Introduction
Mexico is a country with a high biodiversity, including fungal diversity. It ranks thirteenth in the world in territorial surface area (1,964,375 km2) yet fifth in biodiversity. Of the 32 federal entities of Mexico, Sonora is the second largest, with an area of 179,355 km2, which is larger than the smallest 106 countries in the world. In regard to number of fungal species, Sonora ranks fifth in Mexico, with 658 recorded; of these, 461 are Basidiomycetes (Aguirre-Acosta et al., 2014). The most studied groups in Sonora are gasteroid and sequestrate Agaricomycetes (GSA), with more than 120 cited species. Of these, 37 correspond with tulostomataceous stalked-puffballs (TSPs), which are the most diverse and abundant group in Sonora (Esqueda, Pérez-Silva, Herrera, Altés et al., 1998; Esqueda et al., 2004, 2010; Hernández-Navarro et al., 2015, 2017, 2018; Piña-Páez et al., 2010).
The main morphological traits of TSPs are the presence of a spore-sac with pulverulent gleba and a well-defined, hollow stipe, with a volva or a volvoid structure; sometimes, rhizomorphs are present. The genera are mainly differentiated based on the continuity of the stipe, peridium layers, gleba type, presence and type of capillitium, dehiscence, and spore ornamentation. Notably, TSPs are especially abundant, diverse, and predominant in arid and semiarid zones, although some species grow exclusively in subtropical, tropical, or temperate regions. All species present terrestrial habits, with the remarkable exception of T. exasperatum Mont., which is lignicolous. Some studies have provided in situ information on fructification sites, which can help to understand the species autecology, although this task is still far from complete (Cortez et al., 2009; Esqueda et al., 2000; Hernández-Navarro et al., 2015; Wright, 1987).
Originally, TSPs were classified as belonging to the family Tulostomataceae, which consisted of 8 genera classified in 3 tribes: Battarreae (Battarrea, Battarreoides), Phellorinae (Chlamydopus, Dictyocephalos, Phellorinia), and Tulostomatinae (Queletia, Schizostoma, Tulostoma) (Long, 1946; Long & Stouffer, 1946; Wright, 1987). Although several species have been described for each genus, most genera are monotypic or have few taxa, except for Tulostoma which, according to the world monograph of Wright, (1987), is composed of 139 spp. Currently, more than 155 species have been described for the genus (http://www.speciesfungorum.org/names/fundic.asp).
Considering molecular data, several changes have occurred in fungal classification, including the invalidation of the class Gasteromycetes and its segregation into several orders such as Agaricales, Boletales, Russulales, Geastrales, and Phallales. Tulostomataceae was also segregated into 2 families, Phelloriniaceae (including Phellorinia and Dictyocephalos) and Agaricaceae (the rest of the genera). Agaricaceae has traditionally been one of the most studied and best recognized fungal families. However, it is currently considered a heterogeneous family with diverse growth forms such as agaricoid, sequestrate, and gasteroid. Despite the lack of molecular information of TSPs and due their distinctive morphological traits, some authors have suggested that they represent a monophyletic lineage, independent from Agaricaceae in the agaricoid clade of Agaricales (Gube, 2009; Martin et al., 2000; Matheny et al., 2006; Vellinga, 2004).
Up until 2018, 7 of the 8 known genera of TSPs have been reported in Sonora and a total of 37 species. Some of these species are cosmopolitan or with a wide distribution, but a few are also infrequent (Battarrea), rare (Chlamydopus, Dictyocephalos), or extremely rare such as Tulostoma gracilipes and T. portoricense, which were cited for the second time worldwide from Sonora (Esqueda, Pérez-Silva, Herrera, Altés et al., 1998; Esqueda, Pérez-Silva, Herrera & Moreno, 1998; Esqueda et al., 2000; Piña-Páez et al., 2010). The aim of the present study was to analyze the diversity, morphological variability, and distribution of TSPs, through examining the specimens deposited in the herbarium of the Sonora State University over 29 years of sampling.
Materials and methods
All TSPs in the macromycetes collection of the Sonora State University herbarium (UES - for its initials in Spanish) were characterized macro- and microscopically according to the methods previously described by Hernández-Navarro et al. (2015, 2017), including scanning electron microscopy. For the microscopic characterization, temporary preparations of the glebal portions were mounted with 10% KOH, which is recommended by Wright (1987) to rehydrate the material, allowing it to recover its shape and making the ornamentation more easily discernible without altering the color. We measured at least 20 spores, capillitia, septa, and elaters per collection. The recognition of morphological species was based on the combination-of-characteristics approach proposed by Wright (1987). Finally, we gathered the annotated information from the collections: location, municipality, altitude, climate, soil, and vegetation type (Table 1).
Table 1. Abiotic characteristics, type of vegetation, and coordinates by locality.
| Municipality/ | A | V | C | P | S | N | W |
| Locality | |||||||
| Álamos | |||||||
| 1 El Aguaje | 734 | TTF | SDSW | 673 | Re | 26.97 | 108.96 |
| 2 El Sabinito | 364 | TTF | SDW | 673 | Li | 27.00 | 108.80 |
| 3 El Encinal | 1494 | OF | SDT | 673 | Re | 26.97 | 108.98 |
| 4 Km 7.5 Álamos-Guirocoba | 372 | TTF | SDW | 673 | Ca | 26.95 | 108.94 |
| 5 Palo Injerto | 415 | TTF | TSW | 673 | Li | 27.05 | 108.73 |
| Bacoachi | |||||||
| 6 Km 146 Mazocahui-Cananea | 1046 | AL | SDT | 421 | Ca | 30.65 | 109.97 |
| 7 Km 164 Mazocahui-Cananea | 1162 | G | SDT | 421 | Ca | 30.74 | 110.01 |
| Baviácora | |||||||
| 8 La Cieneguita | 1012 | SS | SDSW | 502 | Ca | 29.54 | 110.01 |
| 9 Mazocahui | 507 | MDS | DW | 502 | Ca | 29.54 | 110.12 |
| Benjamín Hill | |||||||
| 10 Las Ánimas | 798 | MDS | VDSW | 314 | Li | 30.21 | 111.32 |
| Caborca | |||||||
| 11 Rancho La Chula | 257 | AL | VDSW | 100 | Ye | 30.70 | 112.25 |
| Carbó | |||||||
| 12 San Luis | 500 | M | VDSW | 192 | Ye | 29.56 | 111.08 |
| Cumpas | |||||||
| 13 El Mezquital | 820 | IG | DSW | 474 | Ca | 29.96 | 109.64 |
