Introduction
The marginal bone level around the dental implant is an important factor to examine for its long-term success because a reduction in bone height also represents alteration of soft tissue levels. This situation needs to be avoided or limited to bring about ideal physiological and aesthetic conditions in implant dentistry. Based on clinical experience and scientific research that has emerged over the years, crestal bone loss has been observed at the junction of the implant platform with the abutment. The bone loss varies from 1.0 to 2.5 mm in the first year, after this period, the bone loss pattern decreases to 0.05 to 0.2 mm per year (Hartman and Cochran, 2004; Hürzeler et al., 2007; Becerra and Morales, 2009; Canullo et al., 2010). Many factors can affect the crestal bone level around dental implants (Chuang et al., 2002; Ko et al., 2003), and the initiative to reduce bone loss using different prosthetic attachment alternatives (conventional and platform switching) has caused controversy in recent years (Lazzara and Porter, 2006; Somborac, 2007). Some studies reported that the use of small diameter attachments into the implant platform resulted in minor crestal bone loss or preserved the same bone level around the implant compared to conventional proceedings that showed crestal bone loss up to 2.5 mm and to platform switching procedures with loss of 0.7 mm (Vela et al., 2006). However, there is much controversy about of the effects of platform switching on crestal bone levels coupled to the insufficient evidence from scientific literature (Becker et al., 2009; Hagiwara, 2009). The vast number of studies that have evaluated prosthetic abutments regarding crestal bone levels changes have been conducted using dogs as experimental models; however, the results have been inconsistent. The inconsistency can be attributed to several intrinsic (physiological and health) factors. In this sense, Mascarenhas et al. (2003) and Matthews (2014) argued that age and gender (or gonadal condition) are the most important factors on the alveolar bone level stability responses to implants. In a recent analysis, data of 61 scientific papers that studied responses to dental implants in dogs found only 54% reported gender and 1.6% reported gonadal condition (Valenzuela et al., 2015). Therefore, it is possible to infer that many of the inconsistencies observed in the responses to dental implants are mainly mediated by these factors. Since no information is available relating the effects of gender or gonadal condition to the responses of bone crestal levels in dogs implanted with two types of dental prosthetic abutments, the aim of this experiment was to contribute to the knowledge of the factors that affect crestal bone level changes after restoration, using conventional and platform switching abutments in intact and neutered (male and female) dogs.
Materials and methods
Characteristics of dogs and dog handling prior to implant placement
This experimental study was conducted at the facilities of the Instituto de Investigaciones en Ciencias Veterinarias, Universidad Autonóma de Baja California in Mexicali, B.C., México. The experiment was handled in compliance with ethical rules dictated by local and international animal welfare standards under experimental conditions. (NCR, NOM). Prior to the end of the study, all animals were registered in an adoption program. Once the study was completed, all dogs were adopted.
Fourteen adult mongrel dogs (5 intact and 9 neutered) comprising seven males and seven females were used in a completely randomized design to evaluate crestal bone level changes around dental implants when the animals received two types of abutments (conventional vs. platform switching) and metallic crowns. All dogs were selected from the Animal Control Centre of Mexicali, B.C. The inclusion criterion for selecting dogs for the experiment was performed based on recommendations reported by Valenzuela et al. (2015).
Three weeks before the experiment started, dogs were transferred to the research facilities and were treated for endoparasites (5% Tasasel®, Fort Dodge Animal Health, Mexico City, México), by small animal veterinarian practitioners and were examined by a dentist for healthy oral conditions. Dogs were allotted to individual cages (1.5×1.5 m) with a neoprene floor, individual feeders, and an automatic water dispenser. Dogs were held in cages overnight and were released during day (0800 to 1800 h) into a 14×28m fenced area. Dogs were fed approximately 115-120 kcal/ kg BW0.75 and 9-10 grams of protein/ kg BW0.75. Fresh feed was provided twice daily with commercial diet pellets (Pro-Plan; Purina®, Nestlé-Purina Petcare, Mexico). Feed had low moisture content (< 10%) and covered the nutritional requirements of crude protein, fats, carbohydrates, soluble fibre, and macro and microminerals (NRC, 2006). Dogs were allowed ad libitum access to fresh and clean water. At the start of the experiment, dogs averaged 28.1 ± 4.2 kg of body weight and 24 ± 5 months of age and 36% were intact (5/14) and 64% (9/14) were surgically neutered.
