Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.80 no.3 México dic. 2009
Ecología
Diversity and structure of periphyton and metaphyton diatom communities in a tropical wetland in Mexico
Diversidad y estructura de las comunidades de diatomeas del perifiton y el metafiton en un humedal tropical en México
Claudia Ibarra1, Rosaluz Tavera2,* y Eberto Novelo3
1 Posgrado en Ciencias Biológicas. Facultad de Ciencias, Universidad Nacional Autónoma de México. Apartado postal 70–474, 04510, Coyoacán, México, D.F. México.
2 Departamento de Ecología y Recursos Naturales, Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad Universitaria, Apartado postal 70–474, 04510, Coyoacán, México, D.F., México.
3 Departamento de Biología Comparada, Facultad de Ciencias, Universidad Nacional Autónoma de México. Ciudad Universitaria, Apartado postal 70–474, 04510 Coyoacán, México, D.F., México.
* Correspondent:
rts@hp.fciencias.unam.mx
Recibido: 12 enero 2009
Aceptado: 27 mayo 2009
Abstract
We investigated the structure and diversity of diatoms in communities of metaphyton and periphyton from the wetland of El Edén Ecological Reserve, Quintana Roo, Mexico. In spite of the close association and communication between these communities, our comparisons reveal that the 2 communities have distinct species assemblages, with the periphyton being more diverse overall. We fit abundance curves for periphyton and metaphyton, and argue that our results are consistent with communities where environmental conditions play a more important role than competition in structuring diatom species assemblages.
Key words: Bacillariophyceae, diatom assemblages, competition, random recruitment, karstic wetlands.
Resumen
Investigamos la estructura y la diversidad de las comunidades de diatomeas en el metafiton y el perifiton del humedal de la Reserva Ecológica El Edén, Quintana Roo, México. A pesar de la cercana asociación entre estas comunidades, nuestro análisis revela que tanto el perifiton como el metafiton consisten de distintas asociaciones de especies y el perifiton es el más diverso. Las distribuciones de las abundancias de las especies de diatomeas satisfacen curvas de distribución log–normal; esto significa que en las comunidades estudiadas, las condiciones ambientales juegan un papel más importante que la competencia para determinar su estructura.
Palabras clave: Bacillariophyceae, asociaciones de diatomeas, competencia, reclutamiento al azar, humedales kársticos.
Introduction
El Edén Ecological Reserve (EER), Quintana Roo, Mexico, consists of wetlands noteworthy for their ubiquitous algal growths, which can be divided into periphyton and metaphyton. Periphyton is found growing as crusts during the dry season, and adheres to virtually any substrate during the rainy season when the wetland is inundated. Metaphyton, which floats on the water surface, is only found during the wet season and permanently at the shores of cenotes (sinkholes).
The most conspicuous elements of both communities are filamentous cyanoprokaryotes (Novelo and Tavera, 2003); however, numerous species of diatoms can be found growing on and among these filaments. The majority of the species found in these growths has been documented in tropical areas of low salinity and have been described as alkaliphiles associated with karstic environments or at least indifferent to calcareous substrates (Novelo et al., 2007). Diatom populations found growing in these communities closely follow changes in the hydroperiod as well as differences in substrate and vegetation type present in different parts of the wetland area (Novelo and Tavera, 2003). However, the close association of periphyton and metaphyton communities and the potential for mixture due to annual patterns of inundation create the potential for substantial overlap in species assemblages. In this study we investigate the structure and diversity of diatom species in both communities. We show that the 2 communities are in fact distinct in their diatom assemblages, and argue that these differences are likely due to the change from a sessile to a free–floating life style conditions associated with colonization of the metaphyton.
