Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ciencias agrícolas
versión impresa ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.5 no.spe9 Texcoco sep./nov. 2014
https://doi.org/10.29312/remexca.v0i9.1050
Articles
Secondary metabolites and chlorophyll in cempasúchil in response to salinity stress
1 Colegio de Postgraduados-Campus Montecillo. Carretera México-Texcoco km. 36.5, Montecillo, Municipio de Texcoco, Estado de México. C. P. 56230. México. (mgperalta@colpos.mx; marinie@colpos.mx).
2 Colegio de Postgraduados-Campus Córdoba. Carretera Córdoba-Veracruz, km. 348, Congr. Manuel León, Municipio de Amatlán de los Reyes, Veracruz. C. P. 94946. México. (fernandg@colpos.mx; jcruz@colpos.mx).
3 Universidad Autónoma Chapingo (UACH). Carretera México-Texcoco km 38.5, Montecillo, Municipio de Texcoco, Estado de México. C. P. 56230. México. (serratocruz@gmail.com).
Biotic and abiotic stresses factors influence the biosynthesis of secondary metabolites in some medicinal species. In this regard, it has been reported that salinity stress disrupts the biochemical properties of a large number of crops. In this context, this study aimed to evaluate the effect of treatments of two NaCl levels (10 and 47 mM) added to the nutrient solution that would cause to cempasúchil (Tagetes erecta Linn) var. Inca in flowering stage. The concentration of carotenoids and flavonoids was evaluated in flowers. In leaves, flowers, stems and roots identification of essential oils was made and a qualitative quantification of these was done. In the leaves, the concentration of chlorophyll a, b and total was determined. The high concentration of NaCl tested, reduced by 26.7 and 27.9% carotenoid concentrations and total flavonoids in flowers. The concentrations of chlorophyll a and total were not affected by salinity; while the concentration of chlorophyll b was increased significantly with the supply of 47 mM NaCl. Seven essential oil constituents were identified from leaves of plants treated with 10 mM NaCl; while only four, when NaCl concentration increased; conversely, a larger number of compounds were identified in stems and roots with 47 mM NaCl. The presence of farnesene in flowers is noteworthy, only with the level of NaCl supplied.
Keywords: essential oils; carotenoids; chlorophyll; flavonoids
Factores de estrés bióticos y abióticos tienen influencia en la biosíntesis de metabolitos secundarios en algunas especies medicinales. En este sentido, se ha reportado que el estrés salino altera las propiedades bioquímicas de un gran número de cultivos. En este contexto, esta investigación tuvo como objetivo evaluar el efecto del tratamiento que dos niveles de NaCl (10 y 47 mM) adicionados a la solución nutritiva, ocasionan en plantas de cempasúchil (Tagetes erecta Linn) var. Inca, en fase de floración. La concentración de carotenoides y flavonoides fue evaluada en flores. En hojas, flores, tallos y raíces se realizó identificación de aceites esenciales y se hizo una cuantificación cualitativa de éstos. En hojas, se determinó la concentración de clorofilas a, b y total. La concentración alta de NaCl evaluada, redujo en 26.7 y 27.9% las concentraciones de carotenoides y flavonoides totales en flores. Las concentraciones de clorofilas a y total no fueron afectadas por el nivel de salinidad; mientras que la concentración de clorofila b fue incrementada significativamente con el suministro de 47 mM de NaCl. Se identificaron siete constituyentes esenciales en aceite proveniente de hojas de plantas tratadas con 10 mM NaCl; mientras que solo cuatro, cuando la concentración de NaCl aumentó; por el contrario, un mayor número de compuestos fue identificado en tallos y raíces con 47 mM NaCl. Destaca la presencia de farneseno en flores, solo con el nivel alto de NaCl suministrado.
Palabras clave: aceites esenciales; carotenoides; clorofila; flavonoides
Introduction
A wide range of abiotic stresses such as high and low temperatures, drought, alkalinity, salinity, UV light, among others, are potentially harmful to plants. Specially, salinity is one of the main factors that adversely affect agricultural production globally, given that more than 800 million hectares worldwide are affected by salinity, representing more than 6% of the planet's surface. Salinity affects crop by causing nutrient imbalance, osmotic stress, water deficiency and oxidative stress (Munns and Tester, 2008).