| 14 Km 8 Ajos-Bavispe | 879 | CG | DSW | 474 | Ca | 29.98 | 109.66 |
| 15 Km 8.5, Ajos-Bavispe | 882 | CG | DSW | 474 | Ca | 29.98 | 109.66 |
| 16 La Selva | 884 | M | SDSW | 474 | Re | 29.96 | 109.62 |
| Fronteras | |||||||
| 17 El Frijolito | 2370 | POF | TSW | 687 | Re | 30.94 | 109.96 |
| 18 El manzano | 2296 | OF | TSW | 687 | Re | 30.93 | 109.97 |
| 19 La Sal | 2028 | OF | TSW | 687 | Re | 30.96 | 109.95 |
| General Plutarco Elías Calles | |||||||
| 20 Cráter El Celaya | 264 | MDS | VDSW | 191 | Ye | 31.99 | 113.46 |
| 21 Cráter El Colorado | 204 | SCS | VDSW | 191 | Ye | 31.92 | 113.31 |
| 22 Cráter El Elegante | 167 | SCS | VDSW | 191 | Ye | 31.86 | 113.38 |
| 23 Sierra Los Tanques | 488 | MDS | VDSW | 191 | Ye | 31.77 | 113.01 |
| 24 El Papalote | 319 | M | VDSW | 191 | Re | 31.93 | 113.03 |
| 25 San Juanico | 193 | SCS | VDSW | 191 | Re | 31.83 | 113.34 |
| 26 WPT del Colorado | 197 | HV | VDSW | 191 | Ye | 31.89 | 113.29 |
| Guaymas | |||||||
| 27 Cañón de Nacapule | 173 | SCS | VDW | 328 | Li | 28.02 | 111.05 |
| 28 Ejido Francisco Villa | 168 | SCS | VDW | 219 | Ye | 28.11 | 111.02 |
| 29 Maytorena | 146 | SCS | VDW | 328 | So | 28.23 | 110.81 |
| Hermosillo | |||||||
| 30 Calle 0 La Costa de Hermosillo | 96 | MDS | VDW | 157 | Ye | 28.88 | 111.35 |
| 31 Centro Ecológico de Sonora | 262 | M | VDW | 342 | Re | 29.02 | 110.95 |
| 32 CIAD | 255 | UZ | VDW | 342 | Re | 29.13 | 110.91 |
| 33 El Crucero La Costa de Hermosillo | 21 | MDS | VDSW | 169 | So | 28.83 | 111.72 |
| 34 El Apache | 36 | M | VDW | 123 | So | 28.32 | 111.24 |
| 35 El Papalote | 299 | M | VDSW | 342 | Re | 29.22 | 111.04 |
| 36 Km 90 Hermosillo-Bahía de Kino | 12 | SCS | VDSW | 168 | So | 28.83 | 111.77 |
| 37 La Milla UNISON | 202 | UZ | VDW | 342 | Ye | 29.08 | 110.97 |
| 38 La Pintada | 218 | SCS | VDW | 342 | Re | 28.56 | 111.01 |
| 39 La Primavera | 163 | MDS | VDW | 280 | Re | 28.80 | 111.15 |
| 40 La Tijerita | 296 | M | VDW | 342 | Re | 29.17 | 110.92 |
| 41 La Victoria | 236 | AL | VDW | 342 | Ye | 29.12 | 110.89 |
| 42 Rancho Las Palomas | 216 | M | DSW | 292 | Xe | 28.99 | 110.46 |
| Huásabas | |||||||
| 43 Sierra La Madera | 1304 | SS | SDT | 438 | Re | 29.90 | 109.46 |
| Huatabampo | |||||||
| 44 Huatabampito | 11 | AL | VDW | 326 | Ve | 26.84 | 109.64 |
| La Colorada | |||||||
| 45 Km 31 Hermosillo-Yécora | 290 | M | VDSW | 343 | Ye | 28.85 | 110.71 |
| 46 Km 40 Hermosillo-Yécora | 335 | M | VDSW | 343 | Ye | 28.82 | 110.63 |
| 47 Km 100 Hermosillo-Yécora | 472 | TS | DSW | 489 | Re | 28.62 | 110.12 |
| Opodepe | |||||||
| 48 Tuape | 670 | MDS | VDSW | 354 | Xe | 30.05 | 111.01 |
| Pitiquito | |||||||
| 49 Ejido 15 de Mayo | 269 | MDS | VDSW | 90 | Re | 29.79 | 112.53 |
| 50 Entrada a Puerto Libertad | 9 | MDS | VDSW | 90 | Re | 29.90 | 112.66 |
| 51 Km 158, 36 N a Puerto Libertad | 28 | MDS | VDSW | 90 | Re | 29.88 | 112.64 |
| 52 Las Dunas, Punta Cirio | 40 | MDS | VDSW | 90 | Re | 29.85 | 112.64 |
| 53 Km 115, 36 N a Puerto Libertad | 112 | MDS | VDSW | 90 | Re | 29.64 | 112.30 |
| 54 Km 125, 36 N a Puerto Libertad | 127 | MDS | VDSW | 90 | Re | 29.68 | 112.38 |
| 55 Rancho Punta Cirio | 212 | MDS | VDSW | 90 | Re | 29.82 | 112.57 |
| Puerto Peñasco | |||||||
| 56 Ejido Los Norteños | 119 | SDV | VDSW | 137 | Re | 31.66 | 113.33 |
| 57 Ejido Punta Peñasco | 163 | SDV | VDSW | 137 | Re | 31.76 | 113.27 |
| 58 Sierra Blanca | 69 | MDS | VDSW | 137 | Re | 31.52 | 113.42 |
| Rayón | |||||||
| 59 Rancho La Granada | 640 | MDS | DSW | 464 | Re | 29.69 | 110.50 |
| San Javier | |||||||
| 60 Km 137 Hermosillo-Yécora | 496 | TDF | SDSW | 684 | Re | 28.58 | 109.78 |
| 61 Km 151 Hermosillo-Yécora | 720 | TDF | SDSW | 684 | Re | 28.58 | 109.68 |
| 62 Km 3.5 a San Javier | 760 | TDF | SDSW | 684 | Re | 28.58 | 109.75 |
| San Luis Río Colorado | |||||||
| 63 Cerro Lava / Microondas | 246 | SDV | VDSW | 54 | Re | 32.05 | 113.56 |
| 64 San Luis Río Colorado, ciudad | 42 | UZ | VDW | 78 | Re | 32.45 | 114.76 |
| Soyopa | |||||||
| 65 Km 162 Hermosillo-Yécora | 260 | TDF | SDW | 576 | Li | 28.56 | 109.60 |
| 66 Km 162.5 Hermosillo-Yécora | 287 | TDF | SDW | 576 | Li | 28.56 | 109.60 |
| 67 Km 163 Hermosillo-Yécora | 278 | TDF | SDW | 576 | Li | 28.57 | 109.60 |
| 68 Río Yaqui | 213 | TTF | SDW | 576 | Lu | 28.57 | 109.55 |
| Ures | |||||||
| 69 Km 72 Hermosillo-Mazocahui | 1378 | M | DW | 371 | Xe | 29.45 | 110.29 |
| 70 Sierra Huérfana, Bosque de encino | 886 | OF | SDT | 478 | Ca | 29.10 | 110.20 |
| 71 Sierra Huérfana, Matorral subtropical | 599 | SS | SDSW | 393 | Li | 29.10 | 110.25 |
| 72 Sierra Huérfana, Mezquital | 145 | M | SDSW | 478 | Xe | 29.21 | 110.15 |
| Yécora | |||||||
| 73 Km 200.5 Hermosillo-Yécora | 915 | TDF | SDSW | 651 | Ca | 28.47 | 109.34 |
| 74 Km 205 Hermosillo-Yécora | 900 | TDF | TSW | 651 | Ca | 28.46 | 109.31 |
| 75 Km 258 Hermosillo-Yécora | 1476 | OF | TSW | 1057 | Li | 28.37 | 109.07 |
Headers: (A) Altitude (m asl); (V) vegetation; (C) climate; (P) precipitation (mm); (S) soil type; (N) north latitude; (W) west longitude. Vegetation types: (AL) agricultural lands; (CG) cultivated grassland; (G) grassland; (HV) halophytic vegetation; (IG) induced grasslands; (M) mezquital; (MDS) microphyllous desert scrub; (OF) oak forest; (POF) pine oak forest; (SDV) sandy desert vegetation; (SCS) sarcocaule scrub; (SS) subtropical scrub; (TS) thorn scrub; (TDF) tropical deciduous forest; (TTF) tropical thorn forest; (UZ) urban zone. Climate types: (DSW) dry semiwarm; (DW) dry warm; (SDSW) semidry semiwarm; (SDT) semidry temperate; (SDW) semidry warm; (TSW) temperate subwet; (VDSW) very dry semiwarm; (VDW) very dry warm. Soil type: (Ca) Cambisol; (Li) Lithosol; (Lu) Luvisol; (Re) Regosol; (So) Solonchak; (Ve) Vertisol; (Xe) Xerosol; (Ye) Yermosol.
Data analysis was done in the statistical environment R version 3.0.3 (http://www.R-project.org) using the vegan (Oksanen et al., 2019) and cluster packages (Maechler et al., 2019). Canonical Correspondence Analysis (CCA) was used to elucidate the relationships between TSPs and environmental conditions, and significances were tested by permutational multivariate analysis of variance (PermANOVA) (p < 0.01). Hierarchical cluster analysis (HCA) using Ward’s method was used to group TSPs, taking into account environmental conditions (Ward, 1963).
