Introduction
Panchronic species are those species that remain relatively unchanged in phenotype over evolutionary time (Grassé, 2013; Grandcolas et al., 2014) and thus, raise the question of why they remain with those relatively unchanged traits (Grassé, 2013). One very important hypothesis is that they live in optimal conditions, but these may make them vulnerable to extinction (Grassé, 2013). Some plants such as Ginkgo (aprox. 80 million years ago- Ma) and vertebrates such as the Solenodon (aprox. 60 Ma) and the coelacanth (aprox. 75 Ma) are perhaps the best- known panchronic organisms (Grassé, 2013). Possibly, among the invertebrates, the least studied species is the butterfly Baronia brevicornis Salvin, 1893 (Lepidoptera: Papilionidae) (Fig. 1; Grassé, 2013). Baronia brevicornis evolved about 80-90 Ma and is the oldest member of Papilionoidea, the superfamily that groups 7 families commonly known as butterflies, which diversified about 98.3 Ma (Nazari et al., 2007; Espeland et al., 2018; Kawahara et al., 2019). It is possible that B. brevicornis survived the K/T mass extinction (Labandeira et al., 2002) and has maintained a relatively unchanged morphology at least for about 80-90 Ma (Heikkilä et al., 2012; Machkour-M’Rabet et al., 2014; Espeland et al., 2018; Kawahara et al., 2019). This remarkable and taxonomically important species is included as Endangered (EN) in the IUCN Red List of Threatened Species and, unfortunately, information about its ecology and host plant use is very scarce (Legal et al., 2015; Machkour-M’Rabet et al., 2014; Puttick et al., 2018). Therefore, natural history data on this species are needed for population programs and to help establish plans for its conservation (Legal et al., 2015).
The restricted natural range of B. brevicornis (Soberón & Townsend-Peterson, 2005) makes it a vulnerable endemic species within tropical dry forest in Mexico (Michán et al., 2004; Legal et al., 2015). Among Mexican tropical dry forest plants, members of the family Fabaceae are abundant (Torres et al., 2009), such as the thorny bush Vachellia campechiana (Mill.) Seigler & Ebinger (Fabaceae), which is used by B. brevicornis larvae almost exclusively as a host (Vázquez & Pérez, 1961; León-Cortés et al., 2004). For example, larvae rarely feed on the closely related species Vachellia pennatula (Schltdl. & Cham.) Seigler & Ebinger and is even rarer to find it on Vachellia hybrids (Vázquez & Pérez, 1961; León-Cortés et al., 2004). Caterpillars have 5 developmental instars in approximately 1 month; pupation occurs on the ground, and adults emerge the following summer, approximately 10.5 to 11 months after pupation (Vázquez & Pérez, 1961, 1966). The adults are polymorphic, with 3 distinct male and female morphs, for a total of 6 color morphs (Vázquez & Pérez, 1966; Vázquez, 1987). Males have 3 parallel bands of yellowish spots on the upper side of their forewings, transverse to the wing venation from the submarginal to the basal zone (Vázquez, 1987). The 3 male morphs differ slightly in the background color of the wing: light brown, dark brown, and yellow. In the yellow morph, the spots are barely visible. There are also 3 female morphs (Fig. 2). The brown female morph (Fig. 2a) is very similar to the males (Fig. 2b), but it lacks the 3 parallel bands of yellow spots (distinguishing it from males) and has 2 or 3 nonparallel bands of spots, with the spots on the basal wings being more elongated. The ‘melanic’ female morph (Fig. 2c) has dark brown wings with small white spots. The orange female morph has large orange spots (Fig. 2d) that almost completely cover the wing. After adults mate, females lay their eggs 1 at a time on the underside of leaves of V. campechiana, spreading them widely; on these plants, eggs hatch approximately 5 days after oviposition, and the life cycle starts again (Vázquez & Pérez, 1961).