Salt tolerance in higher plants is a physiologically multifaceted trait, therefore involves different mechanisms. Among the most important osmotic adjustment, reduction in Na + uptake by the roots and increasing the ion efflux from the cell, intracellular sequestration of Na+, accumulation of K+ in cytosol; sequestration specific-tissue of Na+, charge control of this cation in the xylem, excluding Na+ and oxidative stress tolerance (Zhu, 2003).
Particularly, for the tolerance to stress caused by salinity, the plants modified synthesis of secondary metabolites (Wahid and Ghazanfar, 2006). Secondary metabolites are often referred to as compounds not having a key role in maintaining life processes of plants, but they are important for the interaction of the plant with its environment, adaptation and as a defence (Ramakrishna and Ravishankar, 2011).
Thus, in salt tolerant species increased anthocyanin content is a response, when subjected to salt stress (Parida and Das, 2005). Likewise as a consequence of osmotic stress, the osmotic adjustment in the cell cytoplasm to be induced, which leads to the accumulation of low molecular weight osmolytes such as raffinose, betaine glycine and proline, as well as hydrophilic proteins of high molecular weight the superfamily of abundant proteins during late embryogenesis (Kosova et al., 2011). In medicinal plants it has been associated an increase in the concentration of aromatic compounds such as isoprenoids, phenols and alkaloids, as part of its response to salt stress due to the antioxidant properties of these compounds. Furthermore, derivatives of phenylpropanoid phenols, such as flavonoids, tannins and hydroxycinnamates (esters); which are produced under various stress conditions, are major free radical scavengers (Selmar, 2008 ).
With respect to the synthesis of essential oils in response to salinity, the results are contradictory. In chamomile (Matricaria Chamomila), irrigated with water with different salinity levels (0, 84, 168, 252 and 336 mM NaCl), the highest content of essential oils in the control treatment (Razmjoo et al., 2008) was recorded. On the other hand, in Tunisian marjoram (Origanum majorana ) a significant increase in the performance of essential oils was recorded when plants were irrigated with water with conductivity of 5 dS m-1 compared with the control (0 dS m-1); the increase was 55.5%; while the increase in the irrigation water conductivity to 10 dS m-1 significantly reduced the yield (Jelali et al., 2011).
Although the effects of salt stress have been extensively investigated in crops, generating information concerning changes of secondary metabolism in medicinal plants such as the cempasúchil is needed in response to salinity. In this context, this study aims to evaluate two levels of salinity from NaCl concentrations of carotenoids and flavonoids in flowers, leaves chlorophyll; as well as the identification and quantification of essential oils for body, in cempasúchil (Tagetes erecta Linn.) var. Inca.
Materials and methods
Experimental conditions and plant material. The research was conducted under greenhouse conditions. Seedlings of cempasúchil (Tagetes erecta Linn.) Var. Inca pots were transplanted into black plastic of 1 litter capacity as a substrate containing a mixture of volcanic rock: perlite (60:40, v: v).
Experiment management. Once transplanted, the seedlings were irrigated with tap water (pH 7.3 and EC 0.37 dS m-1) for seven days. They were then irrigated with nutrient solution of 25% (Steiner, 1984) supplemented with micronutrients from AZ Tradecorp™ commercial product at the concentrations described by Trejo-Téllez et al. (2013). The pH of the nutrient solution was adjusted to 5.5 and this was supplied by a drip irrigation system. Two irrigations were made each day in every pot, each of which has a volume of 150 mL.
Treatments evaluated. At the beginning of flowering were added to the nutrient solution with NaCl: 10 and 47 mM NaCl, with conductivity values of 1.25Y 5.25%, respectively. Addition of 10 mM of NaCl (1.25 dS m-1) is not harmful or significantly affected whatsoever in this species (Valdez-Aguilar et al., 2009) therefore can be considered as a reference treatment.
Experimental design. The experimental design was completely randomized, and each treatment had 32 replicates. The experimental unit consisted of a potted plant.
