Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ciencias agrícolas
versão impressa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.8 no.7 Texcoco Set./Nov. 2017
Articles
Effect of different phosphorus levels on avocado inoculated with arbuscular mycorrhizal fungi
1Universidad Veracruzana-Facultad de Ciencias Agrícolas. Circuito Gonzalo Aguirre Beltrán s/n, Zona Universitaria Xalapa, Veracruz. CP 91000. México. Tel. (228) 8421700, ext. 11621. (llacoob@gmail.com; wsangabriel@gmail.com; samuel-agro@hotmail.com; ycabud@gmail.com).
2Instituto de Investigaciones en Ecosistemas y Sustentabilidad-Universidad Nacional Autónoma de México. Antigua Carretera a Pátzcuaro núm.8701, Morelia, Michoacán. (mgavito@cieco.unam.mx).
3Facultad de Biología-Universidad Michoacana de San Nicolás de Hidalgo. Ciudad Universitaria, Morelia, Michoacán.
The arbuscular mycorrhizal fungi (HMA) are a biological alternative to increase the absorption of phosphorus and reduce the excessive use of phosphate fertilizers. The objective of this work was to evaluate the effect of different concentrations of phosphorus fertilization on avocado plants with and without mycorrhiza. A factorial design was used with two factors: mycorrhizal inoculant (with three levels: Rizofermic-UV, Pacispora scintillans and uninoculated) and fertilization with phosphorus (with five levels: without fertilizer, 20, 40, 80 and 160 ppm; using H2PO4 as fertilizer source), each treatment with four replicates. 401 days after inoculation, height, diameter, number of leaves, leaf area, growth rate, fresh and dry weight and percentage of mycorrhizal colonization were evaluated. Variables were analyzed using a Fisher’s test, with a significance level of p< 0.05. being considered significant. The treatment with 160 ppm caused the death of the plants at 150 days, while inoculated seedlings plus 20 ppm showed an increase in significant growth variables (p< 0.05) with respect to the control. Both plants inoculated with Rizofermic-UV and P. scintillans showed a significant increase in growth variables with respect to the control (p< 0.05). The plants inoculated with P. scintillans and also with a treatment of 20 ppm of phosphorus, promote the greatest increase in growth variables. Mycorrhization had positive and significant effects on the development of the inoculated plants compared to the control plants.
Keyword: inoculant; fertilization; phosphorus; mycorrhiza
Los hongos micorrízicos arbusculares (HMA) son una alternativa biológica para aumentar la absorción del fósforo y reducir el uso excesivo de fertilizantes fosfatados. El objetivo de este trabajo fue evaluar el efecto de diferentes concentraciones de fertilización fosforada en plantas de aguacate con y sin micorriza. Para el experimento se utilizó un diseño factorial con dos factores: inoculante micorrízico (con tres niveles: Rizofermic-UV, Pacispora scintillans y sin inocular) y fertilización con fósforo (con cinco niveles: sin fertilizante, 20, 40, 80 y 160 ppm; utilizando H2PO4 como fuente de fertilizante), cada tratamiento con cuatro repeticiones. A 401 días después de la inoculación se evaluó altura, diámetro, numero de hojas, área foliar, tasa de crecimiento, peso fresco y seco y porcentaje de colonización micorrízica. Las variables se analizaron con una prueba de Fisher, considerando significativo un valor de p< 0.05. El tratamiento con 160 ppm causó la muerte de las plantas a los 150 días, mientras que plántulas inoculadas más 20 ppm mostraron un incremento en variables de crecimiento significativo (p< 0.05) con respecto al testigo. Tanto plantas inoculadas con Rizofermic-UV como con P. scintillans mostraron un incremento significativo en variables de crecimiento con respecto al testigo (p< 0.05). Las plantas inoculadas con P. scintillans y además con un tratamiento de 20 ppm de fósforo, promueven el mayor incremento en las variables de crecimiento. La micorrización tuvo efectos positivos y significativos sobre el desarrollo de las plantas inoculadas respecto de las plantas control.
