Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de biodiversidad
versión On-line ISSN 2007-8706versión impresa ISSN 1870-3453
Rev. Mex. Biodiv. vol.80 no.1 México abr. 2009
Ecología
Rocky–reef fish assemblages at San José Island, Mexico
Asociaciones de peces de arrecifes rocosos en Isla San José, México
Carmen Amelia Villegas–Sánchez, Leonardo Andrés Abitia–Cárdenas*, Francisco Javier Gutiérrez–Sánchez and Felipe Galván–Magaña
Departamento de Pesquerías y Biología Marina, Centro Interdisciplinario de Ciencias Marinas–Instituto Politécnico Nacional. Av. Instituto Politécnico Nacional s/n Col. Playa Palo de Santa Rita, 23096 La Paz, B.C.S., México.
*Correspondent:
labitia@ipn.mx
Abstract
We analyzed the composition, diversity, and abundance of marine fish at rocky reefs off San José island, Mexico between October 2001 and August 2002. Fish species were recorded using a visual census in 5 sampling areas of 50 by 5 meters at 2 depths, shallow (1–3 meters) and intermediate (5–7 meters). A total of 26 946 organisms were counted, belonging to 84 species. The families Serranidae (9 species), Labridae (8), Pomacentridae (7), and Haemulidae (6) were the most representative. We measured the rugosity of the bottom surface, which showed a positive relationship with abundance, richness, and fish diversity. The ocean bottom off San José island is covered with various size rocks that offer more feeding and refuge areas to fish assemblages than other areas. The ecological index increased during the warm season. Diversity and richness showed significant variations (P<0.05) by depth, with the highest values in exposed locations around the island (Conejo, Pardito, and San Francisquito). The biological value index indicated that the most representative fish species were Stegastes rectifraenum, Abudefduf troschelii, Thalassoma lucasanum, Scarus ghobban, and Mulloidichthys dentatus. The depth and wave exposure were the 2 environmental variables with the most influence on the structure of rocky–reef fish assemblages.
Key words: fish diversity, rocky reef, Baja California Sur, Gulf of California.
Resumen
Se analizó la estructura íctica de los arrecifes rocosos de Isla San José, México entre octubre del 2001 y agosto del 2002. Las especies fueron registradas por medio de censos visuales, los cuales se realizaron sobre transectos de 50 m de largo X 5 m de ancho en 2 profundidades: somera (1–3 m) e intermedia (5–7 m). Se contabilizaron un total de 26 946 individuos pertenecientes a 84 especies. Las familias mejor representadas fueron Serranidae (9), Labridae (8), Pomacentridae (7) y Haemulidae (6). La rugosidad del sustrato presentó una correlación positiva con la abundancia, riqueza y diversidad de especies de peces, lo que podría indicar que zonas con altos valores de rugosidad ofrecen a los peces más áreas de alimentación y refugio. Los índices ecológicos mostraron una tendencia de incremento de especies durante la temporada cálida. La diversidad y riqueza específica presentaron variaciones significativas entre profundidades (P<0.05), con valores más altos en los sitios expuestos de la isla ( Conejo, Pardito y San Francisquito). El índice de valor biológico indicó que las especies más representativas fueron: Stegastes rectifraenum, Abudefduf troschelii, Thalassoma lucasanum, Scarus ghobban y Mulloidichthys dentatus. La profundidad y exposición al oleaje fueron las variables con mayor influencia en la estructura íctica de los arrecifes rocosos.
Palabras clave: diversidad de peces, arrecifes rocosos, Baja California Sur, golfo de California.
Introduction
Rocky reefs are complex coastal marine ecosystems with interactions between fish species. They are marine structures that offer greater protection to fish along the coast than in the open sea, allowing the development of ecosystems such as mangroves and sea grasses (Vázquez–Domínguez, 2000). Reefs are widely recognized as productive and heterogeneous biomes and consequently have numerous associated species (Álvarez–Filip et al., 2006). In addition, reefs are well–known locations for economic activities , such as fishing (including of ornamental species) and tourism in various countries (Vázquez–Domínguez, 2000).
The Gulf of California recently has been ranked as sixth in the world's top 10 marine biodiversity "hot spots" for tropical reefs (Roberts et al., 2002; Campos–Dávila et al., 2005; Rodríguez–Romero et al., 2005). Some of the highest marine biodiversity values there are found on rocky reefs, which support a wide variety of endemic species (Thomson et al., 2000).
