Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 no.7 Texcoco sep./nov. 2016
Articles
Growth and yield of Capsicum annuum L. inoculated with endomycorrhiza and rhizobacterias
1Universidad Autónoma de Chiapas- Facultad de Ciencias Agrícolas. Entronque carretera costera y Estación Huehuetán. CP 30660. Huehuetán, Chiapas, México. (elcampirano@yahoo.com).
The effectiveness was evaluated of mycorrhizal inoculation-rhizobacteria on growth, yield and colonization of jalapeno pepper Capsicum annuum L. This study was conducted from September 2013 to May 2014. The mollic-andosol soil was used, which sand washed and sieved was added, in the ratio 1:1 (V/V) and yet it is added 30% chicken manure (v/v). The treatments consisted of inoculation and co-inoculation of mycorrhizal and rhizobacteria and the witness. In total, eight treatments with five replicates distributed in a completely randomized design. A plant was considered as repetition. Three destructive samplings were made every 28 days. The results showed a positive effect of co-inoculation on plant growth, with initial variations and contrasting finals in interaction with various microorganisms. Individual inoculation of microorganisms Pseudomonas fluorescens, Azospirillum brasilense and co-inoculation of R. intraradices +A. brasilense increased the number of fruits. The co-inoculation of R. intraradices + P. fluorescens and A. brasilense induced larger fruits.
Keywords: Rhizophagus intraradices; Pseudomonas fluorescens; Azospirillum brasilense; mycorrhizal colonization
Se evaluó la efectividad de la coinoculación micorriza-rizobacterias en el crecimiento, rendimiento y colonización del chile jalapeño Capsicum annuum L. El presente estudio se realizó de septiembre de 2013 a mayo de 2014. Se utilizó suelo Andosol- mólico al cual se agregó arena de rio lavada y tamizada, en proporción 1:1 (V/V) y al mismo se le adicionó 30% de gallinaza (v/v). Los tratamientos consistieron en la inoculación y la coinoculación de micorriza y rizobacterias y el testigo. En total, ocho tratamientos con cinco repeticiones distribuidas en un diseño completamente al azar. Se consideró una planta como repetición. Se realizaron tres muestreos destructivos cada 28 días. Los resultados mostraron un efecto positivo de la coinoculación en el crecimiento de las plantas, con variaciones iniciales y finales contrastantes en interacción con los diversos microorganismos. La inoculación individual de los microorganismos Pseudomonas fluorescens, Azospirillum brasilense y la coinoculación de R. intraradices +A. brasilense incrementaron el número de frutos. La coinoculación de R. intraradices + P.fluorescens y A. brasilense indujeron frutos más grandes.
Palabras clave: Rhizophagus intraradices; Pseudomonas fluorescens; Azospirillum brasilense; colonización micorrízica
Introduction
The interaction between the components of a microbial community in agricultural systems may manifest or not any morphological or physiological attribute of anthropocentric interest of the host plant. In some annuals and perennials found in the different components increase performance by inoculating fungi and bacteria together (Aguirre-Medina et al, 2012). These benefits have been tried in other crops in order to identify synergism between some associations of soil microorganisms and growing interest. Some of the benefits on plant growth are expressed with the combination of associative bacteria and mycorrhizal fungi.
The interaction between rhizobacteria promoting plant growth and fungi endomycorrhizal, can be selective and dependent bacteria and fungus involved (Azcon, 2000). The positive interaction in the growth and yield of some crops has encountered inoculating of Rhizophagus intraradices, Azospirillum brasilense and/or Rhizobium etli (Aguirre Medina, 2006), along with increases in the nitrogen content (N) and phosphorus (P) in plant tissue and grain (Aguirre-Medina et al, 2012). The pseudomonas are an important group of microorganisms that can promote the growth of plants and protect them from pathogens (Unno et al, 2005).
For the production of chili is of great importance the overall evaluation of the use of bio-fertilizers based fungi-rhizobacteria that have been evaluated in other cultures and have proven effective action on plants. Given this background, this study aimed to study agricultural biological effectiveness of mycorrhiza-rhizobacterias co-inoculation on the growth and yield of chile.