Palabra clave: inoculante; fertilización; fósforo; micorriza
Introduction
The phosphorus (P) is one of the limiting nutrients in the soil due to its low availability and low mobility among macro nutrients (Souchie et al., 2006). In the plant, phosphorus is required in the formation of nucleic acids, phosphates of sugars and membranes, protein synthesis and formation of the new protoplasm, so an adequate supply of this element in the soil is necessary (Condron and Tiessen, 2005). The absorption of P in plants is carried out through diacid or monoacid ions of the substrate, due to the low solubility of the phosphate compounds, there is a tendency of displacement of the equilibrium towards the solid phase, so that the concentration of P in solution at a specific time can become very low (0.02 to 0.1 mg L-1) (Chesworth, 2008).
These characteristics have led the plants to develop different physiological mechanisms (accumulation of carbohydrates in the root) and biochemical (root exudates) to increase the acquisition of nutrients, especially P. One such mechanism is the formation of the symbiotic association with arbuscular mycorrhizal fungi (HMA; Smith and Smith, 2011), because it is known that plants can acquire P by (1) direct absorption that occurs through transporters in the epidermis of the root and (2) mycorrhizal absorption translocating P from the extra radical hyphae of the fungi to the interior of the root (Smith et al., 2004). In soils with limited phosphorus content, plants can completely depend on the mycorrhizal association to acquire this nutrient (Smith et al., 2003).
Arbuscular mycorrhizae are associations of the mutualistic type between plants and a great variety of fungi (Smith and Read, 2008); the HMA are soil organisms that form symbiosis with 80% of terrestrial plants (Smith et al., 2004). This association benefits the plant by increasing the availability of nutrients with low availability or low soil mobility, has biocontrol effects against some pathogenic microorganisms, increasing nutritional status, tolerance to water stress, and other benefits (Smith et al., 2011). At the same time, this association plays an important role on soil physical characteristics, increasing particle aggregation and soil stability (Khan, 2006).
Numerous studies have demonstrated the advantages of inoculation with arbuscular mycorrhizal fungi in low fertility soils (Herrmann et al., 2015). The most important effect of HMA on crops is an increase in the uptake and translocation of soil nutrients such as N, P, K, Ca, and Mg (Säle et al., 2015), which translates into higher growth and development of plants, as well as a higher percentage of transplant survival (Rodríguez et al., 2017).
Under conventional production schemes optimum crop development demands high application of mineral fertilizers and pesticides (Weih et al., 2011). Phosphate fertilizer application is sometimes superior to the needs of such crops, resulting in excessive accumulation of readily available phosphorus in the soil (Withers et al., 2001). Therefore, it is necessary to know the optimum phosphorus dose so that the plant and the mycorrhizal association can be efficiently developed, so that the benefits that this symbiosis can be obtained (Taffouo et al., 2014).
Mexico is the center of origin of avocado (Persea americana Mill; Gutiérrez et al., 2010) with a great diversity of species, up to 20 different and three races have been reported: Mexican, West Indian and Guatemalan (Chen et al., 2009). It has been documented that avocado is a myotrophic culture that responds favorably to mycorrhizal inoculation (Barcenas et al., 2007; Bañuelos et al., 2013). However, it is known that there is a great genetic and functional diversity of HMA species (Helgason and Fitter, 2009), and that the genera of HMA associated to the same host species present differences in relation to the level and capacity of sporulation (Bever, 2002), ability to colonize roots (Klironomos and Hart, 2002) and to acquire phosphorus (Smith et al., 2000).
In addition, varieties of the same plant may also differ in their response to mycorrhization. In wheat, colonization by Glomus intraradices varied between 16 and 37% between cultivars (Zhu et al., 2001) and in barley, two varieties inoculated with G. intraradices differed in more than 100% in acquisition of inorganic phosphorus (Pi) (Zhu et al., 2003).
In avocado cultivation the results may be compelling, for example, in a study to evaluate the functional differences between arbuscular mycorrhizal fungi Glomus intraradices, Scutellospora heterogama and their effect on avocado plants (Persea americana), it was found that the joint inoculation with G. intraradices and S. heterogama reduced plant growth rates and the absorption of P, zinc (Zn) and iron (Fe) in relation to plants inoculated with G. intraradices, evidencing in this case the absence of functional complementarity (Violi et al., 2008).