San José island has rocky reefs along its coast with a high diversity of fish and is one of the largest islands in the southern Gulf of California. To date, there is no published detailed description of fish communities. The only information is from Sánchez–Ortíz et al. (1997), who studied the population densities and community structures of fish at 11 locations, including San Francisco Island (located close to San José Island), where they reported 101 fish species. In this work we describe the seasonal structure of fish assemblages associated with rocky reefs off San José Island.
Material and methods
San José Island is between 24°52' and 25°06' North latitude and between 110o43' and 110o35' West longitude (Bourillón–Moreno et al., 1991). It is of volcanic origin, 194–km2 in area, and approximately 28 km long by 7.5 km wide. Its highest elevation is 633 m and in some areas it is covered with vegetation (Anonymous, 1987). Its coasts are quite steep on the eastern side, whereas on the west there are some creeks with small beaches in the interior, protected from winds. Bahía Amortajada on the southwestern coast has extensive sandy beaches and to the south is a mangrove area (Bourillón–Moreno et al., 1991) (Fig 1).
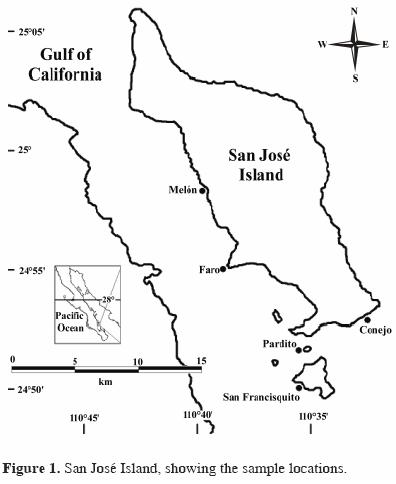
Six bimonthly samplings between October 2001 and August 2002 were made at 5 locations because of their extensive rocky reef coverage (Fig. 1). The 6 samplings were done during both cold and warm seasons because the Gulf of California has only these 2 distinct seasons yearly (Álvarez–Borrego and Schwartzlose, 1979; Anonimous, 2001). January, March, and May were in the cold season and June, August, and October in the warm season.
The visual census method was used because it is a nondestructive technique that provides both qualitative and quantitative descriptions of the reef–fish structure and is acceptable for studies concerning reef management and assemblage patterns (Bortone et al., 1991; Ackerman and Bellwood, 2000; Chávez–Comparan and Macías–Zamora, 2006 ).
Visual transects were made using free or scuba diving during daylight (10:00 to 16:00) over areas 50–m long and 5–m wide (250 m2 per census). Censuses were done from fixed locations, with geographic positions recorded by a global positioning system (GPS) and a coastal mark. All species of fish and their observed abundances were recorded on acrylic sheets. At each selected location, 2 censuses with replicas were taken parallel to the coast at 2 depths, shallow (1–3 m) and intermediate (5–7 m). The density of organisms was obtained dividing the total number of individuals between the total samples surfaces.
Physiographic characteristics, such as water temperature, type of substrate, and rugosity were recorded at the sampling locations. The bottom was assessed visually to estimate the percentage coverage by rocks and sand and rugosity was determined by calculating the ratio L/l of the estimated real distance over the surface (L) to the straight line length or horizontal range (l) in the same vertical plane. To measure the real length, a chain was laid in a straight line over the bottom surface, allowing it to follow the projections and depressions (Luckhurst and Luckhurst, 1978). Measured rugosity has a minimum value of 1 for a flat bottom and increases with the degree of roughness of the bottom surface.
We use the following ecological indices based on species abundance: Shannon–Weaver's diversity (H'), was used to determine the change in the fish community structure. Given that the H' value depends on species richness (number of species present in each sample) and evenness (index of Pielou, 1975), these values were also calculated.
An analysis of variance was used to determine differences in richness and diversity patterns between sites, season of the year, and depths (Clarke and Warwick, 1994). Where significant differences appeared, the Fisher test (Zar, 1996) for multiple comparison was used to determine the pairs of averages that differed significantly. The numbers of individuals for all statistical analyses were log transformed (Log10 n + 1) to homogenize the variances and to normalize the distribution.