Materials and methods
Study area. El Edén Ecological Reserve is located 25 km NNE of the town of Leona Vicario, in the municipality of Lázaro Cárdenas, Quintana Roo, Mexico (Fig. 1). The rock substrate consists of carbonate from the Cretaceous/ Tertiary boundary (Urrutia–Fucugauchi et al., 1996; Campos–Enríquez et al., 2004; Keller et al., 2004). Soils, varying in depth from 0–1m, have a paucity of organic material. Bedrock is composed of carbonate fragments and melted materials of low permeability (Urrutia–Fucugauchi et al., 1996). The water table is very close to the surface, such that elevation and soil depth determine the variability in patterns of inundation in wetland zones during and after the rainy season.
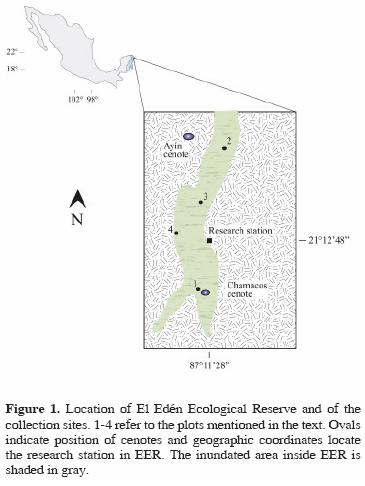
The numerous underground systems of water in the Yucatán Peninsula flow towards the coast (Schultz, 2005). These currents, along with the carbonate composition of the zone, promote the formation of karstic depressions which cenotes, caverns, and superficial orifices form (Perry et al., 2003; Campos–Enríquez et al., 2004; Keller et al., 2004) all of which can be found throughout the EER.
Sampling was carried out in July and October 1999 (the rainy season), April 2000 (the dry season), and August and November 2000 (also in the rainy season). Equal–volume random samples of periphyton and metaphyton were separately taken and fixed in 3% formaldehyde from cenotes and from each of 4 plots situated in the wetlands of the EER (described in detail in Novelo and Tavera, 2003). One cm2 of metaphyton or periphyton was macerated by acid digestion (Rushforth et al., 1984) for extraction, cleaning and counting of diatoms. Permanent slides were mounted in Naphrax and observations of cleaned material were made with a Nikon E600 microscope, with differential interference contrast. Sampling sufficiency was tested by resampling individuals from the data to build accumulation curves (Gotelli and Colwell, 2001) for each community. Similarly, rarefaccion curves and Shannon's index of diversity (Patrick, 1968; Krebs, 1999; Gotelli and Colwell, 2001) were used to compare species richness, equitability, and diversity in each of the 2 communities, since the overall sample size of diatom cells was different in each community. Both curves were calculated and graphed using the software package EcoSim (Gotelli and Entsminger, 2004). We calculated measures of equitability (E) and diversity (H) for each community and compared the assemblage of species using the inverse coefficient of Canberra's distance. Absent species were assigned a value of 0.1 following Krebs (1999) to avoid overvaluing species' presence and to equilibrate the data matrix. We used the R statistical software package Vegan 1.8–2 (Dixon, 2003) to fit rank–abundance data from each community to several abundance distributions as suggested in Wilson (1991).
Results
We identified 126 species of diatoms (Novelo et al., 2007) in 107 samples of periphyton and metaphyton from 4 plots in the wetland zone of the EER. Although 75% of the species are shared between the 2 communities (Table 1), an inverse coefficient of Canberra's distance of 0.16 suggests that they are nonetheless distinct. Both communities have unique species (9 in metaphyton and 31 in periphyton), and the frequency and relative abundance of individual species differ between communities as well. Both have a low frequency of rare species (12% in metaphyton vs. 16% in periphyton, Table 1). Brachysira microcephala (Grunow) Compère was the dominant species in the wet metaphyton and very abundand in wet periphyton. Cymbopleura chacii Novelo,Tavera et Ibarra, Encyonema mesianum (Cholnoky) D.G. Mann and Mastogloia elliptica (C. Agardh) Grunow were dominant in the wet periphyton.