Variables evaluated
Total carotenoids in flowers. Harvest of the flowers was manual when they reached their greater openness. The harvested flowers were dried in forced air oven (Felisa, FE291AD, Mexico) at 40 °C for 48 h; then weighed on an analytical balance (Ohaus Adventurer™ Pro, USA) and ground finely and stored in plastic containers with lids at 4 °C for subsequent analysis. The technique of extracting total carotenoids was performed using the method described by Nagata and Yamashita (2002). The absorbances of the extracts of ten repetitions were read in a spectrophotometer (Thermo Scientific Multiskan®, USA) at 453, 503, 645 and 663 nm wavelength; for estimation of total carotenoids, the obtained values were substituted into the following formula (Nagata and Yamashita, 2002):
Total carotenoids (mg/100 mL)= (0.216)(A663)-(1.220)(A645)-(0.304)(A505)+(0.452)(A453)
Total flavonoids. Sample preparation (drying, grinding and storage) is indicated in the previous section for carotenoids. The total flavonoid concentration quantified with a modified method from those described by Ghasemi et al. (2009), Ebrahimzadeh et al. (2008) and Nanyonga et al. (2013). The extracts of the samples were read in a spectrophotometer (Bausch and Lomb, Spectronic 20, USA) at 415 nm. For the preparation of the calibration curve was used to estimate the concentration of total flavonoids, quercetin was used as standard.
Identification of essential oil compounds by gas chromatography coupled to mass spectrometry (GC-MS). For this determination, four plants per treatment randomly selected were sectioned in flowers, leaves, stems and roots. The samples were dried in a forced air oven at 40 °C for 48 h (Felisa, FE291AD, Mexico), the dried samples were ground and processed each plant organs separately.
Gas chromatography coupled to mass spectrometry (GC-MS), with a gas chromatograph (HP-6890) coupled to a mass detector (HP-5973) was used for the identification of compounds. An HP-5MS (30 m length, 0.250 mm ID, 0.25μm film) column, average speed of 36 cm/s was used. The GC operating conditions were: initial temperature of 40 °C for 5 min, first ramp to 150 °C, an increase of 9 C min-1. It is maintained for 3 min, the second ramp up to 220 °C with a rise of 9 °C min-1. The ion source to 230 °C, 150 °C quadrupole. Splitlees Injector at 220 °C, 6.97 psi. Helium as carrier gas was used and injected with 1 µL of concentrated samples manually.
Terpenoids in identifying the Data Analysis program and database that is consulted were NIST (National Institute of Standards and Technology) based on retention times of each compound and the mass spectra were used.
Chlorophyll a, b and total. Chlorophyll concentration of fresh leaves was determined by the method Harbone (1973). The absorbance of the samples was performed on a spectrophotometer (Spectronic 20, Bausch and Lomb) at wavelengths of 663 and 645 nm. The obtained values were substituted into the formulas for estimation of photosynthetic pigments.
Chlorophyll a= (12.7*663A) - (2.59*A645)
Chlorophyll b= (22.9*645A) - (4.7*A663)
Total chlorophyll= (8.2*663A) + (20.2A645)
Results analysis. The data were statistically analysed according to the experimental design, using analysis of variance (ANOVA) of SAS (SAS, 2011). Means were compared with Tukey's test (p 0.05).
Results and discussion
Total carotenoids. Among the non-enzymatic antioxidants are carotenoids, which are presented ubiquitously in plants and have been reported as vital actors in mitigating adverse effects of salinity on plant growth and metabolism (Rawia et al., 2011). Example of this direct relationship between the level of salinity and concentration of carotenoids are the results of Borghesi et al. (2011), who reported that salt stress may lead to similar or higher increases in the fruit of tomato carotenoids and anthocyanins, those made by genetic engineering; therefore, this represents an option with considerable potential exploitation of tomatoes for saline soils with higher levels of secondary metabolites. Also in buckwheat sprouts (Fagorpyrum esculentum M.) treated with 50 and 100 mM NaCl, carotenoid concentration was twice as high in control plants (Lim et al., 2012). Nevertheless, in this study the concentration of total carotenoids in flowers was significantly reduced with the addition of 47 mM NaCl, compared to the supply of 10 mM NaCl; a reduction of 26.7% (Figure 1).
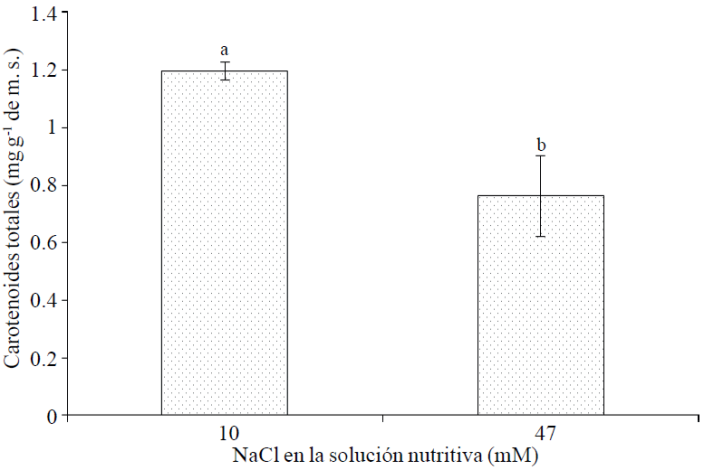
Figure 1 Total carotenoids in cempasúchil flowers var. Inca, treated with two concentrations of NaCl in flowering stage. Mean ± SD with different letters indicate significant differences (Tukey, p≤ 0.05) between treatments.