Results
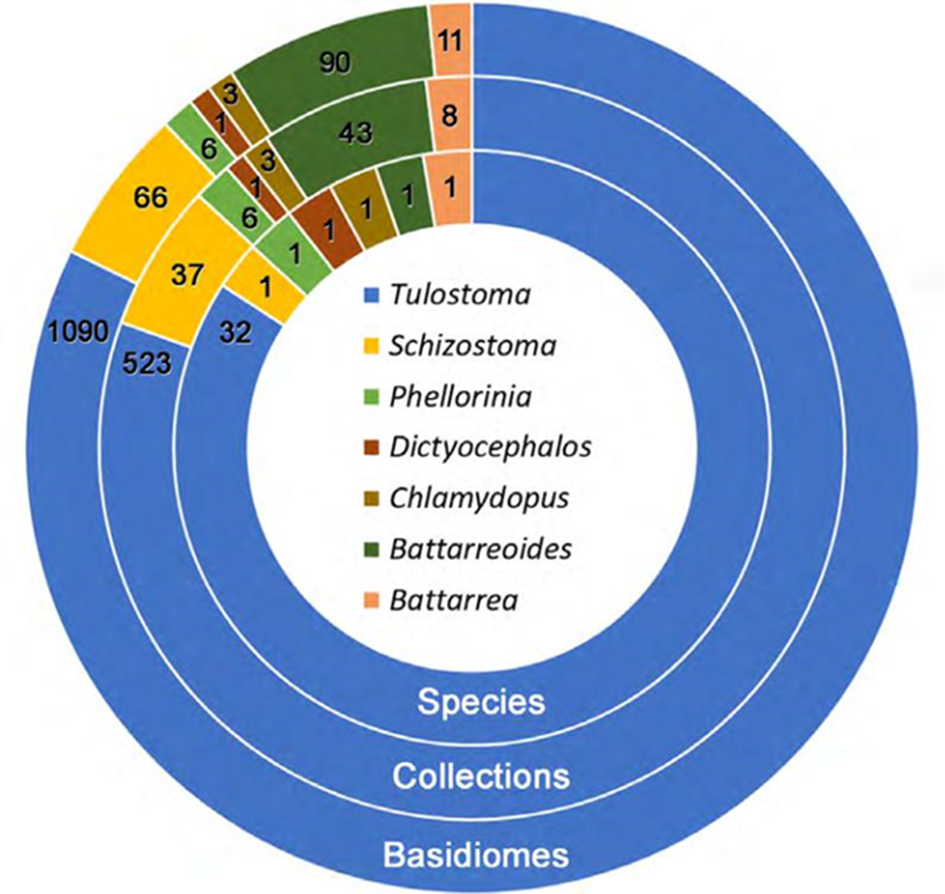
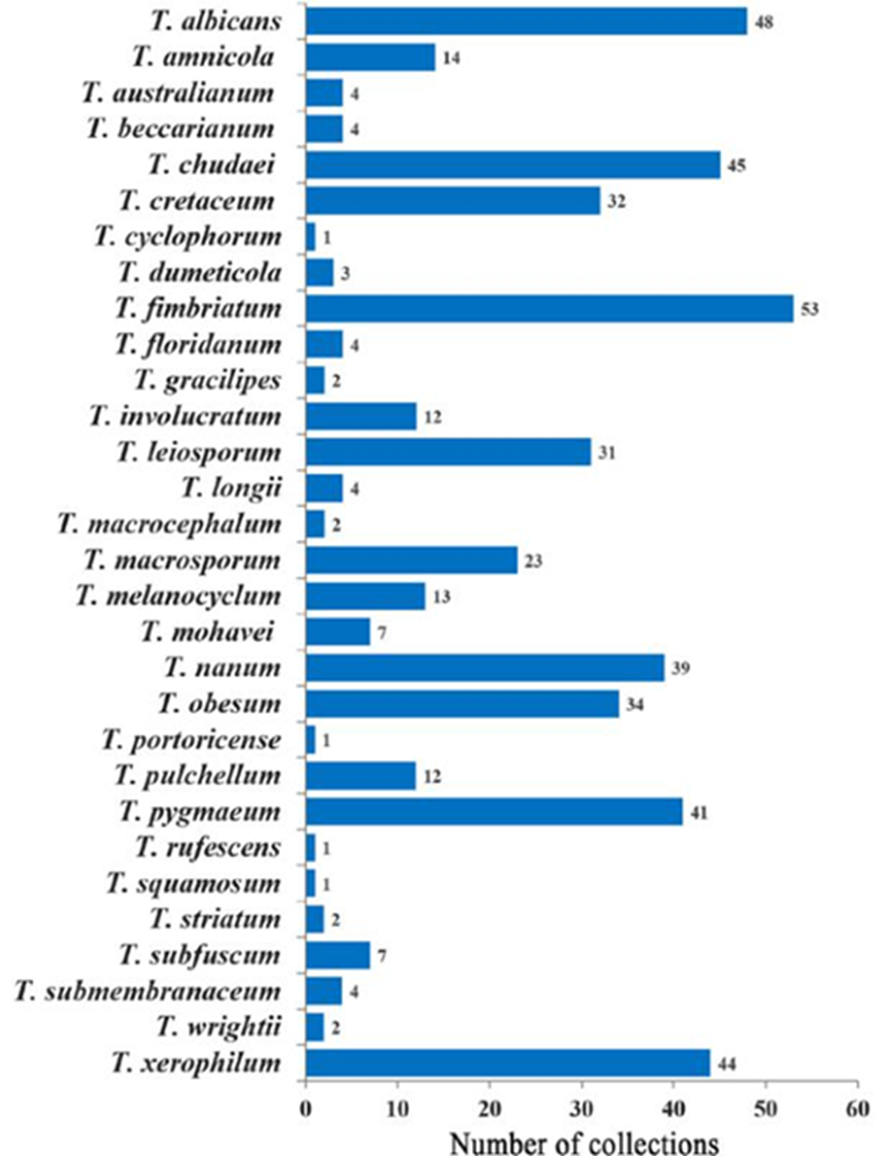
Based on 1,266 specimens in 621 collections, 37 species belonging to 7 genera of TSPs were determined (Table 2; Fig. 1). Tribe Battarreae was represented by both of its genera, Battarrea and Battarreoides with more collections of Battarreoides diguetii than Battarrea phalloides. Tribe Phellorinae was the least abundant group. There was only one specimen of Dictyocephalos attenuatus found in the “El Pinacate” and “Gran Desierto de Altar” Biosphere Reserves. Chlamydopus meyenianus was also a rare species with only 2 specimens. Phellorinia had 6 specimens, one per collection. Tribe Tulostomatinae was the most abundant and diverse, composed of 2 genera, Tulostoma and Schizostoma. The latter genus had only one species (S. laceratum) in Sonora. On the other hand, Tulostoma was the most abundant and diverse genus, with 1,090 specimens in 523 collections corresponding to 31 species. Several Tulostoma species were especially abundant, such as T. fimbriatum (53 collections, 146 specimens), T. xerophilum (44 collections, 98 specimens), T. albicans (48 collections, 88 specimens), T. chudaei (45 collections, 95 specimens), and T. cretaceum (32 collections, 96 specimens) (Fig. 2). Some spore ornamentation under SEM of Tulostoma species are shown in Figures 3 and 4.
Table 2. Distribution of species by locality.
| Species | Locality |
| Battarrea phalloides (Dicks.) Pers. | 2, 11, 28, 31, 33, 37, 38 |
| Battarreoides diguetii (Pat. & Har.) R. Heim & T. Herrera | 8, 11, 27, 28, 29, 32, 34, 38, 39, 47, 48, 56, 58, 72 |
| Chlamydopus meyenianus (Klotzsch) Lloyd | 49, 51, 53 |
| Dictyocephalos attenuatus (Peck) Long & Plunkett | 22 |
| Phellorinia herculeana (Pers.) Kreisel | 23, 24, 47, 72 |
| Schizostoma laceratum (Ehrenb. ex Fr.) Lév. | 21, 22, 23, 23, 24, 29, 35, 39, 41, 46, 47, 48, 51, 53, 56, 57, 63 |
| Tulostoma albicans V.S. White | 10, 11, 12, 13, 14, 23, 27, 29, 35, 39, 47, 48, 53, 70, 71, 74 |
| T. amnicola Long & S. Ahmad | 1, 10, 22, 45, 46, 47 |
| T. australianum Lloyd | 24, 52 |
| T. beccarianum Bres. | 10, 39, 47, 65 |
| T. chudaei Pat. | 12, 13, 15, 22, 24, 29, 38, 39, 45, 46, 47, 48, 52, 56, 59, 60, 63, |
| 72, 72 | |
| T. cretaceum Long | 8, 11, 22, 24, 25, 35, 36, 39, 41, 44, 48, 49, 53, 54, 56, 57, 58, |
| 62, 63, 64 | |
| T. cyclophorum Lloyd | 31 |
| T. dumeticola Long | 3, 60, 61 |
| T. fimbriatum Fr. | 5, 6, 10, 12, 13, 16, 21, 24, 26, 28, 31, 35, 38, 40, 46, 47, 48, 65, |
| 67, 68, 75 | |
| T. floridanum Lloyd | 4, 8, 60, 70 |
| T. gracilipes J.E. Wright | 47, 72 |
| T. involucratum Long | 8, 12, 27, 38, 43, 45, 47, 66, 72 |
| T. leiosporum R.E. Fr. | 14, 20, 22, 23, 24, 29, 39, 45, 47, 48, 57, 60, 63, 65, 66, 68 |
| T. longii Lloyd | 47 |
| T. macrocephalum Long | 22, 34 |
| T. macrosporum G. Cunn. | 7, 10, 14, 29, 33, 38, 41, 45, 47, 48, 72 |
| T. melanocyclum Bres. | 16, 17, 18, 19, 53, 60 |
| T. membranaceum Long & S. Ahmad | 47 |
| T. mohavei Lloyd | 23, 41, 45, 47 |
| T. nanum (Pat.) J.E. Wright | 10, 11, 12, 22, 23, 25, 28, 30, 38, 45, 46, 47, 48, 54, 56, 60, 61, |
| 66, 69, 73 | |
| T. obesum Cooke & Ellis | 10, 20, 22, 24, 25, 34, 38, 41, 50, 55, 56, 57, 58, 63 |
| T. portoricense J.E. Wright | 7 |
| T. pulchellum Sacc. | 14, 45, 46, 47 |
| T. pygmaeum Lloyd | 12, 13, 16, 23, 25, 28, 29, 39, 47, 48, 66, 71, 72, 74 |
| T. rufescens Hern.-Nav. & Esqueda | 43 |
| T. squamosum (J.F. Gmel.) Pers. | 60 |
| T. striatum G. Cunn. | 13, 31 |
| T. subfuscum V.S. White | 8, 13, 31, 47, 63 |
| T. submembranaceum G. Moreno, C. Ochoa & J.E. Wright | 18, 29, 42, 66, 68 |
| T. wrightii Berk. | 13, 63 |
| T. xerophilum Long | 9, 10, 11, 24, 38, 46, 47, 48, 65, 66, 72 |

Figure 3. Sporal ornamentation by SEM. a, Battarrea phalloides (UES 4861); b, Battarreoides diguetii (UES 10479); c, Chlamydopus meyenianus (UES 4400); d, Phellorinia herculeana (UES 10063); e, Schizostoma laceratum (UES 2815); f, Tulostoma albicans (UES 10067); g, T. cretaceum (UES 10314); h, T. fimbriatum (UES 10093); i, T. gracilipes (UES 9100); j, T. leiosporum (UES 8241); k, T. longii (UES 10116); l, T. macrosporum (UES 10112). Scale bar = 1 μm.