Sedation-Anaesthesia Protocol for Surgical Procedures
Dogs received an intramuscular single-dose of clindamycin (11 mg kg-1 BW) as prophylactic, 24 hours prior to surgery. Dogs were examined prior to each procedure; body weight, rectal temperature, heart rate, and respiratory rate were registered and a thorough physical examination was performed. A pre-anaesthetic IM dose of xylazine (0.5-1.0 mg kg-1) was applied 10 min before induction. Subsequently dogs were anaesthetized via IV using a combination of zolazepam + tiletamine (4-6 mg/kg, flow rate 10 mL/h), to induce deep surgical anaesthesia. This is a proven protocol commonly used in dogs due its safety and low impact on cardiovascular and respiratory physiology. Dogs were monitored using a pulse oximeter (NellcorTM N65 Portable Pulse Oximetry System, Covidien, MA, USA) and stethoscope every 5 min during the procedures. Before surgical procedure, both sides of the mandibular nerve were blocked by infiltrating the mandibular foramen zone 0.90 mL HCl mepivacaine 3% (DENTOCAIN® Simple, GRUPO ZEYCO Laboratories, Jalisco, México). This formula gives enough working time and avoids self-mutilating behaviour after surgical procedures, results in profound anaesthesia, and allows for painless procedures in compliance with ethical rules for animals in research and experimental studies. Once anaesthetized, extractions were performed, removing both mandibular fourth premolars, no artificial graft was used (Fickl et al., 2008). To promote healing, only self-absorbing sutures were used. After recovery, meloxicam was orally administered in a single-dose of 0.2 mg kg-1/24 hours for 5 days.
Implants and abutment used
Thirty-two completely osseointegrated implants, Classix 4.2 × 10 mm, were used (Cortex TM Dental Implant Industries Ltd, Shlomi, Israel). The implants used offers both conventional and platform switching options in its prosthetic components.
Surgical implant placement procedures
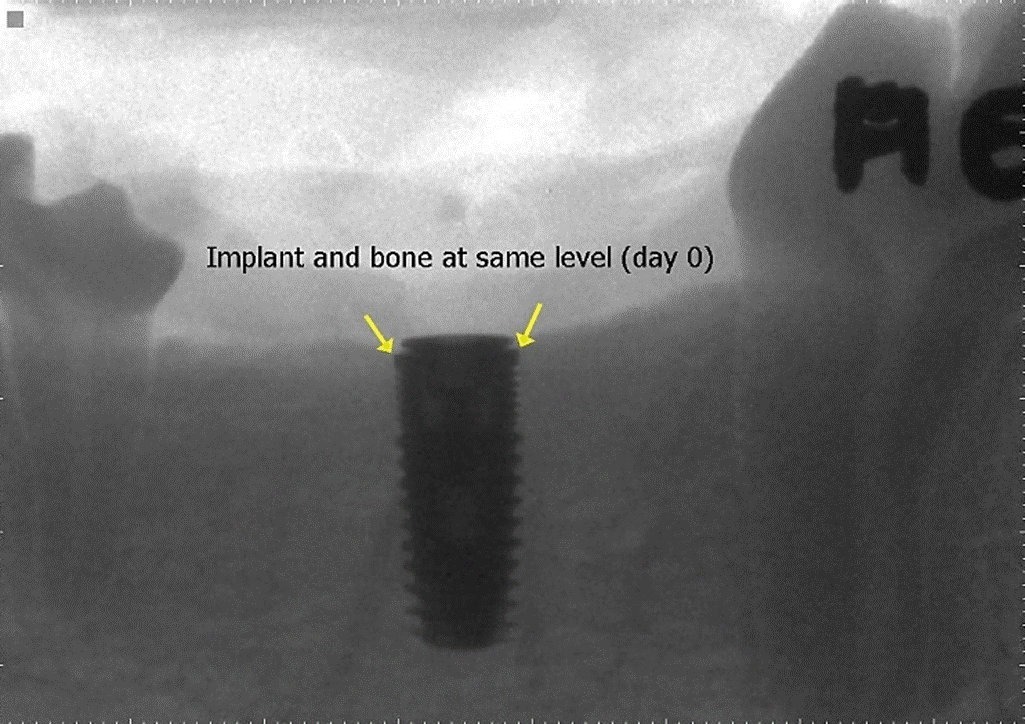