Figure 2 Female morphs. a) Brown, c) melanic, and d) orange morphs. Males are much more similar between morphs and resemble the brown morph of females (b). Images courtesy of Juan Carlos Mata.
Despite the relevance of B. brevicornis, basic knowledge of its life history is lacking. Hence the aim of this paper is twofold: first, we are interested in documenting the results of a 3-year long marking, releasing, and recapturing monitoring program on a population of B. brevicornis during the flight season, and second, we explored why the distributional range of B. brevicornis is narrower than the occupation of its larvae host plant (Soberón & Peterson, 2005).
With our first goal we aim to offer accurate estimates of population structure, sex ratio, and longevity. Data on population structure will provide basic information about the morph frequency (the presence of 3 male and 3 female morphs is unusual in the animal kingdom), longevity will reveal if there are sexual or morph differences according to season, size will reveal if some morphs are larger than others, as well as if females are larger than males, and finally, sex ratio is an important variable in studies of population structure because a skewed sex ratio is the major factor limiting population growth and it is also important forestablishing conservation actions (Donald, 2007; Steifetten & Dale, 2006).
A notable observation about the natural history of this species is that while V. campechiana occupies most parts of the tropical dry forest, the distribution of B. brevicornis is restricted to small patches of a few Vachellia trees. Hence, to determine the possible causes for the mismatch between B. brevicornis distribution and its host plants we explored 2 hypotheses: a) V. campechiana might not be the only host plant, as previously proposed and only sites with plants for both larvae and adults may support the B. brevicornis populations (Vázquez & Pérez, 1961; León-Cortés et al., 2004; Legal et al., 2015). Another non-mutually exclusive possibility, is that b) the V. campechiana water status is lower in the population inhabited by B. brevicornis compared to the plant populations without caterpillars of B. brevicornis. This hypothesis was considered very likely because it is well known that herbivores are more likely to be found feeding on host plants with lower plant water status than on those with higher plant water status (Archer & Detling 1986). To test these hypotheses, we compared localities with or without B. brevicornis to determine differences in the potential plant food sources, recorded the feeding behavior of larvae and adults, and analyzed the plant physiology in terms of the water potential of the plant food source of the larval stage. In sum, we present basic knowledge of one of the least-studied panchronic species, and the oldest butterfly species in the world.
Material and methods
Our study was conducted in a population located in the Reserva de la Biosfera de la Sierra de Huautla (REBIOSH), in the southern part of the state of Morelos, Mexico (Covarrubias-Camarillo et al., 2016). The original vegetation type is tropical dry forest, but approximately 40% of the total surface is currently degraded due to deforestation for agricultural fields, wood extraction, and cattle ranching (Conanp, 2005). The study site is an "acahual" (area with secondary vegetation) with V. campechiana (Fabaceae), and remnants of the original tropical dry forest vegetation specifically maintained by local people for their ethnobotanical value (Maldonado et al., 2013). To protect the species, the exact study site is not specified here, but can be provided upon request.
Mark-release-recapture was performed daily from 10:00 to 14:00 hours, which is the time when B. brevicornis butterflies are the most active (unpublished data). Individuals were captured using entomology nets and marked with a unique number code on the right forewing using enamel paint for model kits (Testors). Consecutive numbers up to 99 were painted on the wing, and a dot was added for every 100 and a line for every 1,000 thereafter. We used a Mitutoyo digital caliper (0.001 mm) to measure the length of the right forewing (from the base to the apex of the wing) and thorax width, which are good indicators of flight ability in butterflies (Chai & Srygley, 1990; Van Dyck & Wiklund, 2002). Individuals were then released. To estimate the sex and morph frequency, we pooled data collected in 2014, 2015, and 2017, because we found very few individuals of some morphs. We performed daily samplings from 9:00 to 16:00 hours, to record the main activity from the moment of emergence from pupae early in the morning to the moment in which they leave the site. We marked these individuals every day and identified their sex to try to follow them throughout their lives.