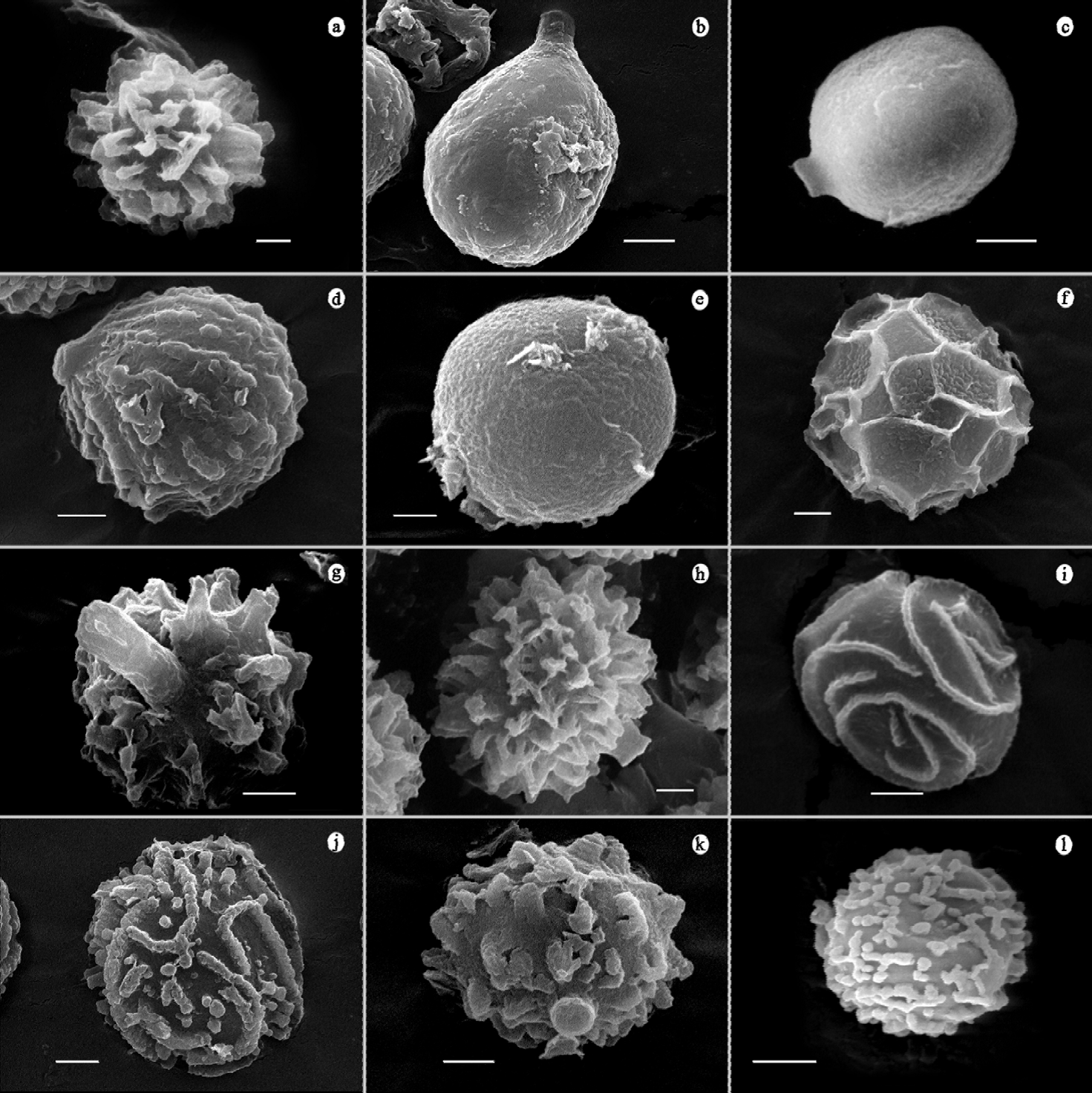
Figure 4. Sporal ornamentation by SEM. a, T. melanocyclum (UES 1688); b, T. membranaceum (UES 10055); c, T. mohavei (UES 5209); d, T. nanum (UES 10048); e, T. obesum (UES 8321); f, T. portoricense (UES 1251); g, T. rufescens (UES 10528); h, T. squamosum (UES 10092); i, T. striatum (UES 5490); j, T. subfuscum (UES 10080); k, T. wrightii (UES 5608); l, T. fimbriatum (UES 906). Scale bar = 1 μm.
The collections were gathered in 75 localities corresponding to 23 municipalities, 16 vegetation types, and 8 soil types with altitudes ranging from 9 to 2,370 m asl. Also, these localities had different pluvial precipitation from 54 to 1,057 mm per year, with 190 mm being the most common. All regimes were associated with precipitation under 700 mm except for locality 75 (1,057 mm). Vegetation type of localities was microphyllous desert scrub (23%), thorn, subtropical, and sarcocaule scrub (19%), mezquital (16%), sandy desert vegetation (11%), oak forest (7%), tropical thorn forest (7%), natural and induced grassland (2%), pine-oak forest (1%), tropical deciduous forest (1%), halophytic vegetation (1%), and perturbed areas (12%; urban zone, agricultural lands, and cultivated grassland) (Fig. 5). Regions varied from very dry and warm to subhumid temperate. Most collections came from very dry, semi-warm climate. In particular, 65% came from localities considered arid, 27% semiarid, and only 8% from temperate environments.
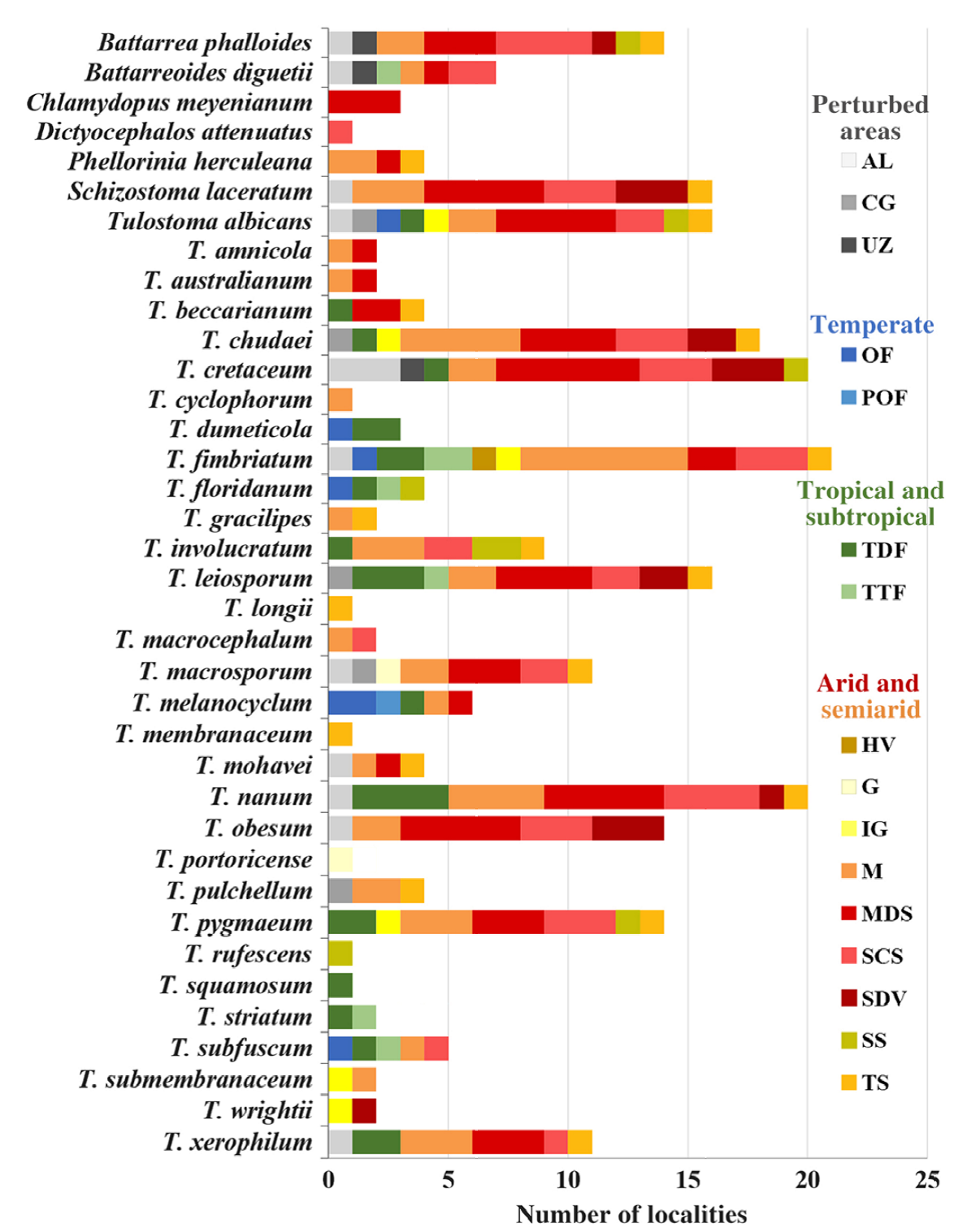
Figure 5. Distribution of tulostomataceous fungi by vegetation type in Sonora. (AL) Agricultural lands; (CG) cultivated grassland; (UZ) urban zone; (OF) oak forest; (POF) pine oak forest; (TDF) tropical deciduous forest; (TTF) tropical thorn forest; (HV) halophytic vegetation; (G) grassland; (IG) induced grasslands; (M) mezquital; (MDS) microphyllous desert scrub; (SCS) sarcocaule scrub; (SDV) sandy desert vegetation; (SS) subtropical scrub; (TS) thorn scrub.
