Introduction
The insect order Hymenoptera includes 3 large groups of parasitic wasps: Chalcidoidea and Braconidae, which are more speciose in the tropics (Horstmann et al., 1999; Noyes, 1989; Wharton et al., 1997) and Ichneumonidae, which are mostly found in temperate zones (Jones et al., 2012; Owen & Owen, 1974; Skillen et al., 2000). Although some authors consider ichneumonids to be an exception to the general trend that on a large-scale species richness increases at lower latitudes (Owen & Owen, 1974; Veijalainen et al., 2012), others have questioned this hypothesis because of description and sampling biases, since small koinobiont species might be overlooked or undersampled in the tropics (Quicke, 2012; Santos & Quicke, 2011; Veijalainen et al., 2013). The abundance and distribution of ichneumonids, similar to all organisms, is determined by climate and availability of resources. However, biodiversity studies are not always related to the sources of variation that influence on distribution patterns of species, such as vegetation types, seasonal climatic changes, and biogeographic distribution (Brown & Maurer, 1989; Clark et al., 2011; Menezes et al., 2015; Poulin et al., 2011; Sääksjärvi et al., 2006; Wolda, 1988).
With a global estimate of 100,000 species (Gauld, 2000), of which more than 24,281 have been described (Yu et al., 2015), the Ichneumonidae is one of the most species-rich animal families in the world (Goulet & Huber, 1993; Triplehorn & Johnson, 2005; Veijalainen et al., 2012). Nearly 7,400 ichneumonid species have been defined as Neotropical, and approximately 7,700 species have been described as Nearctic. In Mexico, where the Nearctic and Neotropical regions converge, approximately 1,300 ichneumonid species have been recorded (Ruíz-Cancino, 2015), of which 59%, 29%, and 10% have Neotropical, both Neotropical and Nearctic and Nearctic distributions, respectively, whereas the remaining species show other trends (Ruíz-Cancino et al., 2014). Extensive faunistic studies of Ichneumonidae have been carried out in Tamaulipas, Veracruz, and Yucatan, which are coastal states on the Gulf of Mexico (Ruíz-Cancino, 2015). However, the fauna of Ichneumonidae have been poorly studied in coastal habitats such as mangroves, which are interesting ecosystems for studying parasitic wasps (Myartseva et al., 2014).
In this study, the community structure of Ichneumonidae in a mangrove area in the southern region of Tamaulipas, Mexico, is described and represents the first study of this family in such habitat. Also, 2 nearby localities with different vegetation types were examined. The study area is characterized as Neotropical, but it is actually on the boundary of the Nearctic region in the northeastern region of the country. Therefore, in this study we evaluated the diversity and species composition of ichneumonid species depending on their biogeographical distribution, type of vegetation, and month of collection.
Materials and methods
Three localities were sampled, which belong to the municipality of Altamira in the state of Tamaulipas, Mexico, at an elevation of 2-15 m asl. These localities correspond to a mangrove area in the Municipality Armenta (22°25’48.30” N, 97°52’34.86” W), a residual low deciduous forest (LDF) in the Congregación Las Prietas (22°31’46.53” N, 97°56’52.86” W), and a low thorny forest (LTF) in the Municipality Aquiles Serdan (22°33’2.87” N, 97°54’13.11” W). The LDF is lush green with dense foliage and a thick herbaceous layer during the rainy season and after the arrival of summer season most trees shed their leaves. The dominant plant genera are Bursera, Phoebe, Chloroleucon, and Acacia (Rzedowski, 2006). The LTF is present in drier sites than those where LDF occurs, with predominance of either evergreen or deciduous thorny trees. The dominant plant genera are Prosopis, Esenbeckia, Pithecellobium, and Acacia (Rzedowski, 2006). The distance among sites is 12-14 km. The weather in the region is predominantly warm and humid with rain in summer. The monthly mean temperature varies from 16.4 °C in January to 25.2 °C in May. The monthly average rainfall varies from 1.8 mm in February to 206.5 mm in September (Table 1) (Conagua, 2012, 2013).