Thirty days after the extractions were performed and following the same anaesthesia procedures, we surgically inserted two 2 × 10 mm implants and cover screws in each dog through Branemark protocol, with gradual milling at both fourth lower premolars’ healed areas at the same crestal bone level and with 30 N/cm2 torque (Figure A), using a “flapless" technique, which decreases crestal bone loss (Blanco et al., 2008).
After implants were placed, dogs received an IM single-dose of clindamycin (11 mg kg-1 BW) as prophylactic with meloxicam (0.5 mg kg-1 BW) daily for 5 days as an analgesic. Dogs received a soft diet for 2 weeks; chlorhexidine (0.20%) was sprayed orally, 1 hour after meals Ten days after implant surgery, the dental prophylaxis was done, and dogs were clinically checked daily for 2 weeks and once a week afterward. Dogs were put out (average of 10 hours) from their individual cages.
Prosthetics procedures
Fifty days after implantation, the soft tissue over the implants and cover screws were removed and healing screws were settled. Full mouth impressions and models were made to build crowns and fixation splints. Ten days later, healing screws were randomly replaced by conventional abutment (4.2 mm Ø) and platform switching abutment (3.7 mm Ø) and metallic crowns were cemented over them.
Crestal bone changes evaluation
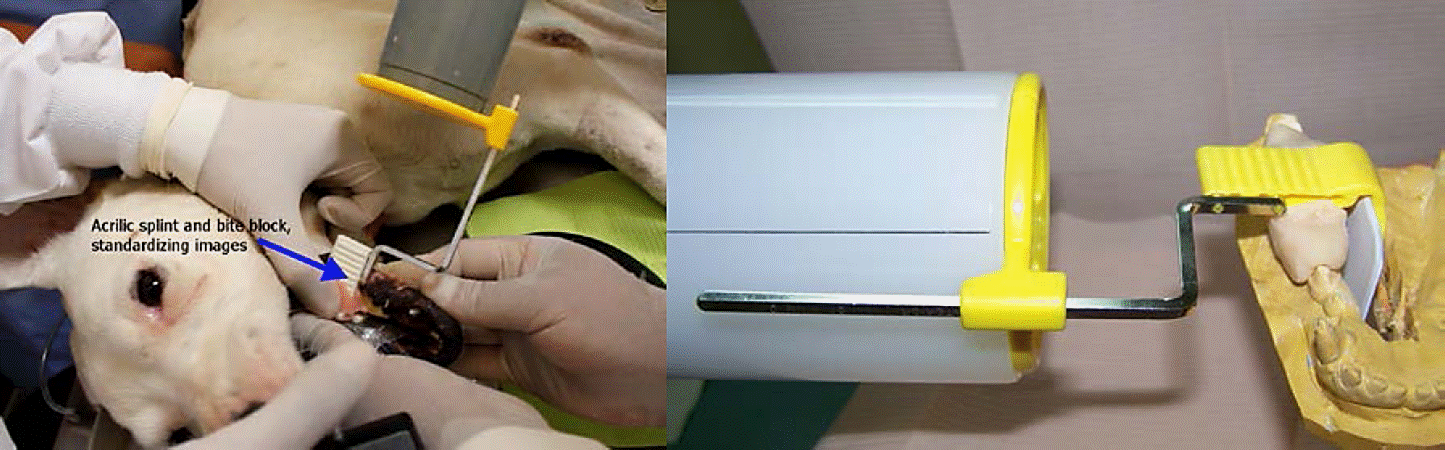
Because periapical radiographs with paralleling technique are more accurate (repeatability and reliability) in assessing marginal bone levels, compared with panoramic radiographs (Romanos, and Javed, 2014), we used a modified procedure from Hürzeler et al. (2007) as follows: The fixation splints for a digital X-rays sensor (No. 2) were constructed over gypsum models with self-curing acrylic and the RINN XCP posterior bite block. These devices were placed over metallic and natural crowns, holding a sensor (Schick sensor, Schick Technologies, Inc., Long Island, NY, USA) in the same position and in relation to an X-ray cone, using RINN XCP accessories (Extension Cone Paralleling, RINN Dentsply Corporation, Elgin, IL, USA) to standardize the collection of samples (Figure B) and obtain a reliable record (Mamoru et al., 2006).
