping of QTL conditioning disease resistance in common bean. Crop Sci . 36(5):1344-1351. [ Links ]
Miklas, P. N.; Larsen, R. C.; Riley, R. and Kelly, J. D. 2000a. Potential marker-assisted selection for bc-1 2 resistance to Bean common mosaic potyvirus in common bean. Euphytica. 116(3):211-219. [ Links ]
Miles, J. W. and Pandey, S. 2004. Long‐term selection in plants in the developing world. In: Plant Breeding Reviews: Long-term Selection: Crops, Animals and Bacteria. Janick, J. (Ed.). v. 24, part 2. John Wiley & Sons. New York, USA. 45-88 pp. [ Links ]
Morales, F. and Niessen, I. 1988. Comparative responses of selected Phaseolus vulgaris germplasm inoculated artificially and naturally with bean golden mosaic virus. Plant Disease. 72(12):1020-1024. [ Links ]
Morales, G. F. J. y Castaño, J. M. 2008. Enfermedades virales del frijol común en América Latina. Publicación No. 364. Centro Internacional de Agricultura tropical (CIAT), Cali, Colombia. 86 p. [ Links ]
Morales, F. J. and Anderson, P. K. 2001. The emergence and dissemination of whitefly-transmitted geminiviruses in Latin America. Arch. Virol. 146(3):415-441. [ Links ]
Morales, F. J. and Castaño, M. 1987. Seed transmission characteristics of selected bean common mosaic virus strains in differential bean cultivars. Plant Disease. 71(1):51-53. [ Links ]
Naderpour, M.; Lund, O. S.; Larsen, R. and Johansen, E. 2010. Potyviral resistance derived from cultivars of Phaseolus vulgaris carrying bc-3 is associated with the homozygotic presence of a mutated eIF4E allele. Mol. Plant Pathol. 11(2):255-263. [ Links ]
Pasev, G.; Kostova, D. and Sofkova, S. 2013. Identification of genes for resistance to Bean common mosaic virus and Bean common mosaic necrosis virus in snap bean (Phaseolus vulgaris L.) breeding lines using conventional and molecular methods. J.Phytopathology . 162(1):19-25. [ Links ]
Rodríguez, R. R. y Yoshii, O.K. 1990. Tolerancia varietal del frijol al mosaico dorado y control químico del vector Bemicia tabaci Genn. en Papantla, Veracruz. Agric. Téc. Méx . 16(1):19-32. [ Links ]
Rojas, M. R.; Gilbertson, R. L.; Russell D. R. and Maxwell, D. P. 1993. Use of degenerate primers in the polymerase chain reaction to detect whitefly-transmitted geminiviruses. Plant Dis . 77(4):340-347. [ Links ]
Sastry, K. S. 2013. Plant virus and viroid diseases in the tropics. Volume 1. Introduction of plant viruses and sub-viral agents, classification, assessment of loss, transmission and diagnosis. Springer: 1st (Ed.). Dordrecht, The Netherlands. 109-189 pp. [ Links ]
SIACON (Sistema de Información Agroalimentaria de Consulta). 2015. Programa Informático. Base de datos agrícola, pecuaria y pesquera. http://www.gob.mx/siap/acciones-y-programas/produccion-agricola-33119. [ Links ]
Singh, S. P.; Morales, F. J.; Miklas, P. N. and Terán, H. 2000. Selection for bean golden mosaic resistance in intra- and interracial bean populations. Crop Sci . 40(6):1565-1572. [ Links ]
Whitmer, C. C.; Fisher, M. M.; Taylor, J. C. and Jahn, M. 2000. The I gene of bean: a dosage dependent allele conferring extreme resistance, hypersensitive resistance, or spreading vascular necrosis in response to the potyvirus bean common mosaic virus. Mol. Plant-Microbe Interact . 13(11):1266-1270. [ Links ]
Received: February 00, 2018; Accepted: March 00, 2018











 texto en
texto en 