Recapture histories were analyzed using the Mark software (White & Burnham, 1999) within the framework of information theory model selection (for a rationale for this approach, see Anderson et al., 2001; Burnham & Anderson, 2002). We started by fitting a time-dependent Cormack-Jolly-Seber (CJS) model by group [Phi (g*t) p (g*t)]. We then used Akaike’s information criterion (AIC) as computed by Mark software to select the best models to describe the variation in the data. Overdispersion was estimated by dividing the parameter ĉ of model Phi (g*t) p (g*t) by the ĉ value obtained from bootstrapping and was found to be negligible (ĉ = 1.117). This value was used to obtain the QAICc of each model in Mark.
At the beginning of the flight season, we found adults feeding on flowers of plants (Burseraceae, Fabaceae, Malvaceae, and Boraginaceae) and larvae feeding on V. campechiana. Thus, we compared 4 localities of V. campechiana: 2 with the presence of larvae of B. brevicornis (150 × 85 m and 115 × 55 m) and 2 without (150 × 85 m and 115 × 55 m). Each locality with butterflies was next to a locality without butterflies. First, we explored if the 4 localities included all species on which the butterfly adult stage feeds and we determined the species. Second, we selected only 2 localities, one with B. brevicornis (150 × 85 m) and one without it (150 × 85 m), and compared the physiology of V. campechiana trees (food source of the larval stage) in terms of plant water status. Specifically, during the rainy season, predawn (ΨPD), and midday (ΨMD) plant water potentials were measured before sunrise and between 13:00 and 15:00, respectively, with a Scholander pressure chamber (1000, PMS Instrument Co., Albany, OR, USA). The determinations were performed on 2 last- growth terminal shoots ca. 3.5 mm in diameter from 2 to 3 leaves of 5 V. campechiana trees at each locality, namely, those without and with B. brevicornis. ΨPD is an indicator of soil water availability, as plant and soil water potential tend to equilibrate given minimum transpiration during the night (Cavender-Bares et al., 2007; Fallon & Cavender- Bares, 2018). ΨMD indicates the plant water status after a day of gas exchange.
Because our measurements of wing length and thorax width were not normally distributed, we used generalized linear models (gamma distribution with a log link) to test for statistically significant effects of sex, morph, and their interaction. Differences in plant water potential between V. campechiana localities were explored with one-way ANOVA. We assumed that each plant is an independent sampling unit to explore the water potential of each locality. Increasing the number of localities is nevertheless needed to test the generality of our results, but due to logistical problems it could not be done in this study. Analyses were performed using SPSS Statistics 23.
Results
The most supported model included time-dependence in survival and sex dependence in recapture rates (Table 1). Estimates indicate that daily survival (phi) varied over time, with values ranging between 0.19 and 0.763 (Table 2). The mean survival per sex was 0.602 for males and 0.468 for females, estimated from the model Phi(g) p(t), although this model has low support, which suggests that sex did not affect survival. Recapture was very low: 9.0% for males and 3.3% for females (Table 2).
Table 1 Model ranking by QAICc as estimated by MARK. The most supported model suggests that survival was time-dependent and recapture rate sex-dependent. The remaining models had almost no support, as indicated by their AICc weights.