To determine the dominant species through the annual cycle, we used the Biological Value Index (BVI) proposed by Sanders (1960). To calculate this index, a value of importance, expressed in points, was assigned to each species as a function of its numerical abundance in each sample. This allows the species to be ranked in order of importance based on the spatio–temporal constancy of their abundance (Loya–Salinas and Escofet, 1990).
The similarity in the reef community structure of the different locations of the island was done using cluster analysis with the Q–mode matrix and an agglomerative–method complete linkage, using the fish species with 95% accumulated of relative abundance. The principal components were analyzed by each variable, using a Q–mode correlation matrix. The variables used in this analysis were depth, temperature, percentage of rock coverage, rugosity, species richness, abundance (square root transformed), and fish diversity .
Results
A total of 26,946 fish were recorded during 6 bimonthly samplings (15,261during the warm season and 11,685 in the cold season) from 84 species, 60 genera and 31 families (Table 1). The families most represented by number of species were Serranidae (9 species), Labridae (8), Pomacentridae (7), Haemulidae (6), Scaridae (5), Lutjanidae (4), and Muraenidae (4). The fish families of highest abundance were Pomacentridae (38 %), Labridae (18 %), and Haemulidae (10 %).
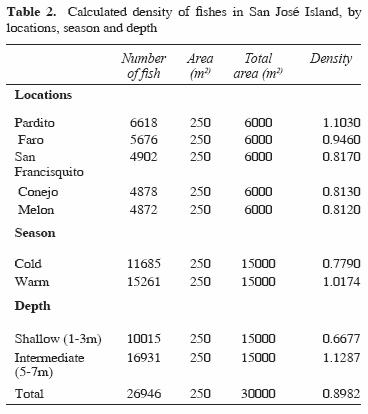
From 84 species identified, only 12 were recorded at all reefs and at both depths sampled. These common species off San José Island were Abudefduf troschelii, Balistes polylepis, Bodianus diplotaenia, Canthigaster punctatissima, Fistularia commersonii, Holacanthus passer, Hoplopagrus guntheri, Lutjanus argentiventris, Mycteroperca rosacea, Scarus ghobban, Stegastes rectifraenum, and Thalassoma lucasanum. The total fish density was of 0.89 organisms/m2. In Table 2 we indicate the density values obtained by locations, season and depth.
The average sea temperature varied by season. The minimum temperature in the cold season was 18.5 °C and the maximum was 31 oC during the warm season. The rugosities in 5 locations were similar, but the highest values were at locations with intermediate depth (Table 3). There was a significant positive correlation between rugosity and fish abundance (R = 0.678, Pα0.05 0.031), richness (R = 0.653, Pα0.05 0.040), and diversity (R = 0.700, Pα0.05 0.024).
Three sampling locations had rock coverage of 80% or more. Whereas Pardito at the intermediate depth was 65% and San Francisquito at the same depth had 50%. The rock coverage was not correlated with abundance (R = 0.285, Pα0.05 0.424), richness (R = 0.542, Pα0.055 0.105), or diversity (R = 0.535, Pα0.055 0.110).
No significant variation was found in diversity or in specific richness between seasons (P>0.05), but they increased with the increase in the water temperature (Fig. 2a, 2b).
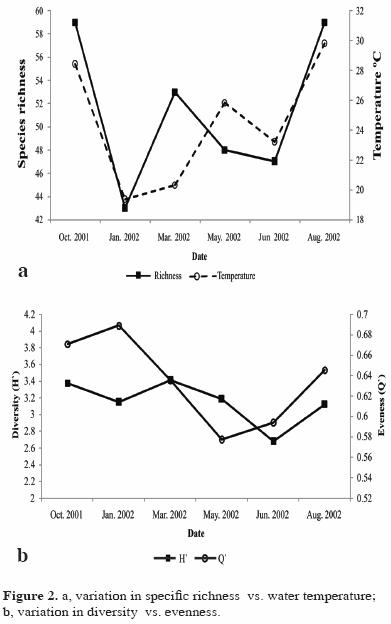
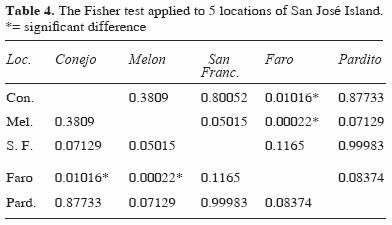
Diversity and species richness showed significant differences by depth, with higher values at intermediate depth. Among the different locations, the diversity was low in the Faro area and was significantly different from other locations (Faro–Conejo and Faro–Melon) (Table 4). Locations in exposed areas of the island ( Conejo, Pardito, and San Francisquito) had higher values of richness and diversity at both depths (Table 5).