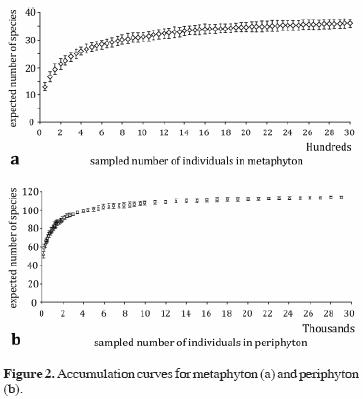
Accumulation curves suggest that sampling in each community was sufficient: both curves leveled off to the asymptote at less than 80% of the observed species (Fig. 2).
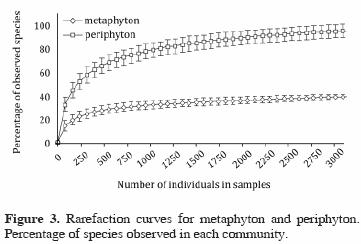
Using the rarefaction curves, we can compare equal–sized samples from both communities and show that the periphyton has higher species richness (Fig. 3). Shannon's index of diversity suggests that the periphyton is also slightly more diverse (H=3.55) compared to metaphyton (H=3.41).
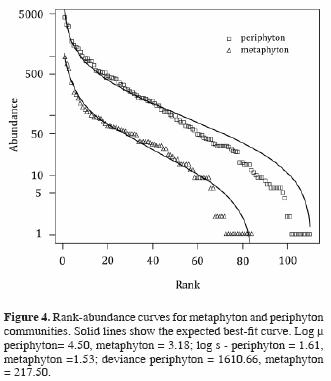
Rank abundance curves of diatoms in both communities better fit a log–normal distribution than any of the other distributions tested (broken stick, pre–emption, Zipf and Zipf–Mandelbrot models), using the Akaike information criterion (Fig. 4). This is reflected in the high equitability (E>0.75) of both communities. Neither highly abundant nor singleton species are particularly frequent, and the distribution is dominated by groups of species of intermediate abundance (between 14 and 80%, Fig. 4). This is consistent with a random recruitment of species in both communities.
Discussion
The majority of the diatoms in the EER can potentially grow in either periphyton or metaphyton. In fact, many species observed in the EER have been previously reported in both communities in other areas (Patrick and Reimer, 1966, 1975; Foged, 1984; Krammer, 1997a, 1997b; Krammer and Lange–Bertalot, 1985, 1986, 1988, 1991a, 1991b). During the brief periods of inundation, the development of diatom populations in both communities is expansive and rapid, with diatoms flourishing even in shallow waters. The difference in the composition of the diatom assemblages was evident from the list of species observed (Table 1), and substantiated by calculation of Canberra's distance.
The development of diatom populations in the metaphyton depends on the presence of a water column, present only during the rainy season in most parts of the EER. Sample size is therefore lower for the metaphyton, making a comparison of richness difficult; rarefaction curves circumvent this problem and confirm the higher species richness of the periphyton.
Rank abundance distributions of diatoms from both periphyton and metaphyton fit well to a log–normal model, which describes communities in development, where interspecific competition is less important in causing species rareness. In the EER, diatom community structure is undoubtedly affected by changes in the hydroperiod. Not only does inundation dislodge diatoms in the periphyton, thus contributing to the formation of the metaphyton and a change from a sessile to a free–floating life style, but also brings about changes in light, temperature, and nutrient availability (Novelo and Tavera, 2003). The response of diatom populations in the metaphyton to these varied changes must be rapid: in spite of environmental change in a short period of time, diatoms in the metaphyton are nearly as diverse and equitable as their counterparts in the periphyton.
Therefore, in the EER, it is likely not interspecific competition but the ecophysiological response to a changing environment that determines species distributions. Caswel (1976) shows that biological interactions such as interspecific competition generally reduce diversity within communities. This is not seen in communities in the EER, even when considering the effect of inundation. This interpretation also finds support in May (1975), who argues that under a lognormal distribution model, species are generally independent and sufficiently numerous to survive small migrations.