All TSPs from Sonora are terricolous. Regosol was the most represented soil type with 43% of the localities; 34 out of the 37 identified species were collected in this soil type, with the exception of D. attenuatus, T. macrocephalum, and T. portoricense, which are extremely rare species with only one record each. Yermosol was representative of 17% of the localities and 54% of taxa were collected in this soil type. Tulostoma melanocyclum was gathered in several localities that all had regosol soil type. Meanwhile, vermosol, cambisol, and lithosol represented 17, 15, and 12% of sampled localities, respectively. Notably, T. cretaceum was observed in all soil types except lithosol and was the only species found in vertisol. Finally, solonchak and xerosol were present in 4 localities, and vertisol and luvisol were present in one locality.
The first 2 CCA axes had eigenvalues of 0.36 and 0.28, respectively, which accounted together for 60% of total variance in weighted averages of species. Axes were highly significant (p = 0.001, 999 permutations in the permANOVA test), which suggests a strong relation between TSPs and environmental conditions on both axes. The first axis had the highest correlation coefficients with altitude (0.86) and precipitation (0.85), and the second axis with type of climate (0.53). The ordination diagram of CCA shows the main pattern of variation in community composition, which is strongly correlated and best explained by the environmental variables considered, and shows the approximate centers of TSPs and localities distributions along each environmental variable included
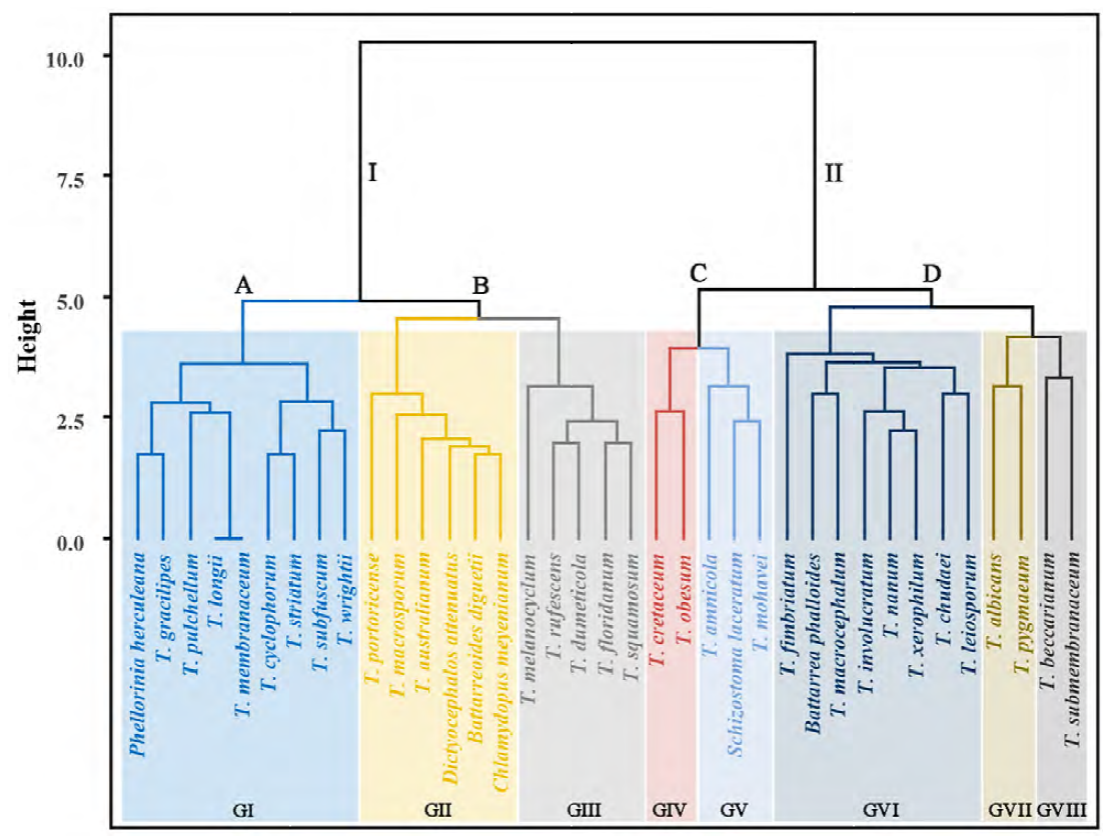
The resulting hierarchy tree with TSPs associated with environmental conditions showed 2 clades each with 2 subclades (ScA-ScD), and each subclade with 1, 2, 2, and 3 groups, respectively (GI-GVIII) (Fig. 7). ScA is composed of species with strictly fibrillose to fimbriate stoma and membranous exoperidia. ScA-GI is composed of species with asperulate to verrucose spores except T. membranaceum with smooth spores. ScA-GI also includes rarely collected species with subreticulate, striate, substriated and verrucose spores. ScB-GII is composed of species with asperulate and verrucose spores except T. portoricense with reticulate spores. ScB-GIII included species strictly with tubular stoma and echinulate spores. ScC included species with smooth spores and indefinite stoma. ScD included species with asperulate to verrucose spores, mainly tubular some fimbriate stoma, and membranous or hyphal exoperidium.
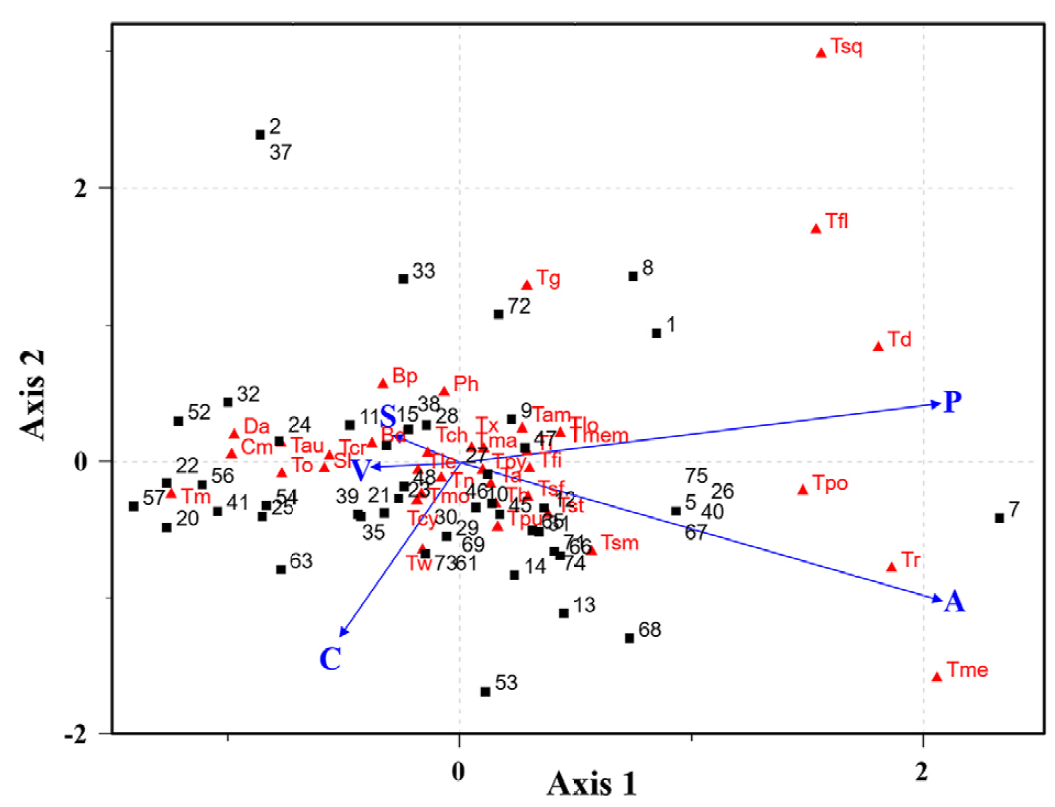
Figure 6. Canonical correspondence analysis (CCA) integrating 37 TSPs (triangles), 75 localities (squares), and environmental conditions (arrows). P, Precipitation; A, altitude; C, climate type; V, vegetation type; S, soil type.