Four of all fish species found were 50% of the BVI; S. rectifraenum, A. troschelii, T. lucasanum, and S. ghobban. Similar results were found during the warm and cold seasons of the year, although higher dominance was observed in the cold season. In the cold season, 6 species (A. troschelii, T. lucasanum, S. rectifraenum, Mulloidichthys dentatus, S. ghobban, and Paranthias colonus) were 80% of the BVI, whereas in the warm season 8 species accounted for 80% (the 6 previous species plus Microlepidotus inornatus and Haemulon maculicauda).
The analysis of the 2 depths revealed that 4 species at the shallow depth were 50% of the BVI; S. rectifraenum, A. troschelii, T. lucasanum, and S. ghobban, whereas at the intermediate depth, 5 species were 50% (the 4 previous species and M. dentatus) (Fig. 3).
A cluster analysis distinguished 3 groups. The first was at the shallow depth at 5 sites. Of these Faro was the most remote. The second group was made up of the sites Faro and Melón (intermediate depth), which are located in protected areas of the island. Group 3 included Conejo, San Francisquito, and Pardito, all at the intermediate depth but located at exposed areas of the island (Fig. 4).
The principal component analysis showed that 2 components were 84% of the variance. The first component explained 73% of the variance associated with the spatial distribution of species abundance, where the depth was the dominant variable. The shallow locations are located on the right side of figure 5 and the intermediate depth locations on the left side. The second component explained 10% of the variance and no variables dominated as they did in the first component (Fig. 5).
In the fish assemblages, the first component, the depth and richness eigenvectors, were more negative, whereas the temperature was more positive. In the second component, the rock coverage and rugosity yielded values on the positive side of Figure 5, whereas the abundance and depth were negative. The separation of Conejo at the intermediate depth, in the upper left of the figure 5, is caused mainly by the high values of richness (51) and rugosity (1.44). The grouping in shallow locations is mainly caused by their similar water temperatures.
Discussion
At San José Island there is less influence from tourism than at Espiritu Santo and Cerralvo Islands in the lower Gulf of California, and therefore less disturbance to these fish associations. which makes San José Island an ecologically and economically important location. We found that from 19 ornamentally important fish species reported by Almenara–Roldán (2000) in the Gulf of California, 12 species (63%) were found here.
The depth was the most influential variable in the structure of fish assemblages around this island, where the specific richness, diversity, and abundance increased with depth. This variable was the only one of all the ecological indices analyzed that showed significant variation. Other studies on reef–fish communities in the Gulf of California also found a positive relationship between species richness, abundance, diversity, and depth (Sánchez–Ortiz et al., 1997; Arreola–Robles and Elorduy–Garay, 2002; Sala et al., 2002; Rodríguez–Romero et al., 2005; Álvarez –Filip et al., 2006).
The changes observed in the fish assemblages with depth could be related to visiting fish species at the intermediate depth. These species were seen only once at 1 location. Some pelagic species were found at this intermediate depth (Caranx caballus, Caranx caninus, and Sphyraena lucasana among others) that were not seen at the shallow depth. Another factor that could have influenced this result was the bottom rugosity, which was generally higher at the intermediate depth. Other studies have found that rugosity adds complexity to the habitat, resulting in more feeding areas for fish assemblages and refuge in caves and cracks (Anderson et al., 1989; Aburto–Oropeza and Balart–Páez, 2001).
The only location that had significant variation in ecological indices compared to others was Faro, maybe because of its particular depth characteristics. This location is very shallow, with a maximum depth of 4.6 m, and is found between 2 sandy areas, which would explain the low fish diversity. Some fish species characteristic to this location with a sandy bottom were Haemulon steindachneri, Paralabrax maculatofasciatus, and Sphoeroides lobatus, which were found at Faro but at no other location. Also Mugil cephalus, Mulloidichthys dentatus, and Microlepidotus inornatus were found to be more abundant at this location with its sandy areas.