Total flavonoids. The concentration of flavonoids in flowers had a negative relationship with the concentration of NaCl in the nutrient solution; observing an increasing of NaCl from 10 to 47 mm 27.9% reduced in their concentration (Figure 2). Similarly, in chamomile (Matricaria chamomilla L.) treated with varying levels of NaCl (40, 80, 120 and 190 mM), there was a reduction in the concentrations of flavonoids than the control’s (without NaCl); however, with a NaCl concentration of 40 mM, we found the highest content of flavonols 3 -O-glycosides, flavonoids most abundant flowers of this species (Afzali et al., 2007).
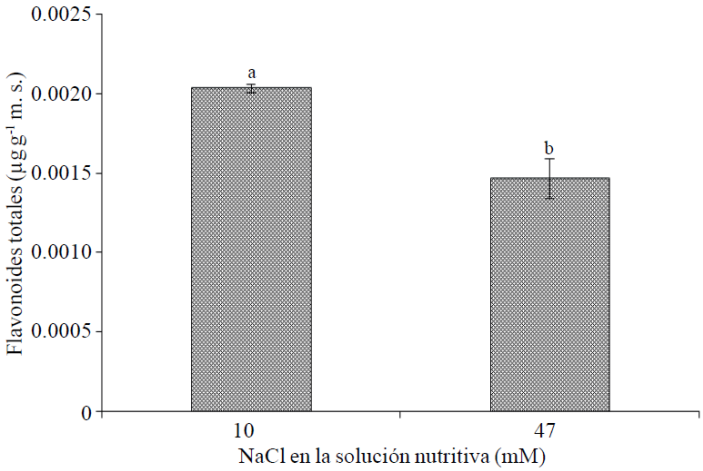
Figure 2 Total flavonoids in flowers of cempasúchil var. Inca, treated with two concentrations of NaCl in flowering stage. Mean ± SD with different letters indicate significant differences (Tukey, p≤ 0.05) between treatments.
Opposite to the results of flavonoids obtained in this study, in hypocotyls and cotyledons of seedlings of tomato (Solanum esculentum cv. H-2274) and red cabbage (Brassica oleraceae convar. capitata (L.) Alef. Var. Rubra DC. Cv Möhrenkopf), gradual increases were observed in the concentrations of anthocyanins, as the NaCl concentration increased from 0 to 150 mM (Eryılmaz, 2006).
Total chlorophylls. The Table 1 shows the leaf chlorophyll concentrations under two levels of NaCl. Total concentrations of chlorophyll a and were not affected by the level of NaCl added to the nutrient solution; while the concentration of chlorophyll b was statistically superior 47 mM NaCl, 10 mM compared.
Table 1 Leaf chlorophyll concentrations under two levels of NaCl.
| NaCl, mM | Clorofila | ||
| a | B | total | |
| 10 | 67.42 ± 1.46 a | 23.1 ± 0.51 b | 91.43 ± 1.87 a |
| 47 | 48.8 ± 2.73 a | 15.02 ± 0.39 a | 64.48 ± 5.82 a |
Medias ± DE con letras distintas en cada columna indican diferencias significativas (Tukey, p≤ 0.05) entre tratamientos.
Studies on the effects of salt stress in plants have mainly focused on growth, proline accumulation, chlorophyll content, relative K+/Na+, Ca2 + ratio /Na+, accumulation of Na + and Cl-. It has been said that genotypes with a high accumulation of proline and chlorophyll contents, as well as high ratio K+/Na+ and low accumulation of Na+ and Cl- are more tolerant (Mane et al., 2011). Considering the above, we can say that Tagetes erecta var. Inca is a tolerant species at levels of 47 mM NaCl.
Essential oils. The retention times of the essential oil compounds identified in leaves, flowers, stems and roots are presented in Table 2.