In contrast to these results, inoculation with consortia of HMA species (Glomus fasciculatum, Glomus constrictum, Glomus tortuosum, Glomus geosporum, Acaulospora scrobiculata Glomus mosseae and Glomus cubense) favored a greater development in Mexican creole avocado plants (Persea americana var. drymifolia) with respect to control plants, increasing plant height up to 54%, stem diameter (up to 36%), number and leaf length (48% and 40% respectively), as well as fresh root weight up to 85%) (Castro et al., 2013).
It is known that avocado cultivation is susceptible to attack caused by root pathogens, and that in the nursery stage the soil is sterilized prior to planting, thereby eliminating all types of microorganisms, so that reintroduction of beneficial microorganisms such as arbuscular mycorrhizal fungi may play an important role in the production of this plant. Due to the above, the present work has as objective: to evaluate the effect of different concentrations of phosphorus fertilization, in the development of avocado plants with and without mycorrhizal inoculant, expressed in growth variables.
Materials and methods
Vegetative material
The Creole avocado seeds (Persea americana) were pregerminated in sterile sand, in trays with a capacity of 8 L.
Inoculant
Two inocula were used; the Rizofermic-UV consortium containing twelve species of arbuscular mycorrhizal fungi (Acaulospora morrowiae, Acaulospora scrobiculata, Acaulospora spinosa, Claroideoglomus etunicatum, Funneliformis geosporus, Funneliformis mosseae, Gigaspora decipiens, Gigaspora rosea, Glomus aggregatum, Glomus macrocarpum, Rizhophagus intraradices, and Scutellospora pellucida) produced by the laboratory of beneficial organisms, FCAUV and Pacispora scintillans, produced in the plant-microbial-environment interactions laboratory of IIES, UNAM.
The isolate for both inocula was obtained from spores directly extracted from soil cultivated with maize. It was propagated in pots with autoclaved and autoclaved and 1:1 autoclaved soil with alfalfa as host plant for eight weeks, when colonization of more than 50% was allowed to dry and stored at room temperature, both inocula containing spores, mycelium and fragments of colonized root.
Sterilization of the substrate
A substrate composed of sand, soil and tepetzil (liparite) in proportion (25:50:25), sterilized by autoclaving at 120 °C (14- 15 lb in-2 vapor pressure) for 1 h, for three days consecutive.
Transplantation and inoculation
The plants 15 cm (30 days of germination) were selected were transplanted to containers 2 L and 10 g of the mycorrhizal inocula were added.
Description of treatments
The experiment had a completely random factorial design; with two factors. The first: mycorrhizal inoculant with three levels (Rizofermic-UV, P. scintillans and uninoculated) and the second fertilization with phosphorus, with five levels (without fertilizer, 20 ppm, 40 ppm, 80 ppm, 160 ppm), each treatment with five repetitions.
Fertilizing
The fertilization was carried out from 85% H3PO4 phosphoric acid stock, where 26% was phosphorus (P), mixed with distilled water to obtain the different concentrations that were applied to the seedlings (20, 40, 80, 160 ppm). The application of the fertilizer was done every 8 days, starting the application once the mycorrhizal colonization was established, and 3 mL of the phosphorus fertilizer were applied per plant.
Growing conditions
The experiment was carried out under greenhouse conditions, irrigated with running water at field capacity, and performed this activity daily for the first four months after that date until the experiment was concluded every third day.
Soil characteristics
The soil used, according to Official Mexican Norm 021 RECNAT 2000, has the following physico-chemical properties: sand-sandy with the following sand (73.2%), clay (6.8%), silt (20%), pH 6.04 organic matter 5.93%, organic N 22 ppm, P 4.4 ppm, K 885 ppm, Ca 13.575 ppm, Mg 237 ppm, Fe 6.7 ppm, Cu 0.5 ppm, Zn 1.2 ppm and Mn 3.9 ppm. In accordance with NOM 021 RECNAT 2000 as-09 the soil was classified as sandy grit.