Discussion
Within the tribe Battarreae, Battarrea phalloides and Battarreoides diguetii are highly morphologically related. Both species present verrucose spores and elaters in the gleba but are easily distinguished by the pores present in the peridium of B. diguetii, while in B. phalloides, the dehiscence is characterized by irregular cracks. The exoperidium can be absent in both taxa when mature and intemperized, being difficult to discern. However, B. diguetii usually has slender basidiomes with a dry delicate thin volva, whereas the sporocarps of B. phalloides can reach up to 65 cm in height and present a conspicuous thick volva that can be gelatinous when fresh (Esqueda et al., 2002). B. phalloides has been observed in urban areas of Hermosillo (Esqueda et al., 1995) and recently in both the downtown and suburban area of same municipality. It has also been cited in the urban areas of several other federal entities in Mexico. Even though B. phalloides is a cosmopolitan species that has been reported on all continents except Antarctica, it is considered rare and endangered (Rimóczi et al., 2011).
In Mexico, Battarrea has been collected between sea level and 2,550 m asl (Esqueda et al., 2002), yet this genus seems to be restricted to arid zones worldwide. However, Battarreoides has a more limited distribution. It was first discovered in San Luis Potosí, Mexico, but has since been reported in a few other arid zones of Mexico and Africa (Coetzee & Eicker, 1994; Esqueda et al., 2000; Guzmán & Herrera, 1969). Jacobson et al. (1999) studied the autecology of Battarrea and remarked that it is commonly associated with riparian forests on silt floodplain terraces but discarded the existence of mycorrhizal associations with dominant woody species such as Faidherbia albida (Delile) A. Chev. or Tamarix usneoides E. Mey. Furthermore, there is much discussion about the number of species in Battarrea. Currently, more than 15 species have been described. Yet, most mycologists disagree as to whether B. stevenii and B. phalloides should be considered as 2 distinct species or as monotypic (B. phalloides). This has also been argued from a morphological and molecular perspective (Garrido-Benavent, 2015; Martin & Johannesson, 2000; Martin et al., 2013).
The members of Phellorinae share the presence of scales in the peridium that usually fall off, warty and yellowish spores, and persistent, fasciculate basidia at maturity. However, they differ in 3 main aspects: 1) the continuity of the stipe (continuous in Phellorinia and discontinuous in the others), 2) the gleba (cellular in Dictyocephalos and dusty in the rest), and 3) the mode of dehiscence (occurs in Chlamydopus through an apical orifice but is irregular in the others) (Gube, 2009; Long & Plunkett, 1940; Long & Stouffer, 1946). In Chlamydopus, only 2 species are currently accepted, C. meyenianus and C. clavatus, while Dictyocephalos is considered monotypic with D. attenuatus. In Phellorinia, the variable shape of the scales, among other characters, led to the naming of 21 taxa (15 spp., 2 subspecies, and 4 varieties), although this genus is currently considered monotypic (P. herculeana), as discussed by Sharma et al. (2015). Sonoran specimens present peridia with either overlapped or pyramidal scales. The 3 genera have been reported in various countries, but with considerably less frequency than Tulostoma.
The 3 genera of Tulostomatinae develop basidiomes with detachable stalks through a socket of spore-sacs that produce abundant capillitia. This is the most diverse group within the TSPs. The main differences between these genera are found in the dehiscence as well as in several microscopic features, such as the type of capillitium and spore ornamentation.
Schizostoma species are characterized by substelliform dehiscence and dark, pulverulent gleba composed of short, dark, and disarticulated capillitial threads at the unswollen septa (Schizostoma-type) concolored with the smooth spores as well as a conspicuous volvoid structure that resembles a rhizomorph. There are 2 commonly accepted species worldwide: S. laceratum and S. mundkurii (S. Ahmad) Long & Stouffer. The latter has also been classified within Queletia. S. laceratum grows in Sonora, and is morphologically close to some species of Tulostoma that inhabit the most arid zones such as T. cretaceum or T. obesum, which share Schizostoma-type characters; mouth trends to indefinite, dark gleba with short pigmented capillitia and smooth, concolor spores. These Tulostoma species are difficult to delimit and can usually coexist.
Tulostoma obesum differs in its initially tubular mouth with a thinly membranous exoperidium. Meanwhile, T. cretaceum presents a fibrillose mouth and hyphal exoperidium, although it is commonly found weathered with an indefinite mouth, and its exoperidium may be lost or indistinct. T. cretaceum also presents a conspicuous rhizomorph that can be lost if carelessly collected. This rhizomorph differs from that of T. pygmaeum or T. rufescens, which are conformed by thick, loose hyphal filaments strongly intermixed with debris. T. cretaceum and T. albocretaceum Long & S. Ahmad (the latter is not recorded in Sonora) have rhizomorphs that more closely resemble a main root with attached sand and less organic matter. These 3 species also differ in their mouth, which is fibrillose in T. cretaceum and circular with an entire lip in T. albocretaceum and T. obesum, even though it tends toward indefinite in the 3 species. During its immature stage, S. laceratum can be confused with any of the aforementioned taxa but, in its mature stage, the dehiscence is substelliform with larger spores (Esqueda et al., 2004; Hernández-Navarro et al., 2018; Wright, 1987).
Most Tulostoma species have ferruginous to dark brown gleba composed of hyaline to slightly colored capillitial threads that are sometimes swollen at the septa (Tulostoma-type) and a regular apical ostiole that can be circular or oval (either planar or tubular), fibrillous (or denticulated, with or without a margin), or indefinite. Some species can form definite ostioles, although these may become indefinite after weathering; in other species, spores are released from a simple thorn-like aperture. Other smooth-spored Tulostoma species can be easily separated from the aforementioned species by their light yellowish spores and hyaline to subhyaline underarticulated capillitium with or without swollen septa. Two examples are T. amnicola and T. mohavei, which are close and not as common as the taxa with dark spores in the most arid zones. Both present a hyphal exoperidium and circular mouth, although the basidiomes of the first are usually slender (< 10 mm vs. > 10 mm) and have smaller spores (3.6-4.1 vs. 3.9-5.5 µm). Because of their similarities, a more in-depth analysis of these species must be performed. T. leiosporum also has delicate basidiomes and apparently smooth spores when viewed under light microscopy (LM) but differs due to its fibrillose mouth and asperulate spores, which are only observable under scanning electron microscopy (SEM). T. membranaceum has a distinctly membranous exoperidium and fibrillose mouth with slightly colored capillitium with round septa and dark, irregular spores. This latter species is an ill-defined taxon only known in India and doubtfully in the USA (Wright, 1987), so the Mexican material could be a new record for the American continent or even a new species (Esqueda et al., 2004; Hernández-Navarro et al., 2015).
Asperulate to slightly verrucose or spiny sporal species seem to be dominant in semiarid regions, and the boundaries among these taxa are thin. Of these, T. albicans and T. xerophilum are 2 common and similar species. Both present a typical membranous exoperidium, white endoperidium, tubular mouth, and yellowish spores that vary from subsmooth, asperulate to verrucose. Their main difference is in size; T. xerophilum has slender basidiocarps, the exoperidium falls-off in large scales and spores are smaller. Meanwhile, in T. albicans, basidiomes and spores are usually larger, and the exoperidium falls off in small patches, with some remaining in endoperidium as scales. In our experience, T. albicans presents capillitia with slightly swollen and pigmented septa, whereas T. xerophilum presents rounded hyaline septa. Some doubtful specimens of T. albicans do not share this trait, and in combination with the spore size and ornamentation variability, could evidence intraspecific morphological variation or a complex cryptic species. Likewise, T. longii has circular stoma, asperulate spores, and membranous exoperidium, but spores < 3.5 µm diam., one of the smallest for the genus. Rarely, the stoma loses its lip, appearing fibrillose. T. involucratum also has a membranous exoperidium and circular mouth, but the spores usually have a coarser ornamentation with conic verrucae. In addition, its stipe is usually longer. The character that gives this latter species its name is the involute nature of its membranous exoperidium that rolls although some collections lack this character because of weathering. Likewise, this species can be found at higher elevations in less arid regions and is considerably less abundant than the previous 2 species (Esqueda et al., 2004; Hernández-Navarro et al., 2015; Wright, 1987).
Tulostoma submembranaceum presents small basidiocarps but differs from the aforementioned species because it presents slightly verrucose spores and a thinly membranous exoperidium, although it has a fibrillose mouth. It can also be confused with T. fimbriatum, which also presents a fibrillose mouth and verrucose spores but differs in its hyphal exoperidium. Although T. fimbriatum is considered a cosmopolitan species, it was recently proven to be polyphyletic (Jeppson et al., 2017). Thus, the molecular characterization of this common species and other common species of Tulostoma is likely to result in several new records and species in Sonora.