Both mandibular X-ray implant images (right and left) were evaluated using CDR DICOM for Windows software (ver. 3.5.0.150, Schick Technologies Inc.), drawing a vertical line measuring from the implant platform to the first bone contact with the implant body in mesial and distal zones. Measurements were made by the differential (expressed as mm) between the crestal bone level X-ray images at the immediate moment of implant surgical placement (Day 0). The images were compared at 30, 60, and 90 days after crowns were cemented.
Statistical analysis
The crestal bone level data are shown as the mean of both measures (mesial plus distal /2) before carrying out any statistical analysis. Total repetitions for general (n = 28), neutered (n = 18) and intact (n = 10) dogs were enough to detect accuracy differences between treatment (δ) means of ± 0.09, 0.16, and 0.30 mm, respectively, detectable as significant for an expected 0.88 mm CBLs average. This estimation was derived as follows: # reps = 2 (Zα/2+Zβ) (σ/δ) 2, where Zα/2 tabular value of 0.05 for α =1.96 and 0.10 for α = 1.65, Zβ value (probability type II error) of 20% = 0.84 and σ = 0.4861, obtained through weighted variance of 38 previous observations reported in scientific studies.
Experimental data were analysed using a completely randomized design with 2 stratification criteria (gender and gonadal condition); the experimental unit was the dog (random effect), the treatment was the abutment type (fixed effect), and crestal bone level of each implant side (mesial and distal) was the observation. The data were analysed using the MIXED procedure (SAS Institute Inc., Cary, NC, USA). The linear statistical model was Yij= µ + τi+ ρj+ εij; where µ is the true mean effect, τi is the fixed treatment effect, ρj is the random of period effect (30, 60, and 90 days) with mean 0 and variance σb2, and εij is the random experimental error with mean 0 and variance σ2. The following comparisons of abutment type (conventional vs. platform switching) were performed: (a) general response, (b) gonadal condition (intact vs. neutered), and (c) gender (male vs. female). Additionally, the interactions gonadal condition × abutment type and gender × abutment type were analyzed. When significance was detected, interactions were informed. Comparisons were considered significant when the P-value was ≤ 0.05, and tendencies were identified when the P-value was > 0.05 and ≤ 0.10.