Table 1 Monthly temperature and rainfall.
| Months | Average monthly temperature (°C) |
Monthly rainfall (mm) |
| May | 25.2 | 14.8 |
| June | 24 | 30.7 |
| July | 24.9 | 115.6 |
| August | 24.5 | 81 |
| September | 24.1 | 206.5 |
| October | 21.1 | 23.6 |
| November | 18.7 | 45.3 |
| December | 18.6 | 2 |
| January | 16.4 | 11.5 |
| February | 19.1 | 1.8 |
| March | 17.1 | 30.3 |
| April | 21.2 | 3 |
Wasps were captured from May 2012 to April 2013 with Malaise traps that were fabricated based on the design published by Townes (1972). The height of the alcohol pot was approximately 2 m. The Malaise trap color was black, and measured 1.8 m wide by 1.1 m high with a depth of 60 cm for each side. The plastic pot was half full of 95% ethyl alcohol and the samples were collected every 15 days and conserved in refrigeration until examination. One trap was placed in each site. All traps were placed in open areas, oriented with the collector pot toward the east.
Taxonomic determination was carried out using the relevant keys (Brambila, 1997; Dasch, 1979, 1984, 1988, 1992; Gauld, 1988, 1991, 1997, 2000; Gauld et al., 2002; Heinrich, 1977; Kasparyan & Ruíz-Cancino, 2005, 2007, 2008a, 2008b; Khalaim & Ruíz-Cancino, 2012; Reshchikov; 2011; Townes, 1969, 1970a, 1970b, 1971; Townes & Townes, 1978). The specimens that did not match with the morphological features of a described species were regarded as morphospecies. All insects were deposited in the collection of Instituto Tecnológico de Ciudad Victoria, Tamaulipas, Mexico.
Data analysis
Species accumulation curves were generated for each site using the Clench model, which is an adaptation of the Michaelis-Menten equation that is used to describe the kinetics of enzymatic reactions (Jiménez-Valverde & Hortal, 2003). Because the sites are small, all species have the same probability of being caught, and thus we used the negative exponential equation Clench model (Soberón & Llorente, 1993). The accumulation of species as a function of effort was obtained by the Mao Tau estimator, and the adjustment of the Clench equation was performed with the Quasi-Newton simplex algorithm (StatSoft Statistica 8.0). Alpha diversity was determined at each site from the richness and abundance values using the Simpson index of species evenness and the Shannon-Wiener index of diversity (Magurran, 2004). A t-test was carried out to determine statistically significant differences in Simpson Index data. The Berger-Parker dominance index was used to estimate the proportions of the most abundant species (Moreno, 2001). Differences among sites in the collected species and their abundances were determined by a Permanova multifactorial analysis, which is employed to test the simultaneous response of 1 or more variables to 1 or more factors using permutation methods. This statistic test directly uses the distance matrix to partition the diversity among sources of variation, and is especially suitable for analysis of composition data from ecological studies (Tang et al., 2016). The distance index used was Sorensen-Dice because it assumes imperfect sampling. This analysis was performed with PAST software (version 3.0; Anderson, 2005).
The association of species on type of vegetation, month of collection, and their assigned biogeographical distribution was performed with a multiple correspondence analysis (Abdi & Valentine, 2007; Legendre & Legendre, 2003). The biogeographical distribution of each species was obtained from Yu et al. (2015), and those cataloged as Neotropical-endemic to Mexico (Nt-End) were based on Ruíz-Cancino (2010). The multiple correspondence analysis is a modification of X2 used to analyze multiple categorical variables and creates a Cartesian diagram based on the association among more than 2 categorical variables (Legendre & Legendre, 2003). The diagram displays simultaneously the relative position of the categories of variables studied (Gotelli, 2001). The positions of the categories reflect the degree of association among them, nearest categories of different variable represent a high association, while distant categories show lower association. This analysis was performed with the program Statistica version 12 (StatSoft, 2007).
Results
A total of 770 specimens of Ichneumonidae belonging to 19 subfamilies, 66 genera, and 129 species were collected at the 3 localities (Appendix). The overall capture rate for the study was 0.70 individuals per day-trap and the capture rate of species relative to specimens was 16.8 per 100. The highest relative abundance, with 49.6% of all wasps, was observed in the mangrove habitat; the relative abundances in the LTF and the LDF were 25.7% and 24.7%, respectively. The fewest number of specimens for the 3 sites was obtained in January (25 wasps), whereas March was the most abundant month (107 specimens). The months with more specimens were August, July, and March for the mangrove habitat, LDF, and LTF, respectively. Cryptinae was the most abundant subfamily, represented by 39.6% of the collected specimens (Table 2). Cryptinae was common in the 3 sites, whereas Rhyssinae was only collected in LDF. Specimens of Brachycyrtinae, Mesochorinae, and Metopiinae subfamilies were only collected in LTF. The most abundant species were Anomalon ejuncidum Say (72 specimens), Eudeleboea subflava Davis (42 specimens), and Pachysomoides stupidus Cresson (42 specimens) (Appendix).