Four similar species that share a membranous exoperidium, fibrillose to denticulate stoma, and verrucose spores are T. pulchellum, T. gracilipes, T. subfuscum, and T. wrightii. T. subfuscum has been considered a valid species, a variety of T. pulchellum, and recently synonymous with T. punctatum by Jeppson et al. (2017). According to Wright (1987), T. subfuscum presents spores with fused verrucae that are discernible under both LM and SEM, whereas T. pulchellum presents independent verrucae as observed in Sonoran collections. Some specimens of T. subfuscum could be mistaken with T. striatum. In the latter, the ornamentation is ribbed, whereas the former has fused verrucae. The last 3 species have denticulate stoma with delimited peristome. T. gracilipes has been cited 3 times worldwide and somewhat matches the description of T. pulchellum; however, it has smaller basidiomes, delicate stalk, fibrillose stoma without delimited peristome, rounded unswollen capillitia septa, and slightly ovoid spores. Since the holotype of this species is from South Africa, we cannot discard the possibility that the Sonoran material is a different species or even a small form of T. pulchellum. On the other hand, T. wrightii presents non delimited denticulate stoma, submembranous exoperidium, and conic wart spores.
Tulostoma nanum s.l. is characterized by its small basidiomes, asperulate spores, tubular mouth, and hyphal exoperidium. Wright (1987) mentioned that the spore ornamentation of the holotype is similar to T. giovanellae Bres. (not known in Sonora) and variable in size (up to 8 µm in diameter). Some Sonoran collections show this character, although others have asperulate spores without a pattern of verrucae, presenting instead an irregular pattern. Therefore, given the heterogeneity of these Sonoran collections, T. nanum s.l. is likely to be a species complex. Two other similar common species with circular mouth and hyphal exoperidium from the semiarid zones are T. chudaei and T. pygmaeum. T. pygmaeum and T. nanum both present small spore-sacs, but T. pygmaeum clearly differs from the rest because its spores have a coarser ornamentation consisting in conic and blunt irregular verrucae that are easily differentiable under LM. In addition, the Sonoran specimens present a conspicuous rhizomorph strongly intermixed with sand and debris, which T. nanum lacks. T. chudaei is easily distinguished by its acorn-like hyphal exoperidium, conformed of sand particles, and asperulate spores, although its ornamentation seems to vary from asperulate to conic verrucose between collections. Another characteristic that identifies this species is its easily detachable stem; in fact, of 95 specimens, only 25 presented complete stipes, which were sometimes detached. Other species with asperulate spores but stouter basidiomes are T. macrocephalum and T. australianum. Yet, these differ in their exoperidium (hyphal vs. membranous), mouth (circular vs. fibrillose), and spore ornamentation (appressed verrucae vs. truly asperulate) (Esqueda et al., 2004; Hernández-Navarro et al., 2017; Wright, 1987). A conflictive taxon is T. beccarianum, which is characterized by small to medium spore-sacs and a circular mouth, a hyphal to indistinct exoperidium, and verrucose spores. It was synonymized with T. simulans, but the latter is considered as a separate species based on molecular data (Altés & Moreno, 1993; Jeppson et al., 2017).
Tulostoma macrosporum is easily recognized by the largest spore size in Sonoran Tulostoma species, reaching up to 14 μm in diameter, although ornamentation can vary from spiny to apparently subreticulate in LM. This species can be found in semiarid areas; meanwhile, other species with coarser ornamentation are more likely to be found in subtropical to temperate areas. Notably, T. portoricense was the only species from Sonora with truly reticulated spores. It is found in grasslands in semidry, temperate climates. Meanwhile, T. squamosum was collected in 2 localities in tropical thorn forest and tropical deciduous forest, and T. floridanum, T. melanocyclum, and T. rufescens in subtropical or temperate localities. Several macroscopic traits easily separate T. squamosum, including its scaly, reddish brown stipe, and verrucose exoperidium composed of dark, colored, thick-walled cells as mycosclereids. T. cyclophorum and T. dumeticola share the latter characteristic, but the first species has a fibrillose mouth, uncolored endoperidium, and verrucose, subreticulated spores, whereas T. dumeticola presents a chocolate brown exoperidium, slender sporocarps, and spiny, subreticulated spores. Meanwhile, T. floridanum presents a grayish exoperidium with a reddish stipe and echinulated spores.
Tulostoma melanocyclum has a characteristically obscure peristome but an uncolored endoperidium. In Sonora, only T. dumeticola and T. rufescens present a colored endoperidium that is chocolate and pinkish, respectively, while the others have a whitish, grayish, straw to light yellow color. Most collections of these coarser spore ornamented species show a common trait: the glebal portions tend to be cottony and rich in capillitial threads, which are difficult to disarticulate, with smaller numbers of spores. Species from the most arid zones present a dusty gleba, and the capillitial threads are less abundant and totally disarticulated. In contrast, species from semiarid zones present a powdery but spongy gleba with numerous spores and fewer unarticulated capillitium threads. These characteristics could be correlated with the environments in which these fungi are distributed and with the mechanisms of spore dispersion, which are discussed below.
Accordingly, the morphological characteristics of TSPs are very variable, especially in Tulostoma. Based on the combination of characteristics (CC) concept, Wright (1987) hierarchized morphological traits in Tulostoma as primary (mouth, type of exoperidium, color of the endoperidium, spore size, and ornamentation) and secondary (socket, stipe, capillitium, septa, and lumen). Under this concept, specimens that do not match in any of their primary characteristics are considered separate taxa. Some other characteristics such as size of spore-sac were pointed to as critical, with a slender or robust sac as < 10 or > 10 mm in diameter, respectively. However, some Sonoran collections did show high variability in these characters. For this reason, some “intermediate” specimens, which may likely form part of species complexes, remain unidentified. Despite the great collection efforts made over 29 years, there is still vast territory in Sonora to be explored for new collections.
Certain CC seem to be correlated with the environment and spore dispersion (Gube & Dörfelt, 2012). Wright (1987) described the habitat of Tulostoma species according to vegetation and soil type. These species can be classified as psammophilous (present in sandy soils in arid regions), terricolous (clay-loving species present in pastures or at roadsides), or “forest-soil-loving species” (present in tropical or temperate zones with a high content of organic matter). Wright (1987) also mentioned that Tulostoma species are very sensitive to disturbances or modifications to their habitats, such as the loss of plant species, which can result in the absence of basidiomes for 5 to 7 years. However, several Tulostoma species were also observed in urban and agricultural zones in Sonora (Fig. 5). Contrary to Wright (1987), the first axis of the CCA had the lowest correlation coefficients with soil (-0.12) and vegetation type (-0.16), while the second axis had the lowest correlation coefficients with vegetation (-0.01) and soil type (0.08) (Fig. 6).
In very dry areas (< 300 mm annual precipitation), spore dispersion is more likely to be air-mediated. In the case of TSPs, almost all genera seem to be euanemochorous, meaning that they present an irregular dehiscence and that the sporocarp is well anchored into the ground. This mechanism is the most common in all genera except for Battarreoides and some species of Tulostoma with defined ostioles. An irregular dehiscence or indefinite mouth leaves the gasterothecia exposed, and the short, disarticulated capillitia are interspersed among a pulverulent mass of smooth spores with little friction. In our study, subclade C had the lowest number of species (5), all of them with smooth spores; ScC-GIV included T. cretaceum and T. obesum, which inhabit in the most arid sampled zones with dark yellowish spores and stoma tend to indefinite (Fig. 7). Dark, thick walls of many species may serve to protect from UV damage, as seen in other GSAs from the Sonoran Desert, such as Calvatia pygmaea (R.E. Fr.) Kreisel, G. Moreno, C. Ochoa & Altés, Agaricus deserticola G. Moreno, Esqueda & Lizárraga, and Montagnea arenaria (DC.) Zeller.
In semiarid regions (≥ 300 mm annual precipitation), the boleohydrochorous species present defined mouths and an unarticulated capillitium. This may be one way to protect the gasterothecia and regulate sporal release through water droplets. The asperulate to verrucose ornamentation adds friction to the sporal mass, so a major force is needed to release the spores. Subclade D included the highest number of taxa (12) and the most common collected species such as T. albicans, T. fimbriatum, T. nanum, and T. xerophillum, which have asperulate to verrucose spores and tubular stoma except T. fimbriatum and T. submembranaceum (fibrillose) (Fig. 7). The required force is greater when capillitia are more abundant and spores are more ornamented. Coarser ornamentation types might be useful for specific dispersal modes, such as aerial or animal-mediated, as spiny spores are more likely to attach to surfaces. Or, reticulated spores might aid in aerial dispersion. Subclade B-GIII comprised species strictly with tubular stoma and echinulate spores (T. dumeticola, T. floridanum, T. melanocyclum, T. rufescens, and T. squamosum) (Fig. 7) which inhabit temperate, sub-tropical, and tropical sites (Fig. 5). These dispersal modes are a common feature of species that develop in highlands. However, many tropical and temperate areas in Sonora are located in mountain ranges where slopes are also crucial for spore establishment.
Another strategy is evidenced by the geanemochorous species with easily detachable stems (e.g., T. chudaei) (ScD-GVI; Fig. 7), which allows the spore-sac to tumble freely like some other GSAs common to the Sonoran Desert (e.g., Disciseda spp., C. pygmaea). The dried spore-sac safely guards the spores and randomly disperses them following interactions with animals or as a result of other factors, such as air and water. The presence of strongly mixed debris in the exoperidium might help the spore sac to gain weight and ensure that the mouth remains facing upwards as in Disciseda species (Calonge, 1998; Gube, 2009).