Characterization of the taxonomy and environmental affinities of the diatoms of the EER (Novelo et al., 2007) has shown that species composition of diatom communities in the EER varies across latitude and the physiological capacities of the species change in relation to the environmental factors of the wetland. The present study analyzes the structure of diatom communities in the periphyton and metaphyton, and uses this analysis to describe the changes in the species composition of these communities as a result of the hydrological cycle. We still know little about the ecology of freshwater algae, and this work is among the first to treat the community structure of tropical diatoms in general terms. We suggest that models of algal community ecology in karstic seasonal wetlands need to take into account the movement and colonization of diatom species.
Acknowledgements
We wish to thank Dr. A. Gómez–Pompa and the staff of El Edén Ecological Reserve for facilitating our research there, also to Dr. J. Ross–Ibarra for the translation to English and graphing and to Dr. J. Morrone and the 3 anonymous reviewers for their critical review to the manuscript that improved this work.
Literature cited
Campos–Enríquez J. O., F. J. Chávez–García, H. Cruz, J. G. Acosta –Chang, T. Matsui, J. A. Arzate, M. J. Unsworth and J. Ramos–López. 2004. Shallow crustal structure of Chicxulub impact crater imaged with seismic, gravity and magnetotelluric data: inferences about the central uplift. Geophysical Journal International 157:515–525. [ Links ]
Caswel, H. 1976. Community structure: A neutral model analysis. Ecological Monographies 46:327–354. [ Links ]
Dixon, P. 2003. VEGAN, A package of R functions for community ecology. Journal of Vegetation Sciences 14:927–930. [ Links ]
Foged, N. 1984. Freshwater and littoral diatoms from Cuba. Bibliotheca Diatomologica 5. J. Cramer. Vaduz. 243 p. [ Links ]
Gotelli, N. J. and R. K. Colwell. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4:379–391. [ Links ]
Gotelli, N. J. and G. L. Entsminger. 2004. Ecosim: Null models software for ecology, Version 7. Acquired Intelligence Inc. and Kesey – Bear. Jericho. VT 05465. http://garyentsminger.com/ecosim/index.htm. Last access: 011009. [ Links ]
Keller, G., T. Adatte, W. Stinnesbeck, D. Stüben, Z. Berner, U. Kramar and M. Harting. 2004. More evidence that the Chicxulub impact predates the K/T mass extinction. Meteoritics and Planetary Science 39:1127–1144. [ Links ]
Krammer, K. 1997a. Die cymbelloiden Diatomeen. Eine Monographie der weltweit bekannten Taxa. Teil 1. Allgenmeines und Encyonema Part. Bibliotheca Diatomologica 36. J. Cramer. Stuttgart. 382 p. [ Links ]
Krammer, K. 1997b. Die cymbelloiden Diatomeen. Eine Monographie der weltweit bekannten Taxa. Teil 2. Encyonema part., Encyonopsis und Cymbellopsis. Bibliotheca Diatomologica 37. J. Cramer. Stuttgart. 469 p. [ Links ]
Krammer, K. and H. Lange–Bertalot. 1985. Naviculaceae. Neue und wenig bekannte Taxa, neue Kombinationen und Synonyme sowie Bemerkungen zu einigen Gattungen. Bibliotheca Diatomologica 9. J. Cramer. Stuttgart. 230 p. [ Links ]
Krammer, K. and H. Lange–Bertalot. 1986. Bacillariophyceae. Teil 1: Naviculaceae. Die SüΒwasserflora von Mitteleuropa Band 2/1. H. Ettl, J. Gerloff, H. Heyning and D. Mollenhauer (eds.). Gustav Fischer Verlag. Stuttgart. 876 p. [ Links ]