Grouping and ordering analysis determined that the fish association structure at San José Island is influenced mainly by depth. However, strong currents also must influence the presence of some species in locations as the San José channel, where Faro and Melón are. The locations Conejo, Pardito, and San Francisquito have weaker currents and are exposed to tides. We have not compared the effect of tides and currents on fish species abundance. However, there are studies that indicate the influence of these factors on the reef–fish assemblages (Aburto–Oropeza and Balart, 2001; Carleton et al., 2001; Friedlander et al., 2003).
The locations with the highest diversity and richness were those exposed to tides, but with weak currents (Conejo, Pardito, and San Francisquito). This was found at both depths studied and is consistent with Friedlander et al. (2003) on the reef–fish associations of Hawaii, who observed that areas moderately exposed to tides had greater richness and diversity. In a study done at the Australian barrier reef, Carleton et al. (2001) also determined that locations with weak currents have more prerecruit fish than those with strong currents.
Some fish species found only in exposed locations belong to the family Pomacentridae (Chromis atrilobata, Stegastes flavilatus, Stegastes leucorus, Microspathodon bairdii). They use these areas for feeding. Chromis atrilobata and species of the genus Stegastes have been reported as planktivores and herbivores (Allen and Robertson, 1994; Chávez–Comparan and Macías–Zamora, 2006) and need very productive areas or those with oceanographic features that retain this food type. These species have pelagic larvae and are drawn by the currents to these areas. Other species found in the exposed areas were C. caninus and Trachinotus rhodopus of the family Carangidae, which also have pelagic larvae.
The fish species S. rectifraenum, T. lucasanum, and Scarus ghobban had a higher dominance in the shallow depth than in the intermediate depth. M. dentatus was one of the most dominant species at the intermediate level, probably because of their preference for areas with mixed patches of sand and rocks, a characteristic that is found at greater depths. This species forms small schools and feeds on small fish and other organisms, including worms, shrimp, crabs, mollusks, polychaetes, and echinoderms that live in the sand. With the aid of chemosensory organs in their chin barbels they locate their prey (Allen and Robertson, 1994; Thomson et al., 2000).
Another fish species determined as dominant at 5 to 7 m, which appeared only rarely at 1 to 2 m, was C. atrilobata. This species was never observed at the protected sites Melón and Faro and was found with greater abundance and frequency at Conejo at intermediate depth. They prefer relatively deep areas between 6 and 20 m, although occasionally are found in deeper waters (Thomson et al., 2000).This species prefers areas with abundant plankton, which constitutes its food, and probably appears in these areas during high tide (Arreola–Robles and Elorduy–Garay, 2002; Chávez–Comparan and Macías–Zamora, 2006). The fish species that were found only in 1 or 2 locations show that the reefs are visited or used as transit areas by some species.
From 84 fish species reported in rocky reefs at San José island, only 12 (14%) were found at 5 locations and both depths, which indicates that the fish composition of each location varies according to its particular characteristics. A similar observation was made by Villareal–Cabazos et al. (2000) at the coral reef of Cabo Pulmo in Baja California Sur. The habitat of reef–fish communities in the Gulf of California are structured by depth, with different structural associations at depths of 0 to 4 m and 5 to 19 m. The upper limit of the second category was established by Allen and Robertson (1994), who mentioned 3 depths.
Richness, abundance, diversity, and evenness increase with water temperature, which is consistent with reports about the Gulf of California by Rodríguez–Romero et al. (1994), Pérez–España et al. (1996), Aburto–Oropeza and Balart–Páez (2001), Campos–Dávila et al. (2005), and Rodríguez–Romero et al. (2005). This increase during summer associated with winds from the southwest (Roden, 1964) generates an increase in the amount of food available and a higher abundance of fish. During winter, the northwest winds on the east coast of the Gulf affect the number of species in the Gulf, which begins to decline (Pérez–España et al., 1996).
During June 2002, a reduction in the ecological indices was measured and was associated with a 2.6 oC decrease in temperature from the previous sampling month. With this change in temperature, the fish spend less energy on motor activities because of their ectothermic metabolism (Eckert et al., 1991) and with reduced mobility, they become less visible to divers.
The dominant fish species in these associations may remain constant in time, which has been mentioned by Castro–Aguirre et al. (1995), who wrote that rocky reef fish communities in the Gulf of California are relatively homogenous because the resources and protection offered by the Gulf of California are constant.