Table 2 Retention times of compounds identified in essential oils from leaves, flowers, stems and roots of cempasúchil plants treated with two levels of NaCl.
| Compuesto | Tiempo de retención | Compuesto | Tiempo de retención |
| D-Limoneno | 12.21 | β- Mirceno | 11.36 |
| Ocimeno | 12.59 | Piperitona | 16.5 |
| Cariofileno | 19.47 | Farneseno | 20.04 |
| α-Pineno | 9.95 | Óxido de cariofileno | 23.2 |
| Trans-pineno | 27.5 |
Is indeed quite important to highlight the large difference in the components of essential oils from Tagetes species, since the compounds identified here do not match those reported in Tagetes coronopifolia Willd, which are in roots 2, 7, 7-trimetilbiciclo[3.1.1] heptan-2-ol y (1S, 2R, 5S) -4, 6,6-trimetilbiciclo[3.1.1] hept-3-en-2-ol (verbenol) (38%); (1S) -6,6-dimetil-2-metilen-biciclo[3.1.1] heptan-3-ona, (1R)-cis-4,6,6-trimetilbiciclo-[3.1.1] hept-3-en-2-ona (verbenona), 2-oxo-decanoato de metilo and 2,7,7-trimetilbiciclo[3.1.1] hept-2-en-6-ona (crisantenona) (41%); while in the flowers, (1S)-6,6-dimetil-2-metilen-biciclo[3.1.1] heptan-3-ona and verbenona (Díaz-Cedillo et al., 2013).
The number of compounds and their concentration in the oil from leaves were higher with low salinity level tested (10 mM), the most abundant D-limonene (8.52%) and ocimene (6.33%) and higher at 58.92 and 46.13%, respectively, for the content thereof recorded in leaves of plants treated with 47 mM NaCl. It is also important to note that only by the addition of 10 mM NaCl, trans-pinene, β-myrcene and piperitone (Figure 3A) were identified. Rawia et al. (2011) reported oil from fresh tissue of Tagetes erecta plants subjected to three concentrations of NaCl (salt), 10 compounds: cis-β-ocimene, β-farnesene, trans-linalool oxide, piperetona, β-ionones, trans-cariofilasa oxide cariofilasa , eugenol, and hexa-decanoice linallool acid methyl ester; in all these, the relative concentration was reduced as the concentration of NaCl is increased. Regardless of the treatment, the most abundant compounds were trans-caryophyllene and eugenol. Therefore, this study overlaps with the results of this research; lower relative concentration of essential oils increases as the concentration of NaCl; and differ in the amount and type of identified compounds.
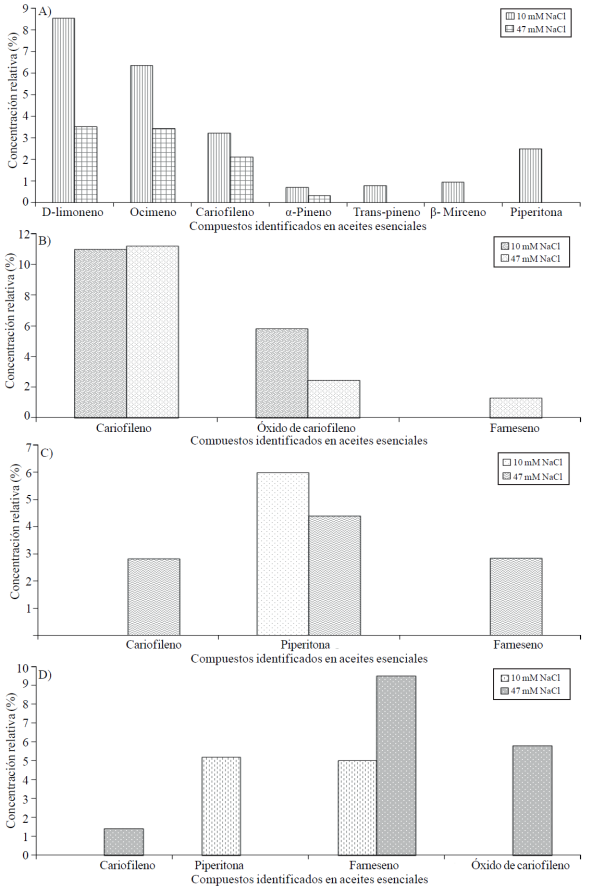
Figure 3 Essential oils identified in leaves (A), flowers (B), stems (C) and roots (D) of cempasúchil var. Inca, treated with two concentrations of NaCl in flowering stage.