| Model | QAICc | Delta QAICc | AICc weights | Model likelihood | Num. Par | QDeviance |
|---|---|---|---|---|---|---|
| {Phi(t) p(g) PIM} | 962.17 | 0.00 | 0.88 | 1.00 | 15.00 | 123.82 |
| {Phi(t) p(t) PIM} | 968.53 | 6.36 | 0.04 | 0.04 | 25.00 | 109.64 |
| {Phi(t) p(.) PIM} | 968.70 | 6.53 | 0.03 | 0.04 | 14.00 | 132.39 |
| {Phi(g) p(t) PIM} | 969.97 | 7.80 | 0.02 | 0.02 | 15.00 | 131.62 |
| {Phi(.) p(t) PIM} | 970.96 | 8.80 | 0.01 | 0.01 | 14.00 | 134.65 |
| {Phi(t) p(g*t) PIM} | 971.18 | 9.01 | 0.01 | 0.01 | 38.00 | 85.16 |
| {Phi(.) p(g*t) PIM} | 973.08 | 10.91 | 0.00 | 0.00 | 27.00 | 110.04 |
| {Phi(g) p(g*t) PIM} | 973.48 | 11.31 | 0.00 | 0.00 | 28.00 | 108.36 |
| {Phi(g*t) p(g) PIM} | 975.71 | 13.54 | 0.00 | 0.00 | 28.00 | 110.59 |
| {Phi(g*t) p(.) PIM} | 976.29 | 14.13 | 0.00 | 0.00 | 27.00 | 113.26 |
| {Phi(g*t) p(t) PIM} | 977.70 | 15.54 | 0.00 | 0.00 | 38.00 | 91.69 |
| {Phi(g*t) p(g*t) PIM} | 982.13 | 19.96 | 0.00 | 0.00 | 46.00 | 79.19 |
| {Phi(.) p(g) PIM} | 985.01 | 22.85 | 0.00 | 0.00 | 3.00 | 170.96 |
| {Phi(g) p(g) PIM} | 986.19 | 24.02 | 0.00 | 0.00 | 4.00 | 170.13 |
| {Phi(.) p(.) PIM} | 987.33 | 25.16 | 0.00 | 0.00 | 2.00 | 175.29 |
| {Phi(g) p(.) PIM} | 988.07 | 25.91 | 0.00 | 0.00 | 3.00 | 174.03 |
Table 2 Baronia brevicornis daily survival over time from the 2017 data (sexes combined). Standard error and confidence intervals corrected for ĉ = 1.1168. Real function parameters of {Phi(t) p(g) PIM} and 95% confidence interval.
| Parameter | Estimate | Standard Error | Lower | Upper |
|---|---|---|---|---|
| 1:Phi | 0.227 | 0.082 | 0.105 | 0.422 |
| 2:Phi | 1.000 | 0.000 | 1.000 | 1.000 |
| 3:Phi | 0.614 | 0.150 | 0.316 | 0.846 |
| 4:Phi | 0.556 | 0.161 | 0.258 | 0.819 |
| 5:Phi | 0.394 | 0.126 | 0.188 | 0.646 |
| 6:Phi | 0.763 | 0.243 | 0.188 | 0.978 |
| 7:Phi | 0.656 | 0.200 | 0.252 | 0.915 |
| 8:Phi | 0.443 | 0.148 | 0.197 | 0.721 |
| 9:Phi | 0.189 | 0.083 | 0.074 | 0.403 |
| 10:Phi | 0.468 | 0.236 | 0.121 | 0.849 |
| 11:Phi | 0.505 | 0.183 | 0.195 | 0.812 |
| 12:Phi | 0.476 | 0.199 | 0.160 | 0.812 |
| 13:Phi | 0.149 | 0.115 | 0.029 | 0.509 |
| 14:p | 0.090 | 0.014 | 0.066 | 0.121 |
| 15:p | 0.033 | 0.013 | 0.015 | 0.070 |
Wing length was significantly affected by sex (Wald test, X 2 = 24.18, df = 1, p < 0.0001), morph (Wald test, X 2 = 65.59, df = 5, p < 0.0001), and the sex*morph interaction (Wald test, X 2 = 65.59, df = 5, p < 0.0001). We also found significant differences in thorax width at the level of sex (Wald test, X 2 = 12.83, df = 1, p < 0.0001), morph (Wald test, X2 = 25.72, df = 5, p < 0.0001), and the sex*morph interaction (Wald test, X 2 = 25.72, df = 5, p < 0.0001). Overall, females were larger than males (females, wings: 32.08 ± 1.56 mm, N = 182; thorax: 3.77 ± 0.05 mm; males, wings: 27.36 ± 0.06 mm; thorax: 3.60 ± 0.01 mm). A Bonferroni post hoc test showed that the differences at the level of the sex*morph interaction were explained by the differences between brown females and the 3 male morphs, being the brown female morph the largest (Fig. 3).