After 401 days after inoculation measuring the evaluated variables was performed: height, diameter, leaf number and leaf area through the program Adobe Photoshop CS4 Extended version 11 and an HP Scanjet 5590 also fresh and dry weight was measured of aerial part and root (the samples were dried at 70 ºC until obtaining constant weight), and growth rate with Gujarati’s technique (2004) that consists of using the initial and final values of the series according to the following equation:ecord the number of grains.
Where: r= is the rate of growth; Vf= final value of the series; Vi= initial value of the series; n= number of repetitions.
Fresh samples of harvested roots (the thinnest of each root system) were washed and cut into 2 cm fragments. Before staining the roots were clarified in a 10% KOH solution for 10 minutes at 120 ° C, followed by acidification with 1% HCl for 1 min at room temperature. The roots were stained in a 0.05% trypan blue solution for 5 min at 120 °C (Phillips and Hayman 1970).
Subsequently, they were placed in lactoglycerol and first observed in an optical microscope (100x magnification, Nikon PFX Optiphot-2) to verify the presence of AMF structures, and later on a stereoscopic microscope (60x magnification, Nikon SMZ645) to determine the percentage of colonization by the intersection gradient technique (Giovannetti and Mosse 1980). The presence of any internal fungal structure in the root was considered as colonization (arbours, vesicles or hyphae).
Results and discussion
The mycorrhizal inoculants had significant effects, as well as the different levels of phosphorus in measured variables and only one effect was found by interaction of both factors in height and growth rate (Table 1).
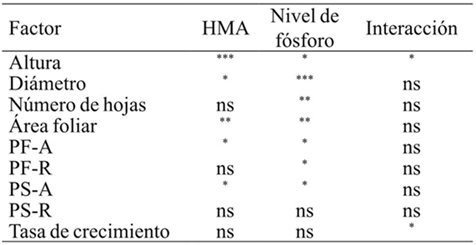
ns= no significativo; *= p≤ 0.05; **= p≤ 0.01; ***= p≤ 0.001; PF-A= peso fresco parte aérea; PF-R= peso fresco raíz; PS-A= peso seco parte aérea; PS-R= peso seco raíz; n= 4.
Table 1. Multivariate ANOVA for each growth variable.
When phosphorus was not added, Rizofermic-UV inoculant showed the highest number of leaves and leaf area. Plants inoculated with P. scintillans had an increase in diameter, height, and leaf area at 20 ppm, compared to the other treatments. Both inoculants increased leaf number, fresh shoot weight, and growth rate at 20 ppm, compared to uninoculated treatment, although this effect was already observed at higher doses of phosphorus. In general, when comparing the effect on the increase of the measured variables between inoculants at different doses of phosphorus, the effect of P. scintillans was more noticeable at 20 ppm, and the effect of Rizofermic-UV occurred at 40 ppm (Table 2).