Materials and methods
Experimental site, soil and biological material
The research was conducted in the nursery of the Experimental Rosario Izapa, the National Institute of Forestry, Agriculture and Livestock (INIFAP), located at km 18 of the Tapachula-Cacaohatan municipality of Tuxtla Chico, Chiapas, geographically Highway at 14° 40' north latitude and 92° 10' west longitude to 435 masl, from September 2013 to May 2014.
On the site the annual average rainfall is 4 700 mm, the average maximum temperature of 25.4 °C, with a minimum of 17 °C with a relative humidity of 85%. The soil used was obtained from 0-30 cm depth in the grounds of Rosario Izapa Chiapas, characterized as andosols (Grajales, De la Piedra and López, 2008).
The substrate was formed with the mixture of soil and sand washed and sifted river, in the ratio 1:1 (V/V) chicken manure was added 30% (v/v). The substrate had the following physical and chemical characteristics; crumbly texture-sand (Bouyucos), (78.66% sand, 18.36% silt, 2.98% clay), porosity (36.12%), true density (1.91 g ml-1), bulk density (1.22 g ml-1), organic matter (07.03%), pH 6.64, N (0.32%), P (136 ppm), K (825 ppm) and with the same plastic bags with a capacity of eight kg were placed on terraces or filled iron beds.
Treatments and application. The seeds Capsicum annuum L., regional jalapeno type trays were seeded with the substrate before planting and were attached to the seed microorganisms Azospirillum brasilense, Rhizophagus intraradices and Pseudomonas fluorescens with carboxymethylcellulose, individually or together. In most treatments of a microorganism, it was first adhered bacteria and the fungus after endomycorrhizal. Transplanting was done 30 days to polyethylene bags with a pair of true leaves. The bacterium A. brasilense was acquired at theAutonomous University of Puebla (UAP) at a concentration of109 bacteria per (g) of peat, P. fluorescens was provided by the INIFAP-Campo Experimental Celaya, with 9 x 106 UFC per gram of peat and R. intraradices developed in the INIFAP Experimental Rosario Izapa-Campo, Chiapas, with at least 40 spores per g soil and 95% of root colonization in the host Brachiaria decumbens L. The data concentrations of microorganisms plant indicate on each product.
The amount of fungus used was calculated based 6% by weight of the seed and the bacteria A. brasilense and P. fluorescens was calculated based 4% by weight of the seed. The treatments were the witness, R. intraradices, A. brasilense, P. fluorescens, R. intraradices + A. brasilense, A. brasilense + P. fluorescens and R. intraradices + A. brasilense + P. fluorescens with five repetitions distributed a completely randomized design. A plant was considered as repetition. Plants were watered with approximately 100 ml of water every other day.
Variables. The morphological variables (plant height, stem diameter, number of leaves and fruits) and physiological (dry biomass of root, stem and leaf) were recorded every 28 days until day 84. Likewise, the radical colonization every 28 days and the number and fresh weight of fruit harvest. The plant height was recorded with tape measure in cm from the root crown to the apical bud. The stem diameter in mm with digital vernier (AutoTECTM, China) to five cm away from the root crown to the apex of after dehydration plant, in addition to the total number of leaves on each plant and the number and weight of fruits at harvest time determining their individual weight in semi-analytical scale (Ohaus, Adventurer Pro, USA).
The biomass dry weight of shoot and root was obtained by a semi-analytical scale (Ohaus Adventurer Pro, USA) after drying in forced air oven at 75-80 °C to constant weight. The mycorrhizal colonization expressed as a percentage, was determined by Phillips and Hayman (1970). The statistical analysis was performed using the Statistical Analysis System package, version 8.1 (SAS, 1999-2000) and the comparison between treatment means by Tukey with α= 0.05.
Results and discussion
In the Table 1 shows the growth of C. annuum L. plant with the treatments applied. The plant height was statistically different (p≤ 0.05) for all three samples. In the first two sampling the plant height increased by inoculating seeds with a microorganism compared to the inoculation of two or all three microorganisms. The maximum height was 63 cm and recorded in plants inoculated with A. brasilense. With Azospirillum is favored the plant growth by the increase in the radical development of the host plant by producing hormones and nitrogen fixation (Bashan and Bashan-2010).