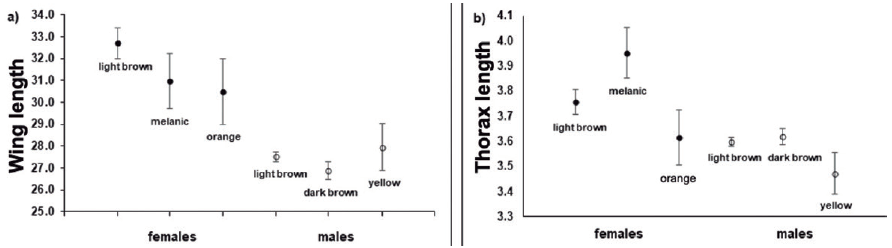
Figure 3 Mean values for a) wing length and b) thorax width (length). Filled and empty points show mean data for females and males, respectively. Error bars show standard error.
The number of males and females varied over the flight season in June (it lasted about 2-3 weeks, starting with the first rains). We captured a total of 1,577 individuals in June 2014 and 2015. Two people carried out the sampling. In this sample, 81% of the captured individuals were male (N = 1,271), and 19% were female (N = 306). However, the sex ratio upon emergence does not seem to be skewed, according to a sample of 53 recently emerged individuals that we captured in 2017 (from June 4-17) because 54.7% of the individuals were male (N = 29), and 45.3% were female (N = 24).
The dark brown morph was the most frequent male morph in 2014 and 2015 (55%, N = 700), followed by the light brown morph (27%, N = 345), and the least frequent male morph was the yellow morph (18%, N = 226). Among females, the brown morph was the most common (67%, N = 206), followed by the melanic morph (23%, N = 69), and finally the orange morph (10%, N = 31).
In 2017, we marked 1,430 individuals from June 4-17. Five people carried out the sampling. Female abundance peaks occurred on the 5th, 8th, and 13th of June, while male population peaks occurred on the 5th, 12th, and 14th of June (Fig. 4). In this sample, 87% of the individuals were male (N = 1,248), and the remaining 13% were female (N = 182). The most common male morph in this sample was the light brown morph (76%, N = 949), followed by the dark brown morph (21%, N = 261), and the yellow morph was the rarest male morph (3%, N = 38). In the case of females, the most frequent morph was the light brown morph (68%, N = 124), followed by the melanic morph (19%, N = 34), and finally the orange morph (13%, N = 24; Fig. 5).
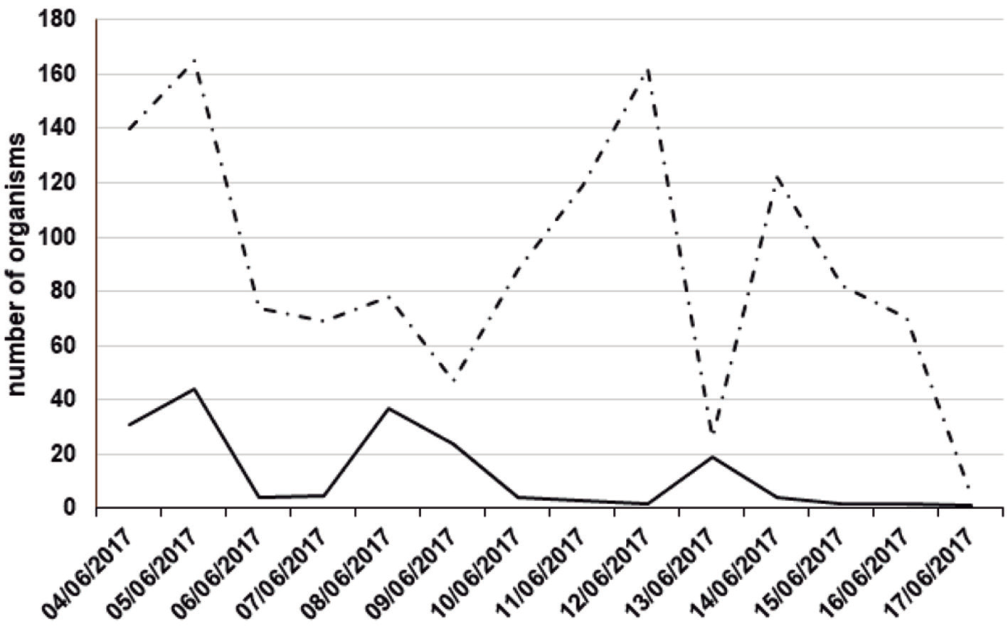
Figure 4 Abundance over time estimated from the number of individuals marked in June 2017. The solid line shows data for females and the dashed line shows data for males.