Results
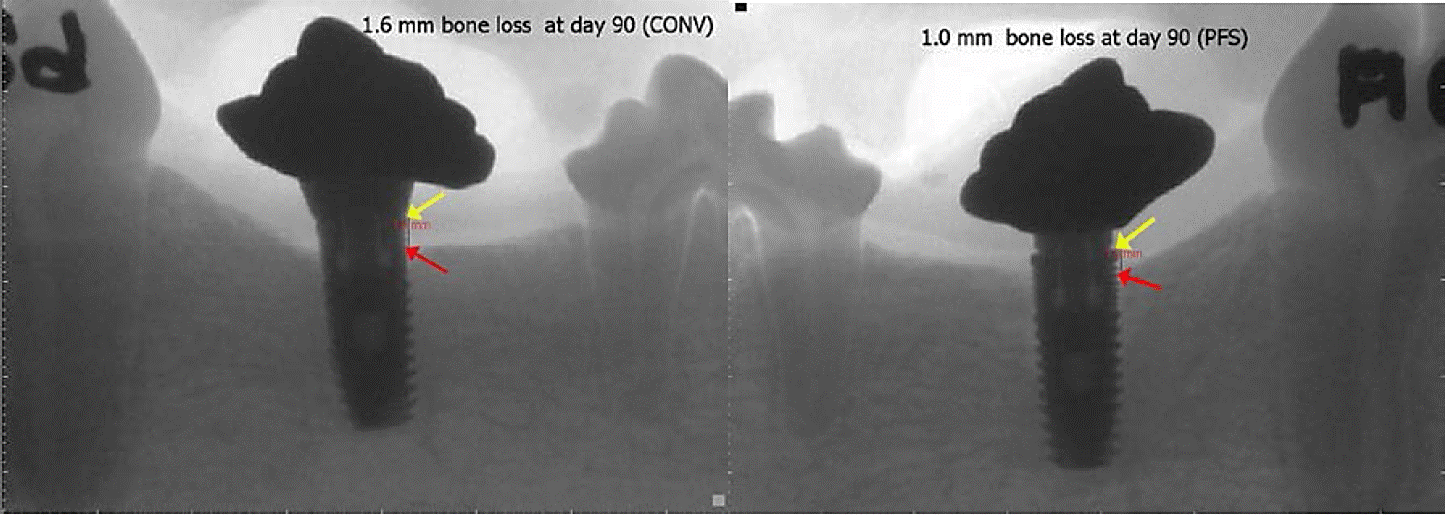
The effects of type of abutment on crestal bone level changes, considering general responses and responses observed to the gonadal condition and gender, are shown in Table 1. The average of crestal bone loss was 0.400 ± 0.186 mm. In general, dogs with CONV had a greater (27.3%, P < 0.05) crestal bone loss than dogs with PFS (Figure C).
Table 1 General effects of different abutment type on crestal bone loss average in implanted dogs (average of measured taken at 30, 60, and 90 days post-restoration)
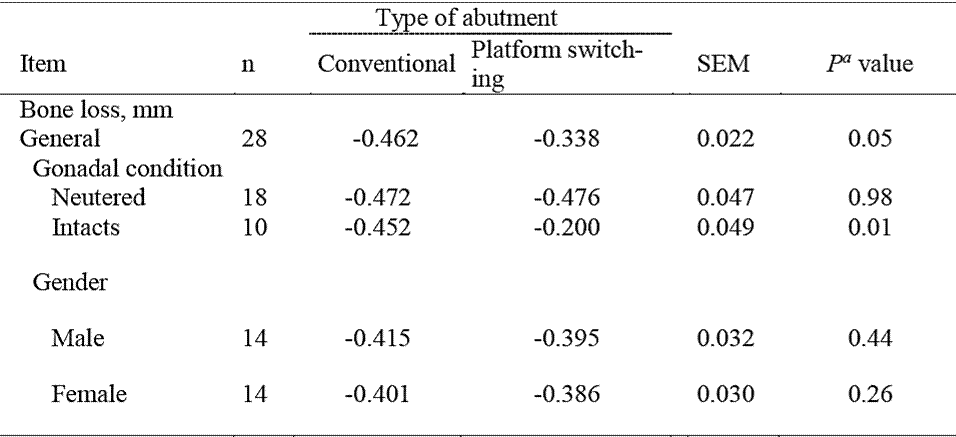
n = Number of observations, SEM = Standard error of the mean, P value = Observed significance level.