Table 1 Comparisons of average plant height, leaf number and stem diameter of Capsicum annuum L. bofertilized with different microorganisms in andosol-mollic of Soconusco, Chiapas soil.
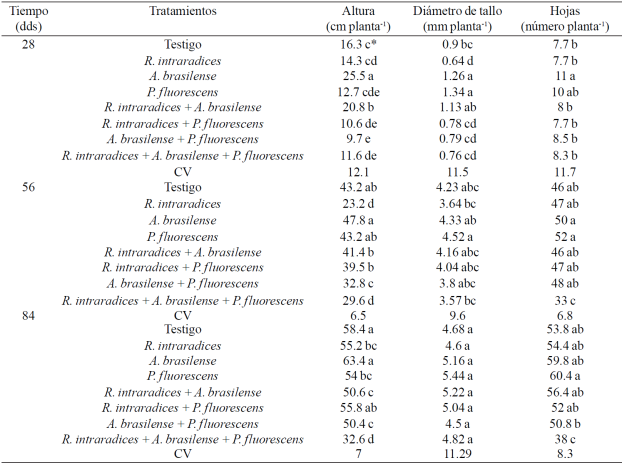
CV= coeficiente de variación (%). 'Valores con diferente letra dentro de cada factor y columna son estadísticamente diferentes (p ≤0.05).
The inoculated with microorganisms three plants showed about half of the height compared to A. brasilense.Alonso and Galán (2006) cite in tomato increase in plant height with A. brasilense.Karthikeyan et al. (2009) recorded increase in plant height to inoculate the seeds of Catharanthus roseus (L.) G. Don. two bacteria, A. brasilense and P. fluorescens and Neetu et al. (2012) cite tallest plants to inoculate Linum usitatissimum L. with P. fluorescens + G. mosseae. Meanwhile Reyes-Ramírez et al. (2014) reported increased height habanero pepper plants (Capsicum chinense Jacq.) after 90 days of being inoculated with Pseudomonas spp. compared with plants inoculated with A. brasilense and the witness.
In some cultures the response to inoculation is higher with a single microorganism and may be related to the increased demand for carbon colonization more symbionts (Sylvia, 2005). In annual crops such as corn and beans, we found differential response to the simple inoculation and co-inoculation of more than one microorganism (Aguirre-Medina, 2006), the previous answer seems to be influenced by the ability of colonization of microorganisms, by interaction with the ground and naturalized microorganisms present in the soil.
Stem diameter also showed statistically significant difference (p≤ 0.05) between treatments during the first two samples. At 28 days after transplanting (ddt), the largest stem thickness was found with inoculation A. brasilense and P. fluorescens. At 56 ddt, stem diameter was very similar among treatments, but the statistical differences favored inoculated with P. fluorescens treatment. Results contrary presented Reyes-Ramírez et al. (2014) in habanero chile (Capsicum chinense Jacq.) Who found no differences between treatments for stem diameter at the first sampling dates, but from 90 ddt and was higher with inoculation of Pseudomonas spp. and compared to the control plants inoculated with A. brasilense.
In other perennial crops it has consigned the same effect but with G. intraradices (Chattopadhyay et al., 2006; Aguirre-Medina et al., 2007 and 2011), and with Gigaspora margarita in plants of C. canephora (Tristáo et al., 2006). The number of sheets presents contrasting response between treatments at 28 ddt and individually inoculated A. brasilense was statistically different (p≤ 0.05) the rest ofthe treatments. In the following samples, the number of leaves was similar between treatments, including the witness, and the least amount of leaves was presented with the inoculation of the three microorganisms. In this regard there is evidence that the combination of more than one microorganisms not necessarily induce an additive or synergistic effect on the host plant (Trabelsi and Mhamdi, 2013), as in our investigation.