The most abundant species in thorny scrub (e.g., T. nanum, T. xerophilum; Fig. 5) were commonly found in uncovered areas in poor soils, while other less abundant species were found under the canopy in soils with higher organic matter content. This suggests that the abundant species are well adapted to semiarid environments, which are considered the origin of GSAs (Gube & Dörfelt, 2012). The differences in the abundance and spore dispersal of the studied taxa might also be explained by a combination of environmental conditions and other ecological interactions, such as competition with other decomposers. However, in this regard, the sampling effort is also relevant. For example, locality 47 with thorny scrub was intensely sampled by Esqueda et al. (2000) and Hernández-Navarro et al. (2015), who reported 19 and ~ 50 TSPs and GSAs, respectively, compared to the number of species (10 to 12 GSA species) reported in other nearby localities.
Finally, further molecular analysis of potential new species and basidiomes with intermediate morphology from tulostomataceous stalked-puffballs must be performed in order to determine the real number of species and to understand the boundaries among closely related genera and species.
Key to Sonoran tulostomataceous stalked puffballs.
1a. Gleba with elaters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
1b. Gleba without elaters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
2a. Peridium dehiscence circumscissile, basidiomes stout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Battarrea phalloides
2b. Peridium dehiscence by multiple definite pores, basidiomes slender . . . . . . . . . . . . . . . . . . . . .Battarreoides diguetii
3a. Spore-sac not detachable from stalk, peridium covered with pyramidal or overlapped scales.. . . . . . . . . . . . . . . . . 4
3b. Spore-sac detachable from stalk through a socket, peridium without scales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
4a. Peridium continuous with stalk, spores verrucose of 4.5-6 μm diam . . . . . . . . . . . . . . . . . . . .Phellorinia herculeana
4b. Peridium not continuous with stalk . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
5a. Dehiscence by an apical fibrillose stoma, gleba pulverulent . . . . . . . . . . . . . . . . . . . . . . . .Chlamydopus meyenianus
5b. Dehiscence by irregular cracks in the peridium, gleba cellular . . . . . . . . . . . . . . . . . . . . . Dictyocephalos attenuatus
6a. Gleba pulverulent, capillitium disarticulated with some ribbon-like threads . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
6b. Gleba pulverulent to cottony, capillitium non-disarticulated without ribbon-like threads . . . . . . . . . . . . . . . . . . . . . 9
7a. Dehiscence by irregular cracks to substelliform at maturity . . . . . . . . . . . . . . . . . . . . . . . . . . .Schizostoma laceratum
7b. Dehiscence by an apical stoma which becomes indefinite with intemperization . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
8a. Stoma circular, exoperidium thinly membranous, without rhizomorph . . . . . . . . . . . . . . . . . . . . . Tulostoma obesum
8b. Stoma fibrillose, exoperidium hyphal, stalk with a distinct rhizomorph . . . . . . . . . . . . . . . . . . . . . . . . .T. cretaceum
9a. Spores perfectly smooth under LM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
9b. Spores ornamented . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
10a. Stoma circular to tubular . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
10b. Stoma fibrillose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
11a. Exoperidium hyphal like an acorn-cup, spore-sac < 10 mm diam., spores 3.6-4.1 μm diam., yellowish . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. amnicola
11b. Exoperidium hyphal non-acorn-cup like, spore-sac > 10 mm diam., spores 3.9-5.5 μm diam., dark yellowish . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. mohavei
12a. Exoperidium membranous, stoma fibrillose, spores smooth under SEM . . . . . . . . . . . . . . . . . . . T. membranaceum
12b. Exoperidium hyphal, stoma fimbriate, spores rugose under SEM . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. leiosporum
13a. Spores reticulate or subreticulate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
13b. Spores asperulate, verrucose, echinulate, or striate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
14a. Exoperidium hyphal, endoperidium without mycosclereids, spores 6.0-8.0 μm diam., strongly reticulate with long spines with a membrane as wing-like structure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. portoricense
14b. Exoperidium membranous, endoperidium with mycosclereids, spores 3.5-4.5 μm diam., verrucose-subreticulate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. cyclophorum
15a. Spores striate or nearly, without spines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
15b. Spores asperulate, verrucose or echinulate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
16a. Spores ornamented by continue ribs sometimes forming spirals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. striatum
16b. Spores ornamented by fused verrucae simulating ribs and spirals . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. subfuscum
17a. Stoma circular or elliptic, planar or tubular . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18
17b. Stoma fibrillose or denticulate, with or without a margin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
18a. Exoperidium hyphal or indistinct . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
18b. Exoperidium membranous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
19a. Basidiome stout, spore-sac < 22 mm diam., spores 4.3-6 μm diam., asperulate at LM and verrucae uneven, appressed some anastomosed under SEM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. macrocephalum
19b. Basidiome slender . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
20a. Spores 6-14 μm diam., asperulate to echinulate some subreticulate at LM . . . . . . . . . . . . . . . . . . . T. macrosporum
20b. Spores size average < 6 μm diam . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
21a. Spore-sac usually > 10 mm diam. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22
21b. Spore-sac usually < 10 mm diam. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
22a. Exoperidium hyphal like acorn-cup; stalk easily detached from spore-sac, spores asperulate to minutely spiny . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. chudaei
22b. Exoperidium indistinct not acorn-cup like, stipe not easily detached, spores with either blunt, digitiform or conic irregular verrucae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. beccarianum
23a. Spores 4.0-5.5 (-7.0) μm diam., asperulate at LM and irregular verrucae arranged in a polar disposition under SEM, stalk without rhizomorph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. nanum
23b. Spores 4.5-5.5 μm diam., irregular echinulate, some spiny and some blunt verrucae, stalk with distinct rhizomorph . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. pygmaeum
24a. Stoma circular, elliptical, planar or tubular . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
24b. Stoma fibrillose, fimbriate, with or without a margin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
25a. Spores subsmooth, asperulate or verrucose with blunt verrucae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
25b. Spores with a coarser ornamentation, distinctly spiny to echinulate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
26a. Exoperidium membranous, papyraceous, falling-off leaving a clean endoperidium . . . . . . . . . . . . . . . . . . . . . . . 27
26b. Exoperidium membranous, falling-off in patches, remaining in endoperidium like scales . . . . . . . . . . . . . . . . ....28
27a. Spore-sac usually > 10 mm diam., exoperidium rolling-in the base of endoperidium, stalk > 4 cm long, spores 4.0-5.0 (-6) μm diam. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. involucratum
27b. Spore-sac usually < 10 mm diam., exoperidium falling-off remaining in the lower third of white endoperidium, stalk < 4 cm long, spores 3.5-4.7 μm diam. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. xerophilum
28a. Spore-sac usually > 10 mm diam., spores 4.5-6.5 μm diam. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. albicans
28b. Spore-sac usually < 10 mm diam., spores 2.8-3.5 μm diam. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. longii
29a. Peridium with hyphal fascicles resembling mycrosclereids of thick wall > 2 μm . . . . . . . . . . . . . . . . . . . . . . . . . 30
29b. Peridium without mycrosclereids-like structures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
30a. Exoperidium with protrusions resembling verrucae, spores echinulate to subreticulate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. dumeticola
30b. Exoperidium verrucose, spores echinulate with spines not coalescing . . . . . . . . . . . . . . . . . . . . . . . . .T. squamosum
31a. Exoperidium hyphal, peristome dark, spores 5.0-8.0 μm diam . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. melanocyclum
31b. Peristome not dark . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
32a. Exoperidium hyphal, endoperidium grayish, spores with free spines . . . . . . . . . . . . . . . . . . . . . . . . . . T. floridanum
32b. Exoperidium thinly membranous, endoperidium pinkish, spores with some spines fusing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. rufescens
33a. Exoperidium hyphal or indistinct, spores verrucose some irregularly fusing . . . . . . . . . . . . . . . . . . . . T. fimbriatum
33b. Exoperidium membranous or submembranous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
34a. Exoperidium typically membranous, dark outside and white inside crust, falling-off in big scales or small patches, even with hydrated exoperidium . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35
34b. Exoperidium submembranous, not entirely solid-white membrane, which might lose integrity when hydrated . 37
35a. Spore-sac up to 26 mm diam., stoma fibrillose to simple, spores asperulate . . . . . . . . . . . . . . . . . . .T. australianum
35b. Spore-sac < 15 mm diam., exoperidium falling-off leaving a clean endoperidium . . . . . . . . . . . . . . . . . . . . . . . . 36
36a. Spore-sac < 9 mm diam., stoma fibrillose, spores 4.1-4.9 μm diam . . . . . . . . . . . . . . . . . . . . . . . . . . . T. gracilipes
36b. Spore-sac > 10 mm diam., stoma denticulate, spores 4.5-6 (-8) μm diam . . . . . . . . . . . . . . . . . . . . . . T. pulchellum
37a. Stoma fibrillose, spores 4.0-6.0 μm diam. minutely verrucose . . . . . . . . . . . . . . . . . . . . . . . . T. submembranaceum
37b. Stoma denticulate, spores 3.5-5 μm diam. with conic verrucae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. wrightii











 nueva página del texto (beta)
nueva página del texto (beta)