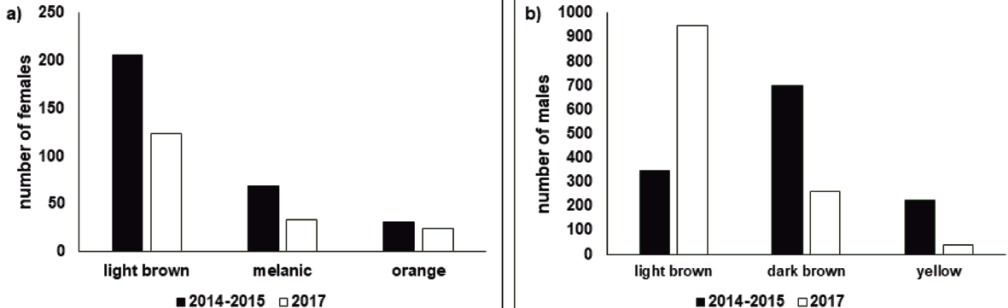
Figure 5 a) Number of female and b) male of Baronia brevicornis individuals of each color morph. Black columns show data for individuals collected in 2014-2015 and white columns for individuals collected in 2017.
According to the literature, B. brevicornis larvae feed on V. campechiana or V. pennatula (Vázquez & Pérez, 1961; León-Cortés et al., 2004). However, as far as we know, V. pennatula and hybrids are absent in our study zone. Thus, the butterflies that we studied fed as larvae exclusively on V. campechiana. We observed that adults fed on the flowers of the trees Bursera copallifera (Burseraceae), Lysiloma sp. (Fabaceae), Pithecellobium dulce (Fabaceae), and Guazuma ulmifolia (Malvaceae) and the bush Tournefortia densiflora (Fig. 6). It is also important to note that although the distribution of V. campechiana is widespread inside the reserve, we found the butterflies only on some patches. In 2 of these patches where B. brevicornis was present, we found the 4 plant species used by the adults, but in 2 other localities where only V. campechiana was present (but there were no nectar sources for the adults), no butterflies were found.

Figure 6 a) Adult feeding on Bursera (image courtesy of Juan Carlos Mata); b) a detail of its mouthparts in electron microscopy (Jorge Contreras-Garduño and Orlando Hernández).
Regarding physiology, we did not detect differences in the ΨPD (F = 0.23; p = 0.63) of V. campechiana trees between localities (with and without B. brevicornis). However, the V. campechiana trees with B. brevicornis had lower mid-day plant water potentials than those without the butterfly (-1.91 MPa ± 0.08 and -1.67 MPa ± 0.03, respectively), indicating a higher daily transpiration (F = 6.31; p = 0.03).