Sampling in two and three the number of leaves of plants inoculated with a microorganism increased and was very similar between the two microorganisms coinoculaciones. It is likely that this time has been improved plant-microbe relationship. Between plants and bacteria there is also a complex and dynamic language for colonization, as their ability to use root exudates as carbon sources and their ability to interact and collaborate with other microorganisms in the rhizosphere (Holguin, 2008). In perennial greenhouse crops, plant microorganisms response thereofis expressed about 90 days after inoculation, as in Tabebuia donnell-smithii Rose (Aguirre-Medina et al., 2014), in Cedrela odorata L. (Aguirre-Medina et al., 2014) and Robusta coffea Coffea canephora (Pierre) ex Froehner (Ibarra-Puon et al, 2004).
For the control treatment, the response is due to the presence of other microorganisms associated naturalized in their root system that favored its development. In this research, only counting the radical colonization was performed by the endomycorrhizal fungus and in the case of the witness, the initial radical colonization was 72% and finally 27%. Very similar values to those found in the treatments where was applied R. intraradices. These facts suggest the contrasting functionality coinoculaciones interacting with plants (Jáderlund et al, 2008).
Dry weight of leaf blade, stem and root. The greatest accumulation of dry biomass of the leaf in plants inoculated was presented with A. brasilense, P. fluorescens and the combination R. intraradices + A. brasilense at 28 ddt, R. intraradices and A. brasilense inoculated separately 56 ddt, and at the end of the evaluation at 84 ddt, all treatments outperformed the treatment inoculated with the three microorganisms (Table 2). Reyes-Ramírez et al. (2014) found no difference in the leaf biomass habanero chile (Capsicum chinense Jacq.) inoculated with Pseudomonas spp., R. irregularis or A. brasilense. In perennial crops such as Leucaena leucocephala (Lam.) De Wit and Theobroma cacao L. coinoculated with Glomus-Rhizobium and Azospirillum microorganisms respectively, increases occur in these same yield components (Aguirre-Medina et al., 2007).
Table 2 Dry weight of leaf blade, stem and root in plants Capsicum annnum L. biofertilizers with different microorganisms in soil andosol-mollic in Soconusco, Chiapas.
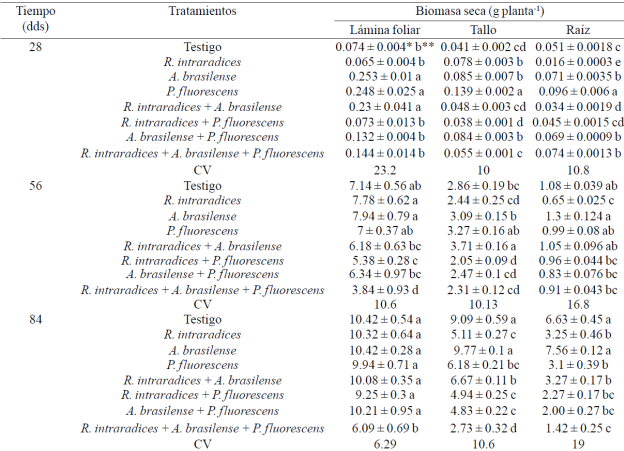
CV= coeficiente de variación (%). * ± error estándar. *Valores con diferente letra dentro de cada factor y columna son estadísticamente diferentes (p≤ 0.05).
The slow initial growth in the treatments inoculated with two microorganisms, is likely related to the increased demand for photosynthates required by them during the establishment phase of symbiosis and consequently decreases its initial growth. In Tabebuia donnell-smithii Rose to evaluate various collections of endomycorrhizal fungi from different regions of Southeast Mexico, he found that induction in the growth of yield components was expressed at different times (Aguirre-Medina et al., 2014).
The dry weight of main stem showed higher biomass in treatments inoculated with P. fluorescens at 28 and 84 ddt and symbiosis R. intraradices + A. brasilense at 56 ddt compared with inoculation of the three organisms and were statistically higher (p≤ 0.05) than the other treatments. With Pseudomonas spp. stem biomass habanero chile (C. chinense Jacq.) increased but not with the combination of endomycorrhizal fungus (Reyes-Ramírez et al., 2014). In Coffea canephora (Pierre) ex Froehner, stem biomass also increased with inoculation separately from the same microorganisms (Aguirre-Medina et al., 2011).