Intact dogs that received PFS had minor (P < 0.01) bone loss than dogs receiving CONV (Fig. C), but in neutered dogs, abutment type had no effect (P = 0.98) on crestal bone loss level; therefore, an interaction (P < 0.01) between type of abutment and gonadal conditions was detected (Table 2). There were no interaction (P > 0.80, data not shown) between gender and abutment type, similar to the sole factor of variation, and had no effect (P = 0.26) on crestal bone level responses to abutment type (-0.401 vs -0.385 mm for males and females, respectively).
Table 2 Interaction between gonadal condition (GC, neutered and intact) and type of abutment on average loss of crestal bone in implanted dogs (average of measured taken at 30, 60, and 90 days post-restoration)
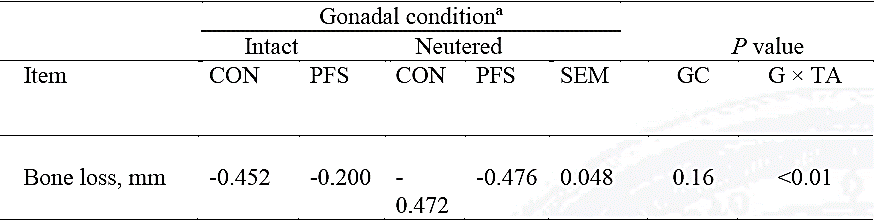
n= 28 observations (10 neutered and 18 intact), CON= Conventional, PFS=Platform swithing SEM= Standard error of the mean, GC= Gonadal condition, G × TA= Interaction Gonadal condition × type of abutment.
The effects of gonadal condition and gender on crestal bone level changes regardless of the type of abutment are shown in Tables 3 and 4. Male and female had a similar (P = .65) crestal bone loss (-0.405 vs. -0.393), while neutered dogs showed a greater (30.8%, P < .01) crestal bone loss compared with intact dogs.
Table 3 Effect of gender (male and female) on crestal bone loss average in implanted dogs (average of measured taken at 30, 60, and 90 days post-restoration).

n = Number of observations, SEM = Standard error of the mean, P value = Observed significance level
Discussion
Crestal bone level changes observed in this experiment are within the range as those reported in previous studies in which the changes in crestal bone level were evaluated over a similar period of time in dogs with dental implants (Blanco et al., 2008; Jung et al., 2008; Cochran et al., 2009). Crestal bone loss in humans has been observed at the junction of the implant platform with the abutment varying from 1.0 to 2.5 mm in the first year (Hartman and Cochran, 2004; Canullo et al., 2010). Similar behaviour of crestal bone loss has been observed in dogs (Jung et al., 2008). However, compared with our results, some studies show a greater crestal bone loss (Canullo et al., 2010). The magnitude of crestal bone loss around implants can be affected by factors, such as the heat generated during drilling and excessive pressure at the crestal region during implant placement, by occlusal overload and periosteal elevation, and by intrinsic factors (such as physiological and health factors). In the present experiment, the experimental units (dogs) were selected for have as uniformity as possible with respect to age, weight, and health status. The implants were placed by an expert (the same person placed all implants) and with the same technique (instruments and procedures); therefore, the changes to crestal bone must be attributed to the factors studied.
Similar to our results, some studies reported that the use of platform switching (small diameter attachments) into the implant platform resulted in minor crestal bone loss or preserved the same bone level around the implant compared to the conventional proceedings (Vela et al., 2006). Although there are two theories that try to explain less crestal bone loss with platform switching, there is nothing definite yet. One of the theories is related to the concentrated stress over the crestal bone when the implant is loaded. This phenomenon was demonstrated by biomechanical simulation in a finite element analysis that suggested the transmission of functional tensile forces to the implant centre (and not to the periphery to the bone) was a result of the reduced diameter abutment in platform switching protocol, thus resulting in less stress transferred to the crestal bone (Khurana et al., 2013).