Table 2 Abundance and species richness per subfamily in the 3 localities.
| Low deciduous forest | Mangrove | Low thorny forest | Total | |||||
| Abundance | Richness | Abundance | Richness | Abundance | Richness | Abundance | Richness | |
| Anomaloninae | 18 | 2 | 49 | 4 | 30 | 4 | 97 | 5 |
| Banchinae | 8 | 2 | 19 | 2 | 19 | 1 | 46 | 2 |
| Brachycyrtinae | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 |
| Campopleginae | 7 | 3 | 37 | 12 | 7 | 5 | 51 | 14 |
| Cremastinae | 8 | 6 | 20 | 8 | 15 | 6 | 43 | 16 |
| Cryptinae | 56 | 24 | 176 | 33 | 73 | 23 | 305 | 45 |
| Ctenopelmatinae | 2 | 1 | 2 | 1 | 1 | 1 | 5 | 1 |
| Ichneumoninae | 27 | 9 | 21 | 8 | 22 | 9 | 70 | 16 |
| Labeninae | 24 | 4 | 12 | 4 | 5 | 2 | 41 | 5 |
| Lycorininae | 1 | 1 | 2 | 1 | 0 | 0 | 3 | 1 |
| Mesochorinae | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Metopiinae | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| Nesomesochorinae | 7 | 1 | 10 | 1 | 8 | 1 | 25 | 1 |
| Ophioninae | 9 | 2 | 12 | 2 | 5 | 3 | 26 | 5 |
| Orthocentrinae | 4 | 2 | 3 | 2 | 9 | 2 | 16 | 2 |
| Pimplinae | 11 | 5 | 15 | 6 | 1 | 1 | 27 | 9 |
| Rhyssinae | 3 | 1 | 0 | 0 | 0 | 0 | 3 | 1 |
| Tersilochinae | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| Tryphoninae | 5 | 1 | 1 | 1 | 1 | 1 | 7 | 1 |
The mangrove area had the highest richness with 88 species of which 31 were exclusive for this site. Sixty-four species (17 exclusive) were collected in LDF, whereas 61 species were collected in LTF (18 were exclusive) (Appendix). The most species-rich subfamilies were Cryptinae (45 species), Cremastinae and Ichneumoninae (both with 16 species), and Campopleginae (14 species). The most species-rich genera were Eiphosoma with 8 species, and Lymeon and Carinodes, with 7 species each.
The maximum values of the Clench function curve for each site are presented in Table 3. The coefficient of determination was greater than 0.9 in all 3 cases, indicating the data were properly matched to the function. This analysis showed that the mangrove site, with 102 estimated species, could potentially be the richest in ichneumonid species of the 3 collected sites. The number of estimated species for the 2 other vegetation types was of 76 in both cases (Table 3).
Table 3 Data from Clench model at the 3 sites.
| Low deciduous forest | Mangrove | Low thorny forest | |
| Collected species | 64 | 88 | 61 |
| Percentage of the collected biota | 84.03 | 62.75 | 80.46 |
| Estimated species | 76.15 | 101.98 | 75.81 |
| Coefficient of determination (R) | 0.9996 | 0.99894 | 0.99895 |
Comparison of the Ichneumonidae communities at the 3 locations by Permanova showed statistically significant differences (F = 1.95, p = 0.0004) among the sites (Table 4). Thus, the 129 species collected in the study area have clearly different spatial distributions.
Table 4 P values from Permanova analysis.
| Sites | Low deciduous forest | Mangrove | Low thorny forest |
| Low deciduous forest | - | 0.0168 | 0.0054 |
| Mangrove | 0.0168 | - | 0.0408 |
| Low thorny forest | 0.0054 | 0.0408 | - |
These community differences at the 3 sites were not detected in terms of the proportional abundance of each species. That is, the Shannon-Wiener and Simpson indexes were similar for the 3 data sets (Table 5). The t-test carried out on the Simpson index data did not indicate a significant difference between the mangrove and LDF (t(434) = -0.924; p > 0.05) or between LDF and LTF sites (t(387) = 1.245; p > 0.05); the only statistically significant difference was detected by comparing the alpha diversity of the mangrove and LTF (t(445) = 2.277; p < 0.05). This indicates that the probability of taking 2 individuals of the same species was greater in LTF than in mangrove, and the uncertainty of knowing the identity of a species was greater in mangrove than in LTF. The Berger-Park index, which expresses the number of individuals in proportion to the most abundant species, did not differ between locations (Table 5).