Most biomass accumulated in the root system was recorded with P. fluorescens and was statistically different (p≤ 0.005) to the other treatments and coincides with that reported by Reyes-Ramírez et al. (2014) in C. chinense Jacq. During the next sampling, this response was also presented with A. brasilense. The greatest growth in the root system may be related to the increased growth of some substances, the result of the symbiosis between the plant and microorganisms. A. brasilense and P. fluorescens promote root development by producing indole acetic acid (Patten and Glick, 2002; Hungria et al, 2004; Ahmad et al, 2006; Neetu et al, 2012) amending root morphology and increase biomass.
Similarly, A. brasilense can induce greater root development in annual plants are inoculated togetherAzospirillum+Glomus in Phaseolus vulgaris L. and Zea mays L. (Dobbelaere et al., 2003). P. fluorescens can stimulate proliferation of roots and has been linked to the promotion of plant growth (Gamalero et al., 2004) and the possible removal of pathogenic microorganisms in the soil (Neetu et al., 2012). The above results indicate that dry matter accumulation in the plant components of Capsicum annuum L., applied microorganism varies over time. At the beginning of the evaluation, the treatments inoculated with A. brasilense, P. fluorescens and double symbiosis R. intraradices+A. brasilense, they accumulated the highest amount of dry matter in different yield components, and this time most mycorrhizal colonization was reached in the combined treatment, which was higher than 90%.
With the individual record of the weight of the fruits were classified into three categories. Of total average fruit per plant, which weighed up to ten g represented 13.7% of total production. 41% of fruits weighed between five and 10 g and less than 5 g, 45.5% of them (Figure 1).
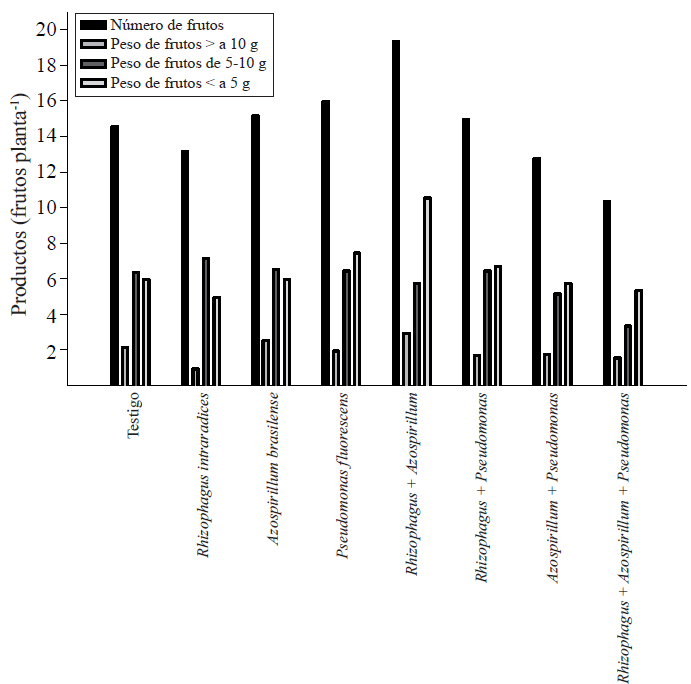
Figure 1 Number and fresh weight of fruits of Capsicum annuum L. biofertilizers with different microorganisms alone or combined in a soil Andosol mollic of Chiapas. The values are averages of five repetitions.
The fruits with greater than 10 g weight was two on average almost in all treatments, except treatment symbiosis R. intraradices, P. fluorescens and the three microorganisms.
Weight in the range of five to ten g, the ratio between treatments was very similar, i.e., there were six to seven fruits. The greatest variation occurred in the fruits that weighed less than 5 g. In this regard, with the double symbiosis R. intraradices+ A. brasilense as many fruits of less than 5 g size was found, and represented approximately 50% of this surplus. This suggests the importance of inoculating a microorganism chili plants to encourage fruiting. If you enter more than one organism, it is likely that the demand for photosynthates by them, reduce the possibility that they are transported to the flowering and fruit set. It has been shown that P. fluorescens in the rhizosphere increased biomass and crop productivity (Sood, 2003), as in the case of habanero (Reyes-Ramírez et al, 2014).