Discussion
Mark-recapture studies are essential for answering key questions on the general biology and population ecology of organisms in their natural habitats (Hagler & Jackson, 2001). These questions are particularly important in the conservation of insect species and their habitats (Molleman, 2018). The data presented in this study are consistent with previous research that defined B. brevicornis as univoltine (Vázquez & Pérez, 1961). Population abundance peaks coincided with the days of heaviest rain (Contreras- Garduño, pers. obs.). Humidity may be an important factor that softens the soil and facilitates adult emergence (Legal et al., 2015). This would also explain why this species starts its flight season with the first strong summer rains (Vázquez & Pérez, 1961).
The data on flying activity obtained from samples taken in 2014, 2015, and 2017 show male-biased operational sex ratios (OSR 4:1). However, recapture histories do not suggest differences in survivorship between sexes, though they do suggest sexual daily variation in flying activity (Table 2). This bias has been explained in other species (e.g., the medfly Ceratitis capitata) by fluctuations in frequency between the sexes throughout the season, for example females being less numerous than males at the beginning of the season but then switching to males being the less abundant sex at the end of it (Carey et al., 1995). In our case however, we observed fewer females than males thoughout the entire season. Furthermore, both males and females were seen flying from the beginning of the season, therefore our findings on male-biased cannot be explained by differences in phenology between the sexes (Lovich & Gibbons, 1990).
Though males and females emerged in similar proportions, female abundance declined from the first day of emergence (day 1 in figure 4) at the mating place defended by males (on the top of V. campechiana). This is in agreement with male biased sex ratios commonly reported for Lepidoptera in field studies, even when in the laboratory the sex ratio is 1:1 (e.g., Brussard & Ehrlich, 1970; Brown & Ehrlich, 1980; Ehrlich et al., 1984). While our results on survival may indicate a slightly higher post- emergence survival in males than in females, estimates of survival could be unreliable due to a female bias in natal dispersal, as has been suggested for other tropical Lepidoptera (Ehrlich et al., 1984; Freitas, 1993; Ramos & Freitas, 1999). We observed that males remain around the areas where virgin females emerge and that females are receptive immediately after adult emergence. Females then, oviposit on Vachellia plants and their larvae feed on the same plant (Vázquez & Pérez, 1961). Thus, males may receive benefits from staying in localities close resources such as Vachellia plants, while females may opt to disperse to avoid sexual harassment. Another hypothesis that does not exclude the former idea of sexual harassment, is that females may opt to stay in sites with food resources for them, and males may opt to stay on plants in which females mate and oviposit. Indeed, it has been suggested that female behavior might be responsible for skewed sex ratios (Vlasanek et al., 2009; Goff et al., 2019). Knowing if females depart from their place of emergence to avoid males or to feed on the flowers from plants other than those of the host plant, will help determine the cause of the operational sex ratio observed here. And this answer in turn will determine the impact of the sex bias on the population structure and the mating system (Kokko & Jennions, 2008; Székely et al., 2014).
Regarding wing length and thorax width, we found that females were larger than males. These traits are associated with flight ability in many Lepidoptera (Chai & Srygley, 1990; Van Dyck & Wiklund, 2002). Again, this result could be associated with the male and female dispersal ecology in this species. Given the sexual differences in the behavior of B. brevicornis, the morphology of the traits associated with flight may also differ between sexes, as occurs in other species of butterflies with similar behavior (Berwaerts et al., 2006; Van Dyck & Wiklund, 2002; Wickman, 1992). Large wings are associated with sustained flights with low energy requirements and the ability to float, while small wings are associated with greater maneuverability and short, fast flights (Chai & Srygley, 1990; Srygley & Chai, 1990; Van Dyck & Wiklund, 2002). If females of B. brevicornis travel greater distances than males, the larger wings may allow them to travel those distances with less investment of energy. Instead, since males defend territories and chase females when they arrive in those territories, they can benefit from greater maneuverability and speed on short flights. Furthermore, in other species of butterflies, a wide thorax is associated with faster flight (Chai & Srygley, 1990; Srygley & Chai, 1990). We propose that the morphological traits of B. brevicornis may be the result of a trade-off to achieve an optimum between speed and duration of flight and that optimum may differ between males and females: large wings in females to fly more and small wings in males for maneuverability. Further study should test whether females fly better than males and among males, for example, whether yellow males fly better than dark brown males.