Krammer, K. and H. Lange–Bertalot, 1988. Bacillariophyceae. Teil 2: Bacillariaceae, Ephitemiaceae, Surirellaceae. Die SüΒwasserflora von Mitteleuropa Band 2/2. H. Ettl, J. Gerloff, H. Heyning and D. Mollenhauer (eds.). Gustav Fischer Verlag. Stuttgart. 576 p. [ Links ]
Krammer, K. and H. Lange–Bertalot. 1991a. Bacillariophyceae. Teil 3: Centrales, Fragilariaceae, Eunotiaceae. Die SüΒwasserflora von Mitteleuropa Band 2/3. H. Ettl, J. Gerloff, H. Heyning and D. Mollenhauer (eds.). Gustav Fischer Verlag. Stuttgart. 576 p. [ Links ]
Krammer, K. and H. Lange–Bertalot. 1991b. Bacillariophyceae. Teil 4: Achnanthaceae, Kritische Ergënzungen zu Navicula (Lineolatae) und Gomphonema. Gesamtliteraturverzeichnis. Teil 1–4. Die SüΒwasserflora von Mitteleuropa Band 2/4. H. Ettl, J. Gerloff, H. Heyning and D. Mollenhauer (eds.). Gustav Fischer Verlag. Stuttgart. 438 p. [ Links ]
Krebs, C. J. 1999. Ecological Methodology. Benjamin Cummings. Menlo Park. 620 p. [ Links ]
May, R. M. 1975. Patterns of species abundance and diversity. In Ecology and evolution of communities, L. Cody and J. M. Diamond (eds.). Belknap – Harvard University Press. Cambridge. p. 81–120. [ Links ]
Novelo, E. and R. Tavera. 2003. The role of periphyton in the regulation and supply of nutrients in a wetland at El Edén, Quintana Roo. In Lowland Maya Area: Three Millennia at the Human–Wildland Interfase, A. Gómez–Pompa, S. Fedick, M. Allen and O. J. Jimenez (eds.). The Haworth Press Inc. Binghampton. p. 217–239. [ Links ]
Novelo, E., R. Tavera and C. Ibarra. 2007. Bacillariophyceae from karstic wetlands in Mexico. Bibliotheca Diatomologica 54 J. Cramer. Stuttgart. 136 p. [ Links ]
Patrick, R. 1968. The structure of diatom communities in similar ecological conditions. American Naturalist 102:173–183. [ Links ]
Patrick, R. and C. Reimer. 1966. The diatoms of the United States, 1. Monographs of the Academy of Natural Sciences of Philadelphia. Philadelphia. 688 p. [ Links ]
Patrick, R. and C. Reimer. 1975. The Diatoms of the United States. 2, Part 1. Monographs of the Academy of Natural Sciences of Philadelphia. Philadelphia. 213 p. [ Links ]
Perry, E., G. Velazquez–Oliman and R. A. Socki. 2003. Hydrogeology of the Yucatan Peninsula. In Lowland Maya Area: Three Millennia at the Human–Wildland Interfase, A. Gómez–Pompa, S. Fedick, M. Allen and O. J. Jimenez (eds.). The Haworth Press Inc., Binghampton. p. 115–138. [ Links ]
Rushforth, S. R., I. Kaczmarska and J. R. Johansen. 1984. The subaerial diatom flora of Thurston Lava Tube, Hawaii. Bacillaria 7:135–157. [ Links ]
R–Vegan 1.8–2. http://cc.oulu.fi/~jarioksa/softhelp/vegan.html (last access April, 2009). [ Links ]
Schultz, G. P. 2005. Vascular flora of El Edén Ecological Reserve, Quintana Roo, Mexico. Journal of the Torrey Botanical Society 2:311–322. [ Links ]
Urrutia–Fucugauchi, J., L. Marín and A. Trejo–García. 1996. Scientific drilling program of Chicxulub impact structure: Evidence for a 300 kilometer crater diameter. Geophysical Research Letters 23:1565–1568. [ Links ]
Wilson, J. B. 1991. Methods for fitting dominance/diversity curves. Journal of Vegetation Science 2:35–46. [ Links ]