Colonization radical. The root colonization of plants at 28 ddt, was high in all treatments where microorganisms (Figure 2) were applied. On average, the range of colonization was the order of 60% to 96%. In the case of control without application of microorganisms initial radical colonization was high, 72%, probably because endomycorrhizal fungi present in the substrate and it is common in species that have adapted to the environmental conditions of the region.
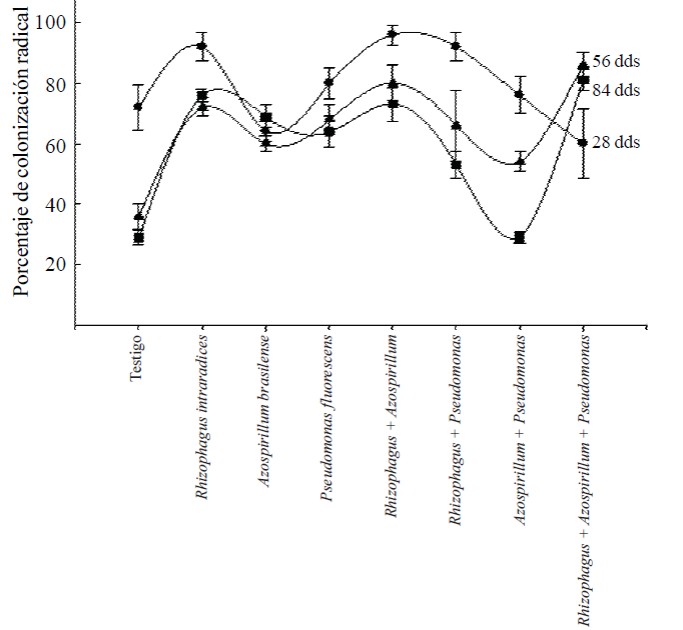
Figure 2 Radical colonization of Capsicum annuum L. biofertilizers with different microorganisms alone or combined in a soil adosol mollic of Chiapas. The values are averages of five replicates ± standard error for each sample.
It was expected that the inoculation of Azospirillum and Pseudomonas bacteria favor increased root colonization, as has happened in other plants (Aguirre-Medina, 2006); however, in the second and third sampling values of root colonization, when applied alone microorganisms, it was 60 to 68% and increased 80 to 86% from when included R. intraradices.
The lowest percentage of mycorrhizal colonization in treatments inoculated with bacteria due to the colonization of endomycorrhizal fungi present in the substrate. When R. intraradices+P. fluorescens, were applied together, also the percentage of colonization was low, on the order of 66% and with the two bacteria together, it was 29%. This indicates that not all microorganisms have the ability to associate to induce greater benefits to the host plant.
In the final evaluation, the percentages of root colonization by mycorrhizal fungi was 76% with R. intraradices, 69% with A. brasilense, 64% with P P. fluorescens and 73% with double symbiosis R. intraradices + A. brasilense and was these treatments where the greatest effect on increasing plant biomass, the thicker the stem diameter, number of leaves, flowers and fruit found. In the other co-inoculation treatments two microorganisms, root colonization was less than 53 and 29% for R. intraradices + P. fluorescens and A. brasilense + P. fluorescens and none of the evaluated variables showed significant or higher values others, not statistically significant differences.
The effects of microorganisms in plant development of plants have been documented (Barea et al., 2002) and in other cases, the application of selected mycorrhizal fungi have favored the performance of various crops, when applied alone, or in combination with bacteria, such as P. fluorescens, which stimulates mycorrhizal colonization tomato and increases production (Gamalero et al., 2004).