The second theory holds that the implant abutment joint always has a microgap, feasibly colonized by bacteria, which leads to the formation of an inflammatory infiltrate in the surrounding tissue, causing resorption of the peri-implant bone crest. In implants with platform switching, as this interface is remote from the implant margin, then bacterial infiltration is moved to the center, favoring the preservation of the peri-implant bone (Lazzara and Porter, 2006).
Currently there is much controversy about of the effects of platform switching on crestal bone levels (Becker et al., 2009; Hagiwara, 2009), since many reports (Rodriguez et al., 2011; Baffone et al., 2012; Bressan et al., 2014;.Lee et al., 2014) indicate a similar crestal bone loss when compared platform switching against conventional methods.
In this experiment, a similar loss was measured between types of abutment only when the abutment type was compared in the group of neutered dogs. Even though the lack of estrogen and its effects on the bone has been widely studied, to our knowledge, there are no scientific studies that analyse changes in bone levels due to dental implants in sterile dogs (that lack of ovaries or testes). The absence of testes and ovaric hormones on the crestal bone levels in neutered dogs in the present study similarly affected both males and females. A study conducted by Kessler et al. (2015) revealed the importance of testosterone in males for maintaining skeletal bone mass. Moreover, Tirabassi et al. (2014) explain the complex relationship between male osteoporosis and hypogonadism, paying specific attention to the role of androgens in the physiology of the male bone. Therefore, even if there was osseointegration in neutered animals (Giro et al., 2011), the low circulating levels of gonadal hormones in neutered dogs eliminates a possible positive effect of the switching platform on crestal bone level.
Due to the limited number of scientific reports, it is difficult to estimate the real effect of sex on the marginal bone level (Chrcanovic et al., 2015). Sex steroids have an important role in skeletal growth and maintenance, so bone mass reaches a peak in both genders at a certain age, after which bone mass decreases. Bone loss is higher and more accelerated in females in whom the oestrous cycle is interrupted (Reece, 2004). A decreases or an absence of estrogen, increases osteoclast activity and decreased osteoblast activity interfering with bone remodelling and architecture (Weitzmann and Pacifi, 2006). Because of abrupt discontinuation of estrogen in human middle-aged females, more frequent reports of crestal bone loss in dental implanted females than in dental implanted males should be expected. Only animal studies in rats have reported the effects of estrogen on bone healing in implants (Eleni and Lazaros, 2014). In a study conducted by Giro et al. (2011), they concluded that estrogen deficiency negatively affected the amount of bone around implants.
A study conducted to evaluate the bone remodelling in dogs determined differences in bone dynamics between non-neutered males and females, finding the highest bone formation in males and an increased bone resorption in females (Belic et al., 2012). However, most reports do not explain if dogs are intact or neutered, and it has been argued that gonadal condition is one of the most important factors concerning alveolar bone level stability and its response to implants (Matthews, 2014). In data of 61 scientific papers that studied responses to dental implants in dogs, only 1.6% reported the gonadal condition (Valenzuela et al., 2015). In this manner, if steroid hormones are present in normal levels, a minimal difference between genders is expected in the response of crestal bone to dental implants. In the present experiment, the lack of effects of both abutment type restoration alternatives on crestal bone levels was not surprisingly when comparing males vs. females.
Conclusions
In conclusion, dogs with PFS showed a lower crestal bone loss than CONV; however, regardless of gender, this response was only maintained in intact dogs. Therefore the gonadal condition has an effect on the magnitude of change in the levels of alveolar crestal bone in response to abutment type in dogs with dental implants. The results obtained in the present experiment can be used as a reference in clinical situations where low levels of gonadal hormones are present. However, more studies are needed to further assess if the dog is an appropriate animal model to extrapolate results to human conditions (such as for elderly patients, bone implant studies, or osteoporosis research). It is recommended to consider these conditions in future studies in which dogs are used when analysing the results of dental implants and crestal bone level stability.











 nueva página del texto (beta)
nueva página del texto (beta)