Contrary to the results of the leaves, in the flowers were found three compounds in essential oils with higher salinity; while with lower salinity only two, caryophyllene and caryophyllene oxide. Farnesene was identified only under higher NaCl concentration of 1.28 percent (Figure 3B). Coincidentally, in peppermint plants (Mentha piperita L.), increases were observed in some essential oil components in response to NaCl treatment, including the trans-β-farnesene is located; with relative concentrations of 0.01 and 0.89%, with the treatment 0 and 50 mM of NaCl, respectively; while with 100 mM NaCl was not identified. In the case of the relative concentration of L-menthol, a major constituent in the essential oil of the species is gradually increased with increasing NaCl dose of 50 and 100 mM (Khorasaninejad et al., 2010).
Similar to those recorded in flower trends are observed in stems; i.e. three compounds identified in plants treated with 47 mM; while only piperitone was detected with the lowest concentration of 10 mM NaCl; however, its concentration exceeded that found with higher concentrations of NaCl in 26.63% (Figure 3C).
In roots, the high dose of NaCl were caryophyllene, farnesene and caryophyllene oxide identified; of these one-farnesene with increasing NaCl. Just the piperitone compound was identified in roots of plants subjected to low salinity levels (Figure 3D).
Consistently, we observe that the relative concentration of piperitone in oil from leaves, stems and roots was significantly affected by increased salinity (Figure 3).
The increase in essential oil content, as seen in flowers, stems and roots of this species has been reported in other, subject to factors that cause osmotic stress. The content of essential oil and basil proline were increased when plants are subjected to drought stress; the growth was inhibited significantly (Baeck et al., 2001). In addition, Hendawy and Khalid (2005) reported that, the essential oil content, total carbohydrates and proline were increased significantly with the increase in the level of salt stress in plants of salvia (Saliva officinalis L. Sage).
Conclusions
The concentrations of carotenoids and flavonoids were adversely affected while increasing NaCl; in contrast, the chlorophyll a and total concentrations were not affected by the level of NaCl, while the concentration of chlorophyll b was increased with increasing NaCl concentration.
The treatment with 47 mM NaCl reduces the essential oil components of leaves and reduces the relative concentrationsas well. In flowers, stems and roots; in contrast, with the increasing level of NaCl, generally essential oil components and their concentrations were also increased, noting the trend in stems and roots.
Literatura citada
Afzali, S. F. A. D.; Shariat, M. H.; Hajabbasi, M. A. and Moatar, F. 2007. Salinity and drought stress effects on flower yield and flavonol-o glycosides in chamomile (Matricaria chamomilla). Iranian J. Medicinal and Aromatic Plants. 23(3-37):382-390. [ Links ]
Baeck, H.; Kuenwoo, P.; Baeck, H. W. and Park, K. W. 2001. Effect of watering on growth and oil content of sweet basil (Ocimum americanum L.). Korean J. Hort. Sci.Technol. 19:81-86. [ Links ]
Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M. L.; Malorgio, F.; Heredia, F. J. and Meléndez-Martínez, A. J. 2011. Effects of salinity stress on carotenoids, anthocyanins, and color of diverse tomato genotypes. J. Agric. Food Chem. 59(21):11676-11682. [ Links ]
Díaz-Cedillo, F.; Serrato-Cruz, M. A.; De la Cruz-Marcial, J.; Sánchez-Alonso, M. G. y López-Morales, V. 2013. Compuestos mayoritarios del aceite esencial en órganos de uma población de Tagetes coronopifolia Willd. Rev. Fitotec. Mex. 36(4):405-411. [ Links ]
Ebrahimzadeh, M. A.; Pourmorad, F. and Bekhradnia, A. R. 2008. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol. 7(18):3188-3192. [ Links ]
Eryilmaz, F. 2006. The relationships between salt stress and anthocyanin content in higher plants. Biotechnol. 20(1):47-52. [ Links ]