Conclusions
The morphological and physiological components chili plant present contrasting initial and final changes in interaction with various microorganisms. Individual inoculation of microorganisms Pseudomonas fluorescens, Azospirillum brasilense and co-inoculation of R. intraradices +A. brasilense increased the number of fruits. The co-inoculation of R. intraradices+P. fluorescens and individual inoculation of A. brasilense induced larger fruits
Literatura citada
Aguirre, M. J. F. 2006. Biofertilizantes microbianos: Experiencias agronómicas del programa nacional del INIFAP en México. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Centro de Investigaciones Regionales Pacífico Sur. Campo Experimental Rosario Izapa. Libro técnico Núm. 2. 201 p. [ Links ]
Aguirre, M. J. F.; Mendoza, L. J. A. Cadena, I. y Avendaño, A. C. 2007. La Biofertilización del cacao (Theobroma cacao) L. en vivero con (Azospirillum brasilense) Tarrand, Krieg et Dobereiner y (Glomus intraradices) Schenk et Smith. Interciencia. 32(8):1-6. [ Links ]
Aguirre, M. J. F.; Moroyoqui, O. D. M.; Mendoza, L. A.; Cadena, I. J.; Avendaño, A. C. H. y Aguirre, C. J. F. 2011. Aplicación de A. brasilense y G. intraradices a Coffea arabica en vivero. Agron. Mesoam. 1(22):1-10. [ Links ]
Aguirre, M. J. F.; Culebro, C. J. F; Cadena, I. y Aguirre, C. J. F. 2014. Crecimiento de TabebuiaDonnell-Smithii (Rose) Inoculada con Hongos Micorrizicos y Azospirillum brasilense. Agrociencia. 48(3):331-345 [ Links ]
Aguirre, M. J. F.; Aguirre, C. J. F.; Cadena, I. J. y Avendaño, A. C. H. 2012. Biofertilización en plantas de la selva húmeda tropical. Colegio de Postgraduados. Montecillo, Estado de México. 99 p. [ Links ]
Ahmad, F.; Ahmad, I. and Khan, M. S. 2006. Screening of free-livingrhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 36:1-9. [ Links ]
Alonso, E. T. y Galán, A. L. 2006. Evaluación agrobiológica de la coinoculación micorrizas-rizobacterias en tomate. Agron. Costarric. 30(1):65-73. [ Links ]
Azcón, R. 2000. Papel de la simbiosis micorrizica y su interacción con otros Microorganismos rizosfericos en el crecimiento vegetal y sostenibilidad agrícola. In. Alarcón, A. y Ferrera-Cerrato, R. (Eds.). Ecología, fisiología y biotecnología de la micorriza arbuscular. Mundi Prensa, México. 251 p. [ Links ]
Barea, J. M.; Azcon, R. and Azcon, A. C. 2002. Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie Van Leeuwenhoek. Int. J. General Mol. Microbiol. 81(1-4):343-351. [ Links ]
Bashan, Y. and de-Bashan, Luz E. 2010. How the plant growth-promoting bacterium Azospirillum promotes plant growth- a critical assessment. Adv. Agron. 108:77-136. [ Links ]
Chattopadhyay, N.; Swain, S. S. and Hore, J. K. 2006. Response of coffee seedlings to nitrogen fixing biofertilizers. Agric. Sci. Digest. 26(2):103-106. [ Links ]
Dey, R.; Pal, K.; Bhatt, D. and Chauhan, S. 2004. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res. 159(4):371-394. [ Links ]
Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Ptacek, D.; Vanderleyden, J.; Dutto, P.; Labandera, G. C.; Caballero, M. J, Aguirre, M. J. F.; Kapulnik, Y.; Brener, S.; Burdman, S.; Kadouri, D.; Sang, S. and Okon, J. 2001. Responses of agronomically important crops to inoculation with Azospirillum. Aust. J. Plant Physiol. 28(9):871-879. [ Links ]
Dobbelaere, S.; Vanderleyden, J. and Okon, Y. 2003. Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit. Rev. Plant Sci. 22: 07-149. [ Links ]
Gamalero, E.; Trotta, A.; Massa, N.; Copetta, A.; Martinotti, M. and Berta, G. 2004. Impact of two Pseudomonas fluorescens and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza. 14:185-192. [ Links ]