The frequencies of the 3 female morphs seem to be very stable, as samples taken in 2014-2015 and 2017 show very similar proportions. In the case of males, the morphs’ relative abundances do not seem to be as stable because the abundance of the dark brown and light brown morphs varied among the sampling seasons. Understanding the ecological and physiological factors that produce such variations is crucial to comprehend how the polymorphism in this species evolved and how it is maintained. Species that present color polymorphisms frequently exhibit relative morph abundances that vary over time and with no more than 2 male or 2 female morphs (Romo-Beltrán et al., 2009; Ruiz-Guzmán et al., 2013; Galicia-Mendoza et al., 2017). However, the color polymorphism in B. brevicornis is especially interesting, not only because it appears in both males and females, which is uncommon in butterflies (McKinnon & Pierotti, 2010), but also because this species is the only survivor in a lineage that diverged early from all other butterflies (Espeland et al., 2018). Thus, studying this polymorphism may be useful to understand how color polymorphisms evolved in other species of Papilionidae.
Finally, we were interested in examining 2 potential explanations for why the distribution of B. brevicornis is narrower than the occupation of its host plant and we proposed 2 hypotheses: a) that V. campechiana water status is lower in the population inhabited by B. brevicornis compared to the plant populations without caterpillars of B. brevicornis, and b) that V. campechiana may not be the only host plant, as previously proposed. Regarding the first hypothesis, it is important to note that the V. campechiana localities with B. brevicornis had greater water expenditure and therefore might experience a greater water deficit, especially at the end of the rainy season. Furthermore, these localities were next to localities without B. brevicornis, which suggests that B. brevicornis may feed preferentially on stressed plants to avoid plant defenses (Blumenthal et al., 2020; Simoes & Baruch, 1991; Stiegel et al., 2017). Further research is needed to determine if the distribution of B. brevicornis is influenced by the level of stress experienced by V. campechiana particularly, because B. brevicornis seems to show philopatry. Regarding the second hypothesis, an important result of this research concerns B. brevicornis feeding behavior, as we identified for the first time that adults feed on the flowers of the trees B. copallifera, Lysiloma sp., P. dulce, G. ulmifolia, and T. densiflora. These plant species (together with plants of Acacia sp.) are maintained by local people in some acahual localities because they are of ethnobotanical value for the population (Maldonado et al., 2013). Additionally, these genera originated with South American tropical dry forest, and then migrated with this type of vegetation to Mexico (Pennington et al., 2000). Both lines of evidence contribute to explain the prevalence of the association between Vachellia and other species with B. brevicornis. Furthermore, we found butterfly patches only in the 2 localities with 4 of 5 plant species; thus, adult feeding behavior may be important for understanding the distribution of this species because it is known that the presence of B. brevicornis is contingent upon the presence of V. campechiana, which the larvae eat. This finding is very important because it may explain why although V. campechiana and V. pennatula have a widespread distribution in Mexico, the distribution of B. brevicornis is narrower. It could be interesting to explore the evolutionary causation of this phenomenon.
Therefore, we suggest that V. campechiana is not the only determinant of the presence of B. brevicornis and that this butterfly species may be additionally limited by the presence of other plants that adults consume. Under this scenario, we propose to look for populations of B. brevicornis with predictive methods (such as in Legal et al., 2015), taking into account the host plants for larvae and adults such as B. copallifera, Lysiloma sp., Pithecellobium sp., T. densiflora, and V. pennatula, as well as for example, soil hardness, which is important for adults to emerge from the ground (León-Cortés et al., 2004). These predictions should then be corroborated in the field, carrying out monitoring programs such as those described here (mark, release, and recapture) and carrying out analyses of genetic variation as well as predictive methods.











 nova página do texto(beta)
nova página do texto(beta)