Table 5 Abundance, shared species, exclusive species, and diversity indexes at each site.
| Sites | Abundance | Richness of species |
Shared species |
Exclusive species |
Shannon- Wiener |
Simpson | Berger-Parker |
| Low deciduous forest | 190 | 64 | 47 (73.4%) | 17 (26.6%) | 3.752 | 0.0355 | 0.11053 |
| Mangrove | 382 | 88 | 57 (64.8%) | 31 (35.2%) | 3.841 | 0.0372 | 0.1021 |
| Low thorny forest | 198 | 61 | 43 (70.5%) | 18 (29.5%) | 3.621 | 0.0401 | 0.096 |
The multiple correspondence analysis (Fig. 1) revealed that biogeographical regions, month of collection, and localities were statistically significant factors (Χ2 d.f.=24,964 = 149,015; p = 0.000) in the community structure of the Ichneumonidae species. The position of categories of biogeographical regions and identity of species was far from the center of graph, and therefore contributes with most of the observed variation. In contrast, the types of vegetation were closer to the center of graph, which means a lower variation. The month of collection had more influence than sites; for example, species cataloged to Nt-End and Nt-Ne regions were most frequent from February to June, and those species from Ne-Nt-Oc-Pa, Ne-Pa-Nt or Nt-Ne-Oc regions were mostly present on August or October. However, some species did not have correspondence with any month or type of vegetation but only with their recorded biogeographical distribution.
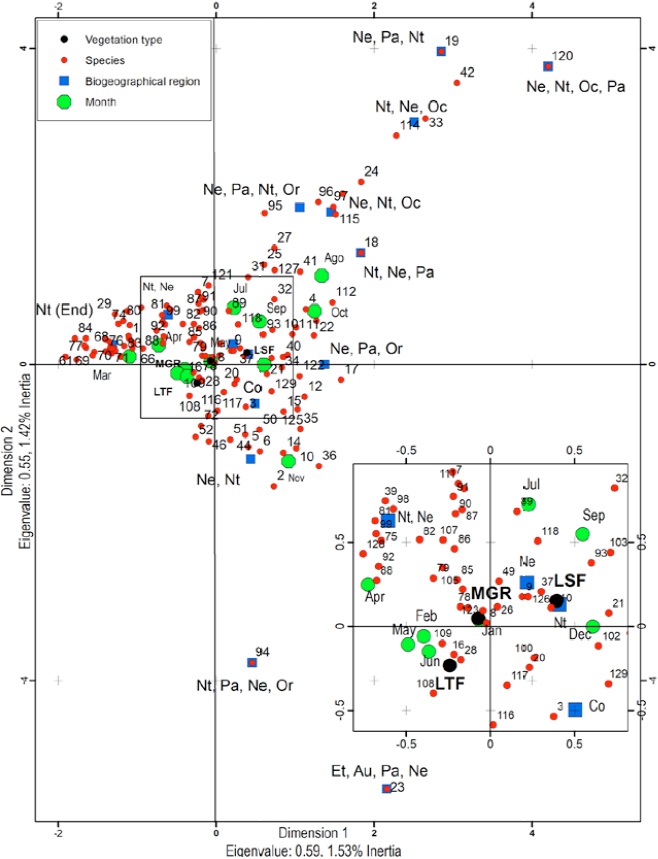
Figure 1 Multivariate correspondence analysis. Ne: Nearctic; Nt: Neotropical; Pa: Palearctic; Or: Oriental; Oc: Oceanic; Co: Cosmopolitan; Et: Ethiopian; Au: Australian; End: Endemic to Mexico; MGR: Mangrove; LDF: Low-Stature Deciduous Forest; LTF: Low-Stature Thorny Forest.
Three species collected in this study represent new records for Mexico: Pristomerus jugulatorGauld, 2000, Physotarsus emarginatus Zhaurova, 2009, and Apechoneura mariae Gauld, 2000. Five species, Brachycyrtus cosmetus (Walker, 1956); Pristomerus sulcatus (Cameron, 1908); Temelucha hilux Gauld, 2000; Chirotica confederatae (Ashmead, 1896); and Enicospilus kleini Gauld, 1988, are new records for Tamaulipas.
Discussion
This study showed that the community composition of the Ichneumonidae in a mangrove area may significantly differ from nearby areas with different types of vegetation, such as LDF and LTF. In addition, the community structure in this group tend to be determined mostly by the biogeographical distribution of species, followed by the temporal variation throughout the year and less by the type of vegetation. Using multivariate analysis techniques, this study provides a relevant contribution for the Ichneumoid diversity in mangrove areas (Burrows, 2003).