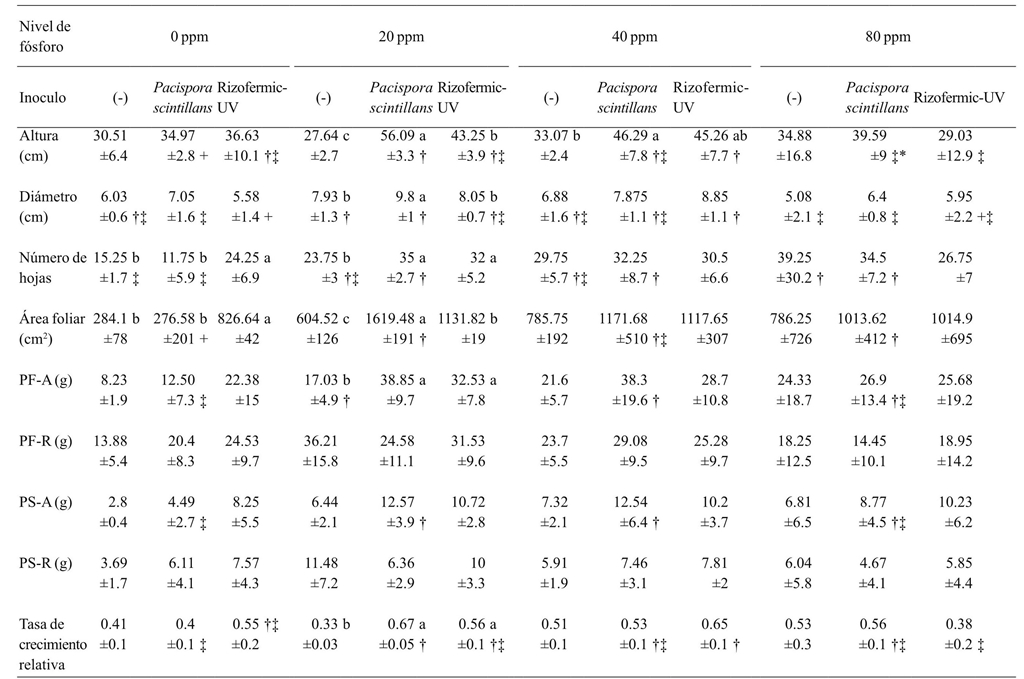
±= indica desviación estándar; PF-A= peso fresco parte aérea; PF-R= peso fresco raíz; PS-A= peso seco parte aérea, PS-R= peso seco raíz; n= 4. Letras distintas muestran diferencias estadísticas entre inóculos por nivel de fertilizante (Fischer, p≤ 0.05); †, ***; ‡, **; +, *= muestra diferencias entre los niveles de fósforo por inoculante.
Table 2 Comparative analysis of the effect of the interaction of two mycorrhizal inoculants and the application of four doses of phosphorus in the development of avocado plants.
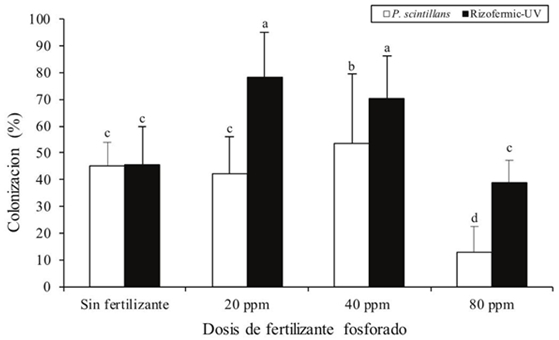
It was found that mycorrhizal colonization decreased with the addition of P doses for both inoculants. Statistical differences (p< 0.05) were observed among the inoculate for this variable. Both the Rizofermic-UV and P. scintillans consortium had a significant decrease in mycorrhizal colonization from 80 ppm, with Rizofermic-UV the inoculant having the highest percentages of root colonization at 20 ppm (78.34%) (Figure 1).
Discussion
According to the results observed in this experiment, the high doses of P (160 ppm) caused the plants to die. It has been reported that high P availability may lead to plant growth depression (Jiménez and Fernández, 2016). In this sense, Marschner (2008) states that high levels of P produce toxicity in the plant by altering the absorption of Zn and Fe. On the other hand, Li et al. (2004) indicate that Zn or Fe deficiencies are more frequent when there are high concentrations of P in the soil, so it is likely that studies that have described micronutrient deficiencies are in fact a reflection of toxicity symptoms due to to high levels of P (Shane et al., 2003).
It was found that the addition of P and mycorrhization promoted plant growth. Within the different doses of P tested, the dose of 20 ppm of P had an effect on the increase of biomass, coinciding with that found by Montoya (2007). Of the two HMA inoculums tested, the plants inoculated with the Rizofermic-UV consortium showed a tendency in the increase of the growth variables, essentially in leaf area (p< 0.05), unlike Pacispora scintillans, which did not have a growth compared to the control.
The differences in effects between HMA strains tested on avocado seedlings, especially in the case of leaf area could be due to functional complementarity between the different species within the Rizofermic-UV consortium and avocado. It is known that HMA species differ widely in their strategies for acquiring P and promoting host plant development (Thonar et al., 2011). In this sense Jaizme and Azcón (1995) found differences of complementarity among HMA species associated with avocado plants.