Ghasemi, K.; Ghasemi, Y. and Ebrahimzadeh, M. A. 2009. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 22(3):227-281. [ Links ]
Harbone, J. B. 1973. Chlorophyll extraction. In: Harbone, J. B. (Ed.). Phytochemical methods. recommended technique. Chapman and Hall, London. 205-207 pp. [ Links ]
Hendawy, S. F. and Khalid, Kh. A. 2005. Response of sage (Salvia officinalis L.) plants to zinc application under different salinity levels. J. Appl. Sci. Res.1:147-155. [ Links ]
Jelali, N.; Dhifi, W.; Chahed, T. and Marzouk, B. 2011. salinity effects on growth, essential oil yield and composition and phenolic compounds content of marjoram (Origanum majorana L.) leaves. J. Food Biochem. 35(5):1443-1450. [ Links ]
Khorasaninejad, S.; Mousavi, A.; Soltanloo, H.; Hemmati, K. and Khalighi, A. 2010. The effect of salinity stress on growth parameters, essential oil yield and constituent of peppermint (Mentha piperita L.). World Appl. Sci. J. 11(11):1403-1407. [ Links ]
Kosova, K.; Vitamvas, P.; Prasil, I. T. and Renaut, J. 2011. Plant proteome changes under abiotic stress contribution of proteomics studies to understanding plant stress response. J. Proteomics. 74:1301-1322. [ Links ]
Lim, J.-H.; Park, K.-J.; Kim, B.-K.; Jeong, J.-W. and Kim, H.-J. 2012. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chemistry 135:1065-1070. [ Links ]
Mane, A. V.; Deshpande, T. V.; Wagh, V. B.; Karadge, B. A. and Samant, J. S. 2011. A critical review on physiological changes associated with reference to salinity. Int. J. Environ. Sci. 6: 1192-1216. [ Links ]
Munns, R. and Tester, M. 2008. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59:651-681. [ Links ]
Nagata, M. and I. Yamashita. 2002. Simple method for simultaneous determination of chlorophyll and carotenoids in tomaito fruit. Nippon Shokuhin Kogyo Gakkaish. 39:925-928. [ Links ]
Nanyonga, S. K.; Opoku, A. R.; Lewu, F. B.; Oyedeji, O. O.; Singh, M. and Oyedeji, A. O. 2013. Antioxidant activity and cytotoxicity of the leaf and bark extracts of Tarchonanthus camphorates. Tropical J. Pharmaceutical Res. 12(3):377-383. [ Links ]
Parida, A. K. and Das, A. B. 2005. Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf. 60:324-349. [ Links ]
Ramakrishna, A. and Ravishankar, G. A. 2011. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signaling and Behavior. 6(11):1720-1731. [ Links ]
Rawia, A. E.; Lobna, S.; Taha, S. M. and Ibrahiem, M. 2011. Alleviation of adverse effects of salinity on growth, and chemical constituents of marigold plants by using glutathione and ascorbate. J. Appl. Sci. Res. 7(5):714-721. [ Links ]
Razmjoo, K.; Heydarizadeh, P. and. Sabzalian, M. R. 2008. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomila. Int. J. Agri. Biol. 10:451-454. [ Links ]
Statistical Analysis System (SAS) Institute. 2011. SAS user’s guide. Statistics. Version 8. SAS Inst., Cary, NC. USA. Quality, and elemental removal. J. Environ. Qual. 19:749-756. [ Links ]
Selmar, D. 2008. Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. Agric. For. Res. 58:139-144. [ Links ]
Steiner, A. 1984. The universal nutrient solution. In: ISOSC Proceedings 6th International Congress on Soilless Culture. The Netherlands. 633-649 pp. [ Links ]
Trejo-Téllez, L. I.; Peralta-Sánchez, M. G.; Gómez-Merino, F. C.; Rodríguez-Mendoza, M. N.; Serrato-Cruz, M. A. y Arévalo-Becerril, A. E. 2013. Cloruro de sodio sobre biomasa seca y absorción de cationes macronutrimentos en cempasúchil (Tagetes erecta Linn.). Rev. Mex. Cienc. Agríc. 5:979-990. [ Links ]
Valdez-Aguilar, L. A.; Grieve, C. M.; Poss, J. and Layfield, D. A. 2009. Salinity and alkaline pH in irrigation water affect marigold plants: II. Mineral Ion Relations. HortScience 44(6):1726-1735. [ Links ]
Wahid, A. and Ghazanfar, A. 2006. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 163:723-730. [ Links ]
Zhu, J.-K. 2003. Regulation of ion homeostasis under salt stress. Current Opinion in Plant Biology 6(5):441-445. [ Links ]
Received: March 2014; Accepted: August 2014











 texto en
texto en 