Grajales, M.; De la Piedra, R. y López, J. 2008. Diagnóstico biofísico y socioeconómico de la parte media y alta de la subcuenca Cohatán en el Soconusco, Chiapas. Avances en Investigación Agropecuaria. 12(1):29-44. [ Links ]
Holguín, Z. G. 2008. La comunicación entre bacterias y plantas. Rev. Ciencia. 72-78 p. [ Links ]
Hungría, M.; Campo, R. J.; Souza, E. M. and Pedrosa, F. O. 2004. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil. 331:413-425 [ Links ]
Ibarra, P. J. C.; Aguirre, M. J. F. ; De Coss, A. L.; Cadena, I. J. y Zavala, M. A. 2014. Inoculación de Coffea canephora (Pierre) ex Froehner con Rhizophagus intraradices (Schenck et Sm.) Walker et Schuessler y Azospirillum brasilense Tarrand, Krieg et Dobereiner en vivero. Rev. Chapingo Ser. Hortic. 20(2):201-213. [ Links ]
Jáderlund, L.; Arthurson, U.; Granhall, V. and Jansson, J. K. 2008. Specific interactions between arbuscular mycorrhizal fungi and plant growth-promoting bacteria: as revealed by different combinations. FEMS Microbiology Letter. 287(2):174-180. [ Links ]
Karthikeyan, B.; Cheruth, A. J. and Azooz, M. M. 2009. Individual and combined effects of Azospirillum brasilense and Pseudomonas fluorescens on biomass yield and ajmalicine production in Catharanthus roseus. Academic J. Plant Sci. 2(2):69-73. [ Links ]
Mohandas, S. 1987. Field response of tomatoes (Lycopersicon esculentum Mill "Pusa Ruby") to inoculationwithV-A fungus Glomusfasciculatum and with Azotobacter vinelandii. Plant Soil. 98:295-297. [ Links ]
Neetu, N.; Ashok, A.; Anju, T. and Alpa, A. 2012. Influence of arbuscular mycorrhizal fungi and Pseudomonas fluorescens at different superphosphate levels on linseed (Linum usitatissimum L.) growth response. Chilean J. Agric. Res. 72(2):237-243. [ Links ]
Patten, C. L. and Glick, B. R. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 68(8):3795-3801. [ Links ]
Phillips, J. M. and Hayman, D. J. 1970. Improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycol. Soc. 55:158-161. [ Links ]
Reyes, R. A.; López, A. M.; Ruiz, S. E.; Latournerie, M. L.; Pérez, G. A.; Lozano, C. M. G. y Zavala, L. M. J. 2014. Efectividad de inoculantes microbianos en el crecimiento y productividad de chile habanero (Capsicum chinense Jacq.). Agrociencia 48:285-294. [ Links ]
Russo, A.; Gotz, C. M.; Felici, C. C.; Moenne, L. Y.; Vanderleyden, A. J.; Toffanin, J. M.; Barea, K.; Smalla, and Nuti, N. 2005. Effect of Azospirillum inoculants on arbuscular mycorrhiza establishment in wheat and maize plants. Biol. Fertility Soils. 41(5):301-309. [ Links ]
Sood, G. 2003. Chemotactic response of plant-growth-promoting bacteria toward root ofvesicular-arbuscular mycorrhizal tomato plants. Microbiol. Ecol. 45:219-227. [ Links ]
Sylvia, M. D. Mycorrhizal symbioses. 2005. In: Sylvia, M.D., Fuhrmann, J. J.; Harte, G. P. and Zuberer, A. D. (Ed.). Principles and applications of soil microbiology. Second Edition, New Jersey, USA. Pearson Prentice Hall. 263-282 p. [ Links ]
Trabelsi, D. and Mhamdi, R. 2013. Microbial Inoculants and Their Impact on Soil Microbial Communities: a review. BioMed Res. Inter. 1-11 pp. [ Links ]
Tristáo, F. S. M.; López de A, S. A. e Parada, D. S. A. 2006. Fungos micorrízicos arbusculares na formajao de mudas de cafeeiro, em substratos orgánicos comerciais. Bragantia. 65(4):649-658. [ Links ]
Unno, Y.; Okubo, K.; Wasaki, J.; Shinano, T. and Osaki, M. 2005. Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of Lupin analysed by phytate utilization ability. Environ. Microbiol. 7(3):396-404. [ Links ]
Received: February 2016; Accepted: May 2016











 texto en
texto en 