Some studies report that increases in P levels reduce the development of mycorrhizal symbiosis; However, the levels at which such a reduction occurs are variable (Almaca and Ortas, 2010) and are based on the identity of the HMA species and the plant genotype (Lacerda et al., 2011). In this case, the interactions between mycorrhizal inoculation and the addition of phosphate fertilizer showed different effects. For example, P. sintillans treatment plus 20 ppm of P increased growth variables more than treatments individually, similar results have been reported by (Dutt et al., 20133). In this sense, the addition of P. scintillans could make fertilizer use more efficient and represent a step forward in reducing the current fertilizer doses used for avocado cultivation.
It is known that mycorrhizal colonization begins to occur at three days after inoculation and strengthened from 21 days (Mustafa et al., 2010); however, both studies conclude that mycorrhizal inoculation needs time to show its beneficial effect. Since avocado plants have a rich nutrient reserve, it is possible that the benefits of the mycorrhizal association do not manifest themselves at an early stage of inoculation (Osorio et al., 2012), so the inclusion of these symbionts must occur at a key low lignification stage (Leskovar et al., 1991) and high susceptibility (Afek et al., 1990) of the plant.
Conclusions
In the present work and under the experimental conditions presented here, it is suggested the fertilization with a minimum dose of phosphorus in interaction with HMA, since in the results obtained here was the concentration where a significant increase in the variables was observed and where there was a increase in height and relatively greater leaf area.
The present study showed that inoculation with HMA, essentially with the strain Pascispora scintillans, promotes greater growth in avocado seedlings when they are fertilized with 20 ppm of phosphorus.
For the fresh and dry weight the interaction of the HMA with the P were those that had more relevance. P. scintillans promoted higher growth in plants compared to plants inoculated with the Rizofermic-UV consortium and control plants.
Literatura citada
Afek, U.; Rinaldelli, E.; Menge, J. A.; Johnson, E. L. V. and Pond, E. 1990. Mycorrhizal species, root age, and position of mycorrhizal inoculum influence colonization of cotton, onion, and pepper seedlings. J. Am. Soc. Hortic. Sci. 115(6): 938-942. [ Links ]
Almaca, A. and Ortas, I. 2010. Growth response of maize plants (Zea mays L.) to wheat and lentil pre-cropping and to indigenous mycorrhizae in field soil. Spanish J. Agric. Res. 8(S1): 131-136. [ Links ]
Bañuelos, J.; Trejo, D.; Lara, L.; Gavito, M. and Carreón, Y. 2013. Effects of seven different mycorrhizal inoculum in Persea americana in sterile and non-sterile soil. Trop. Subtrop. Agroecos. 16(3):423-429. [ Links ]
Bárcenas, A.; Almaraz, C.; Reyes, L.; Varela, L.; Lara, B.; Guillén, A.; Carreón, Y. : Aguirre, S. y Chávez, A. 2007. Diversidad de hongos micorrizógenos arbusculares en huertos de aguacate de Michoacán, In: VI congreso mundial del aguacate. Viña del Mar, Chile. 1 p. [ Links ]
Bever, J. D. 2002. Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proceedings of the Royal Society of London B: Biol. Sci.269(1509):2595- 2601. [ Links ]
Castro, A. E.; Chávez, B. A.; García, S. P. A.; Reyes, R. L. and Bárcenas, O. E. A. 2013. Effect of mycorrhizal inoculants in the development of Mexican landrace avocado rootstocks. Trop. Subtrop. Agroecos. 16(3):407-413. [ Links ]