Acknowledgements
To Instituto Politécnico Nacional (IPN, COFAA, EDI, and PIFI), which provided institutional and financial support for this study. This paper is a contribution from Centro Interdisciplinario de Ciencias Marinas (CICIMAR). Thanks to Dr. Ellis Glazier for editing the English–language text.
Literature cited
Aburto–Oropeza, O. and E. Balart–Paez. 2001. Community structure of reef fish in several habitats of rocky reef in the Gulf of California. Marine Ecology 22:283–305. [ Links ]
Ackerman, J. L. and D. R. Bellwood. 2000. Reef fish assemblages: a re–evaluation using enclosed rotenone stations. Marine Ecology Progress Series 206:227–237. [ Links ]
Allen, G. R. and D. R.Robertson. 1994. Fishes of tropical Eastern Pacific. University of. Hawaii Press, Honolulu, 332 p. [ Links ]
Almenara–Roldán, S. 2000. Demanda internacional de especies marinas ornamentales del Golfo de California. In Recursos arrecifales del Golfo de California, estrategias de manejo para las especies marinas de ornato, O. Aburto–Oropeza and C. Sánchez–Ortíz (eds.). Universidad Autónoma de Baja California Sur, México and Birch Aquarium, Scripps, Research, Estados Unidos. p. 39–47. [ Links ]
Álvarez –Borrego, S. and R. A. Schwartzlose. 1979. Masas de agua del Golfo de California, Ciencias Marinas 6:43–63. [ Links ]
Álvarez –Filip, L., H. Reyes–Bonilla and L. E. Calderon–Aguilera. 2006. Community structure of fishes in Cabo Pulmo reef, Gulf of California. Marine Ecology 27:253–262. [ Links ]
Anderson, T. W. E. E. DeMartini and D. A. Roberts. 1989. The relationship between habitat structure, body size and distribution of fishes in a temperate artificial reef. Bulletin of Marine Science 44:681–697. [ Links ]
Anonymous. 1987. Islas Mexicanas. Régimen jurídico y Catálogo. México, D.F. 154 p. [ Links ]
Anonymous. 2001. Programa de manejo. Complejo Insular Espíritu Santo México, Componente del área de protección de flora y fauna Islas del Golfo de California. Secretaria del Medio Ambiente, Recursos Naturales y Pesca, México, D.F. 194 p. [ Links ]
Arreola–Robles, J. L. and J. F. Elorduy–Garay. 2002. Reef fish diversity in the region of La Paz, Baja California Sur, México. Bulletin of Marine Science 70:1–18. [ Links ]
Bortone, S. A., T. Martin and C. M. Bundrick. 1991. Visual census of reef fish assemblages: a comparison of slate, audio and video recording devices. Northeast Gulf Science 12:17–23. [ Links ]
Bourillón–Moreno, L., A. C. Díaz–Barriga, F. Ecardi–Ambrosi., E. Lira–Fernández, J. Ramírez–Ruiz, E. Velarde–González and A. Zavala–González. 1991. Islas del Golfo de California, Secretaría de Gobernación y Universidad Nacional Autónoma de México. 292 p. [ Links ]
Campos–Dávila, L., V. H. Cruz–Escalona, F. Galván–Magaña, L. A. Abitia–Cárdenas, F. J. Gutiérrez–Sánchez and E. F. Balart–Paez. 2005. Fish assemblages in a Gulf of California marine reserve. Bulletin of Marine Science 77:347–362. [ Links ]
Carleton, J. H. R. Brinkma and P. J. Doherty. 2001. Zooplankton community structure and water flow in the lee of Helix Reef (Great Barrier Reef, Australia). Marine Biology 139:705–717. [ Links ]
Castro–Aguirre, J. L. E. F. Balart–Páez and J. Arvizu–Martínez. 1995. Contribución al conocimiento del origen y distribución de la ictiofauna del Golfo de California, México. Hidrobiológica 5:57–78. [ Links ]
Chávez–Comparan, J. C. and R. Macías Zamora. 2006. Structure of reef fish communities in the littoral of Colima, Mexico. Journal of Biological Sciences 6:65–75. [ Links ]
Clarke, K. R and R. M. Warwick. 1994. Change in marine communities: an approach to statistical analysis and interpretation. Natural Environment Research Council, Plymouth. 144 p. [ Links ]