The community of ichneumonids in the mangrove was different in comparison with the other 2 sites. First, the species assemblages of the 3 types of vegetation were significantly different. Second, the abundance and richness were highest in the mangrove area. Third, the local diversity in mangrove was significant higher than LTF, although not different with LDF.
Malaise traps represent a frequently used collecting technique for sampling Ichneumonidae (Fraser et al., 2008; Hall et al., 2015; Pérez-Urbina et al., 2010; Sääksjärvi et al., 2006; Townes, 1972). The overall capture rate for the study was 0.70 individuals per day-trap. This value is in agreement with those reported in other studies, which vary from 2.1 wasps per day-trap (Pérez-Urbina et al., 2010) to low-rate collections of 0.22 individuals per day (Sääksjärvi et al., 2006). However, the capture rate of species relative to specimens was 16.8 per 100. The values found in other studies range from 7 to 17 species per 100 ichneumonids caught (Hall et al., 2015; Pérez-Urbina et al., 2010; Sääksjärvi et al., 2006; Townes, 1971). In comparison, a study with pan traps captured 0.22 species per 100 specimens from a total of 7,500 ichneumonid wasps collected (Gould et al., 2013). Thus, the values of abundance and richness in the present study using Malaise traps are among those obtained by other studies and allowed the determinations of significant statistical differences.
Mangroves occur in tropical and subtropical latitudes along coastal intertidal areas (Burrows, 2003). In Mexico, they are distributed inside coastal lagoons and deltaic systems (López & Ezcurra, 2002). Mangroves are forests of salt-tolerant woody plants, often comprising 1 species or a mixture of 3 species of these trees (Burrows, 2003). Although their main feature is general floristic simplicity, mangroves are ecosystems of crucial importance for the life cycles of aquatic fauna (Kathiresan & Bingham, 2001). The legal protection in Mexico is based on their ecological relevance (Moral-Padilla et al., 2012). Entomofaunistic studies of these ecosystems usually assess plant-insect relationships through herbivory (Burrows, 2003; Feller & Chamberlain, 2007; Feller & Mathis, 1997; Murphy & Lugo, 1986). However, there are few studies in other trophic guilds. The only faunistic study of parasitioid and predatory insects in mangroves was carried out by Veenakumari et al. (1997) on 2 Indian islands. These authors suggested that these kinds of forests cannot sustain a large population of insects, but they are influenced by their adjacent areas (Berjak et al., 1977; Kathiresan & Bingham, 2001; Veenakumari et al., 1997). Thus, the richness of Ichneumonidae collected in the mangrove area in this study could result from the mutual influences of the mangrove and its surroundings. Rao et al. (1998) had already mentioned that mangrove insect visitors could function as links between these aquatic ecosystems and neighboring areas.
In this study, the community structure of Ichneumonidae was strongly determined by the biogeographical distribution where species were reported. The relative abundances of species showed strongest correspondence with the biogeographical categories compared to collecting month and type of vegetation. Neotropical (Nt, Nt-Ne, Nt-End) species, which were the most abundant, were collected throughout all months of the year, although their frequency was lower in August, October, and November. The frequency of Nt-End and Nt-Ne species was associated with the months from February to June, when the temperature ranged from 19.1-24 °C and the rainfall from 1.8 mm to 30.7 mm. In comparison, the most-rainy months were July (311 mm) and September (314 mm), but their association with the relative abundance of the species was lower, since the associated species tend to be cosmopolites. With respect to types of vegetation, the correspondence was lower than the collecting month. These data indicate that the zone is markedly Neotropical with a good established community of ichneumonids, despite fluctuations of temperature and precipitation through the year.
In conclusion, the community structure of the ichneumonid wasps in the subtropical zones near to Nearctic region strongly depends on the biogeographical distribution of the species in association with climatic variation throughout the year. However, the mangrove and their surroundings appear to be favorable for this group of insects, which is a relevant issue given the limited information of parasitic Hymenoptera in this ecosystem.
Finally, with the new records for Mexico and Tamaulipas presented here, knowledge about the geographical distribution of some species has been extended. Based on this, 1,306 species of Ichneumonidae fauna are now registered for Mexico (Ruíz-Cancino, 2015) and 417 species are reported for the state of Tamaulipas (Ruíz-Cancino, 2010).











 text new page (beta)
text new page (beta)