Chen, H.; Morrell, P. L.; Ashworth, V. E. T. M.; Cruz, M. and Clegg, M. T. 2009. Tracing the geographic origins of major avocado cultivars. J. Heredity. 100(1):56-65. [ Links ]
Chesworth, W. 2008. Encyclopedia of soil science. Encyclopedia of earth sciences series. Springer Firm. 1923 p. [ Links ]
Condron, L. M. and Tiessen, H. 2005. Interactions of organic phosphorus in terrestrial ecosystems. In: B. L.; Turner, B. L.; Frossard, E.; Baldwin, D. S. (Eds.). Organic phosphorus in the environment. Cabi Publ., Cambridge, MA. EUA. 295-308 p. [ Links ]
Dutt, S.; Sharma, S. D. and Kumar, P. 2013. Inoculation of apricot seedlings with indigenous arbuscular mycorrhizal fungi in optimum phosphorus fertilization for quality growth attributes. J. Plant Nutr. 36(1):15-31. [ Links ]
Giovannetti, M. and Mosse, B. 1980. Evaluation of the techniques for measuring vesicular arbuscular mycorrhizal infections in roots. New Phytol. 84(3):489-500. [ Links ]
Gujarati, D. N. 2004. Basic econometrics. 4th edition. McGraw Hill: New York. 1032 p. [ Links ]
Gutiérrez, C. M.; Lara, M. B. N.; Guillén, A. H. and Chávez, B. A. T. 2010. Agroecología de la franja aguacatera en Michoacán. México. Interciencia. 35(9): 647-653. [ Links ]
Helgason, T. and Fitter, A. H. 2009. Natural selection and the evolutionary ecology of the arbuscular mycorrhizal fungi (Phylum Glomeromycota). J. Exp. Bot. 60(9):2465-2480. [ Links ]
Herrmann, L.; Bräu, L.; Robin, A.; Robain, H.; Wiriyakitnateekul, W. and Lesueur, D. 2015. High colonization by native arbuscular mycorrhizal fungi (AMF) of rubber trees in small-holder plantations on low fertility soils in North East Thailand. Archives Agron. Soil Sci. 62(7):1041-1048. [ Links ]
Jiménez, M. M. J. and Fernández, E. R.2016. Response of young olive plants (Olea europaea) to phosphorus application. HortSci. 51(9):1167-1170. [ Links ]
Khan, A. G. 2006. Mycorrhizoremediation an enhanced form of phytoremediation. J. Zhejiang University SCIENCE B. 7(7):503-514. [ Links ]
Klironomos, J. N. and Hart, M. M. 2002. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza. 12(4):181-184. [ Links ]
Lacerda, K. A. P.; Silva, M. M. D. S.; Carneiro, M. A. C.; Reis, E. F. D. e Saggin, O. J.2011. Fungos micorrízicos arbusculares e adubação fosfatada no crescimento inicial de seis espécies arbóreas do cerrado. CERNE [online];. 17(3):377-386. [ Links ]
Leskovar, D. I; Cantliffe, D. J and Stoffella, P. J. 1991. Growth and yield of tomato plants in response to age of transplants. J. Am. Soc. Hortic. Sci. 116(3): 416-420. [ Links ]
Li, L.; Tang, C.; Rengel, Z. and Zhang, F. S. 2004. Calcium, magnesium and microelement uptake as affected by phosphorus sources and interspecific root interactions between wheat and chickpea. Plant and Soil. 261(1-2): 29-37. [ Links ]
Marschner, P. 2008. The role of rhizosphere microorganisms in relation to P uptake by plants. In: White, P. J. y Hammond, J. P. (Eds.). The ecophysiology of plant-phosphorus interactions. Springer Sci. 296 p. [ Links ]
Montoya, A. S. 2007. El modelo agrícola colombiano y los alimentos en la globalización. Ediciones Aurora. Bogotá, Colombia. 221 p. [ Links ]
Mustafa, A. A. A.; Othman, R.; Zinal-Abidin, M. A. and Ganesan, V.2010. Growth response of sweet corn (Zea mays) to Glomus mosseae inoculation over different plant ages. Asian J. Plant Sci. 9(6):337-343. [ Links ]
Osorio, N. W.; Sandra, L. S. G. and Montoya, R. B. E. 2012. Use of soil microorganisms as a biotechnological strategy to enhance avocado (Persea americana) plant phosphate uptake and growth/uso de microorganismos del suelo como estrategia biotecnologica para mejorar la absorción de fósforo y el crecimiento. Rev. Facultad Nacional Agron. Medellín. 65 pp. [ Links ]