Eckert, R., D. Randall and G. Augustine. 1991. Fisiología animal, mecanismos y adaptaciones. McGraw Hill, Madrid. 683 p. [ Links ]
Friedlander, A. M., E. K. Brown, P. L. Jokiel, W. L. Smith and K. S. Rodgers. 2003. Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian Archipelago. Coral Reef 22:291–305. [ Links ]
Loya–Salinas, D. H. and A. Escofet. 1990. Aportaciones al cálculo del índice de valor biológico (Sanders, 1960). Ciencias Marinas 16:97–115. [ Links ]
Luckhurst, B. E. and K. Luckhurst. 1978. Analysis of the influence of substrate variables on coral reef fish communities. Marine Biology 49:317–323. [ Links ]
Pérez–España, H. F., Galván–Magaña and L. A. Abitia–Cárdenas. 1996. Variaciones temporales y espaciales en la estructura de la comunidad de peces de arrecifes rocosos del sur–oeste del Golfo de California México. Ciencias Marinas 22:273–294. [ Links ]
Pielou, E. C. 1975. Ecological diversity. Wiley interscience. Nueva York. 142 p. [ Links ]
Roberts, C. M., C. J. McClean, J. E. N. Veron, J. P. Hawkins, G. R. Allen, D. E. McAllister, C. G. Mittermeier, F. W. Schueler, M. Spalding, F. Wells, C. Vynne and T. B. Werner. 2002. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295:1280–1284. [ Links ]
Roden, G. I. 1964. Oceanographyc aspects of Gulf of California. In Marine geology of the Gulf of California, A symposium, T. H. van Andel and G. G. Shore Jr. (eds.). The American Association of Petroleum Geologists, memoir No. 3. p. 26–45. [ Links ]
Rodríguez–Romero, J., L. A. Abitia—Cárdenas, F. Galván–Magaña and H. Chávez–Ramos. 1994. Composición, abundancia y riqueza específica de la ictiofauna de Bahía Concepción, Baja California Sur México. Ciencias Marinas 20:321–350. [ Links ]
Rodríguez–Romero, J., A. F. Muhlia–Melo, F. Galván–Magaña, F. J. Gutiérrez–Sánchez, and V. Gracía–López. 2005. Fish assemblages around Espiritu Santo Island and Espiritu Santo seamount in the lower Gulf of California, México. Bulletin of Marine Science 77:33—50. [ Links ]
Sala, E., O. Aburto–Oropeza, G. Paredes, I. Parra, J. C. Barrera and P. K. Dayton. 2002. A general model for designing networks of marine reserves. Science 298:1991–1993. [ Links ]
Sánchez–Ortíz, C., J. L. Arreola–Robles, O. Aburto–Oropeza and M. Cortés–Hernández. 1997. Peces de arrecife en la región de La Paz, BCS. In La Bahía de La Paz, investigación y conservación, J. Urbán–Ramírez and M. E. Ramírez–Rodríguez (eds.). Universidad Autónoma de Baja California Sur, Centro Interdisciplinario de Ciencias Marinas y Scripps, Institutions of Oceanography Research. p. 177–188. [ Links ]
Sanders, H. L. 1960. Benthic studies in Buzzard Bay III. The structure of soft–bottom community. Limnology Oceanography 5:138–153. [ Links ]
Thomson, D. A., L. T. Findley and A. N. Kerstitch. 2000. Reef fishes of the Sea of Cortes. The rocky–shore fishes of the Gulf of California. University Texas Press, Arizona. 374 p. [ Links ]
Vázquez–Domínguez, E. 2000. Importancia de la biodiversidad arrecifal. In Recursos arrecifales del Golfo de California. Estrategias de manejo para las especies marinas de ornato, O. Aburto–Oropeza and C. Sánchez–Ortíz (eds.). Universidad Autónoma de Baja California Sur, México and Birch Aquarium, Scripps, Institutions of Oceanography Research, Estados Unidos. p. 9–17. [ Links ]
Villarreal–Cabazos, A., H. Reyes–Bonilla, B. Bermúdez–Almada y O. Arizpe. 2000. Los peces del arrecife de Cabo Pulmo, Golfo de California, México: Lista sistemática y aspectos de abundancia y biogeografía. Revista de Biología Tropical 48:413–424. [ Links ]
Zar, J. H. 1996. Biostatistical analysis, Prentice Hall, New Jersey. 662 p. [ Links ]