Phillips, J. M. and Hayman, D.S. 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55(1):158-161. [ Links ]
Rodríguez, C. G.; Caravaca, F.; Fernández, G. A. J.; Alguacil, M. M.; Fernández, L. M. and Roldán, A. 2017. Arbuscular mycorrhizal fungi inoculation mediated changes in rhizosphere bacterial community structure while promoting revegetation in a semiarid ecosystem. Sci. Total Environ. 584(585): 838-848. [ Links ]
Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; Van der Heijden, M. G. A. and Oehl, F. 2015. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 84: 38-52. [ Links ]
Shane, S. A. 2003. A general theory of entrepreneurship: the individualopportunity nexus. Edward Elgar Publishing Ltd. Cheltenham, UK. 352 p. [ Links ]
Smith, S. E. and Read, D. J. 2008. Micorrhizal Symbiosis. Academic Press 605. San Diego CA, USA. 800 p. [ Links ]
Smith, S. E.; Smith, F. A., and Jakobsen, I. 2003. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 133(1):16-20. [ Links ]
Smith, S. E.; Smith, F. A. and Jakobsen, I. 2004. Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 162(2): 511-524. [ Links ]
Smith, F. A.; Jakobsen, I. and Smith, S. E. 2000. Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago trunculata. New Phytol. 147(2):357-366. [ Links ]
Smith, S. E. and Smith, F. A. 2011. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Ann. Rev. Plant Biol. 62 (2011):227- 250. [ Links ]
Smith, S. E.; Jakobsen, I.; Grønlund, M. and Smith, F. A. 2011. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 156(3):1050-1057. [ Links ]
Souchie, E. L.; R. Azcón, J. M.; Barea, O. J.; Saggin-Júnior and Ribeiro da Silva, E. M. 2006. Phosphate solubilizing and arbuscular mycorrhizal fungi. Pesquisa Agropecuária Brasileira. 41(9):1405-1411. [ Links ]
Taffouo, V. D.; Ngwene, B.; Akoa, A. and Franken, P. 2014. Influence of phosphorus application and arbuscular mycorrhizal inoculation on growth, foliar nitrogen mobilization, and phosphorus partitioning in cowpea plants. Mycorrhiza. 24(5):361-368. [ Links ]
Thonar, C.; Schnepf, A.; Frossard, E.; Roose, T. and Jansa, J. 2011. Traits related to differences in function among three arbuscular mycorrhizal fungi. Plant Soil. 339(1-2):231-245. [ Links ]
Violi, H. A.; Barrientos P. A. F.; Wright, S. F.; Escamilla P., E.; Morton, J. B.; Menge, J. A. and Lovatt, C. J. 2008. Disturbance changes arbuscular mycorrhizal fungal phenology and soil glomalin concentrations but not fungal spore composition in montane rainforests in Veracruz and Chiapas, Mexico. Forest Ecol. Manag. 254(2):276-290. [ Links ]
Weih, M.; Asplund, L. and Bergkvist, G. 2011. Assessment of nutrient use in annual and perennial crops: a functional concept for analyzing nitrogen use efficiency. Plant Soil. 339(1-2):513- 520. [ Links ]
Withers, P. J. A.; Edwards, A. C. and Foy, R. H. 2001. Phosphorus cycling in UK agriculture and implications for phosphorus loss from soil. Soil Use Manage. 17(3):139-149. [ Links ]
Zhu, Y. G.; Cavagnaro, T. R.; Smith, S. E. and Dickson, S.. 2001 Backseat driving? Accessing phosphate beyond the rhizospheredepletion zone. Trends in Plant Sci. 6(5): 194-195. [ Links ]
Zhu, Y. G.; Smith, A. F. and Smith, S. E. 2003. Phosphorus efficiencies and responses of barley (Hordeum vulgare L.) to arbuscular mycorrhizal fungi grown in highly calcareous soil. Mycorryiza. 13(2):93-100. [ Links ]
Received: July 01, 2017; Accepted: August 01, 2017











 texto em
texto em