Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.58 no.2 Ciudad de México abr./jun. 2014
Article
New Polynuclear Nonfused Bis(1,3,4-Oxadiazole) Systems
Yagoub Mansoori, and Raana Sarvari
Department of Applied Chemistry, College of Science, University of Mohaghegh Ardabili, Ardabil, Iran, 56199-11367. ya_mansoori@yahoo.com
Received February 10th, 2013
Accepted March 28th, 2014
Abstract
In the present work, new polynuclear non-fused bis-(1,3,4-oxadiazole) compounds were obtained through Huisgen reaction of bis-(5-oxy-(1H)-tetrazoles with acid chlorides. Bis-tetrazole VIg was obtained from the 1,3-dipolar cycloaddition reaction between suberonitrile and sodium azide in DMF. The prepared compounds have been characterized by IR, 13C NMR, mass spectroscopy and elemental analysis.
Key words: 1,3,4-Oxadiazole, Bis-(1H)-tetrazole, Huisgen reaction.
Resumen
En el presente trabajo se sintetizaron nuevos bis-(1,3,4-oxadiazoles) polinucleares no-fundidos mediante la reacción de Huisgen de bis-(5-oxi-(1H)-tetrazoles con cloruros de ácido. El tetrazol VIg se obtuvo de la reacción de cicloadición 1,3-dipolar entre suberonitrilo y azida de sodio en DMF. Los compuestos preparados fueron caracterizados por IR, 13C RMN, espectrometría de masas y análisis elemental.
Palabras clave: 1,3,4-Oxadiazol, Bis-(1H)-tetrazol, reaction de Huisgen.
Introduction
1,3,4-Oxadiazoles exhibit relevant biological properties and a wide variety of applications, in particular as active compounds in both medicine and agriculture. They are key fragments of various drugs, from antiviral agents to antidepressants [1-2]. The substituted oxadiazoles are heterocyclic compounds, which serve both as biomimetic and reactive pharmacophores and many key elements with potential biological activities such as pesticidal, antiperipheral vasomotility, CNS stimulant, antiinflammatory, hypotensive, insecticidal, bactericidal, hypoglycemic, analgesic, anticonvulsive, antiemetic, diuretic, muscle relaxant, herbicidal and fungicidal activity [3]. Furthermore, 1,3,4-oxadiazoles are potentially versatile compounds converting other hereocycles such as 1,3,4-triazoles [4].
Aromatic oxadiazole based compounds have attracted special attention in the context of research on organic light-emitting diodes (LED). In 1990, Adachi et al. reported 2-(biphenyl-4-yl)-5-(4-ter-butylphenyl)-1,3,4-oxadiazole can act as an excellent electron transport material (ETM) in an organic multilayer EL diode [5-6]. After this report, many researchers began to use various kinds of oxadiazole molecules to obtain high EL performances. The oxadiazoles feature high electron affinities, which in turn, facilitate both electron injection and transport processes that is important in molecular and polymeric LED devices [7-13].
Nonfused bis-oxadiazoles are interested systems due to their luminescent properties and the possibility of using them as scintillators [14]. These compounds are also show biological activities. Maslat et al. demonstrated antifungal and mutagenic activities for bis-oxadiazoles I-IV, Scheme 1 [15].
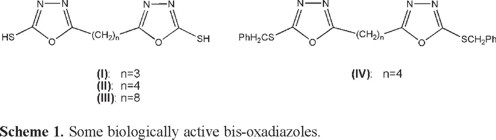
In addition to 1,3,4-oxadiazole compounds, polymers containing oxadiazole units have been widely used in experimental devices as both electron-transporting and hole-blocking materials [15]. These polymers are thermally stable due to presence of oxadiazole moiety [16-20].
These interesting properties of oxadiazole polymers encouraged us to focus on the preparation of thermally resistance polymers containing 1,3,4-oxadiazole units. We recently reported the synthesis and characterization of new polynuclear bis-5-oxy-1H-tetrazoles [21]. In the present work, new nonfused bis(1,3,4-oxadiazole) systems (VIIIa-VIIIg) have been synthesized through Huisgen reaction of the bis-tetrazole compounds with aromatic acid chlorides. These compounds can be considered as model for the polymers obtained from the Huisgen reaction of bis-tetrazoles with bis-acid chlorides [22]. The prepared compounds have been characterized by 13C NMR, IR, and mass spectroscopy methods along with elemental analysis (CHN).
Experimental Section
Instruments
13C NMR spectra (100 MHz) were obtained on a Bruker spectrospin Avanc 400 Ultrashield (Tabriz University, Iran), and Bruker 75 MHz (Urmia University, Iran) spectrophotometers. The IR (KBr) spectra were recorded on a Buck Infrared Spectrophotometer Model 500. Mass spectra (EI, 70 eV) were obtained on a Shimadzu QP-5050 17 A.
Typical procedure for the preparation of bis-tetrazole compounds (VIa-VIf). In a 100 mL two necked round bottom flask equipped with a magnetic stirrer in an ice bath, bis-phenol compound (4.3 mmol) and cyanogen bromide (9 mmol) was dissolved in 10 mL of dry acetone. A solution of triethylamine (0.91 g, 9 mmol) in 5 mL of dry acetone was added drop wise to this solution at 0-5 °C. Triethyl ammonium bromide salt is gradually formed as white precipitate. The reaction mixture was stirred for additional 30 minutes, filtered, and the clear filtrate was added to the suspension of sodium azide (0.96 g, 14.7 mmol) in 5 mL of dry acetone at room temperature. The reaction mixture was stirred and refluxed for 90 minutes. Water (10 mL) was added and the solution was concentrated under reduced pressure. The solution was placed in ice bath and acidified by drop wise addition of concentrated hydrochloric acid, while vigorous stirring. The precipitate of bis-tetrazole compound was filtered, washed with cold water, and re-crystallized from ethyl acetate and hexane [22-23].
Bis 1,3,4-oxadiazole (VIIIa). In a round bottom 25 mL flask equipped with magnetic stirrer bis-tetrazole (VIa) (1.48 g, 6.0 mmol), benzoyl chloride (VIIa) (2.25 g, 16.0 mmol), and pyridine (1.27 g, 16 mmol) were refluxed in 5 mL of sodium dried toluene for 4 hours. Nitrogen cessation was occurred at 69-71 °C. Solvent was removed under reduced pressure and the residue was poured into 20 mL of%5 NaOH. The mixture was stirred for 30 minutes, filtered, and washed with distilled water. The crude product was re-crystallized from mixture of methanol and water.
Yield 31%, mp 153-154 °C. IR (KBr), cm-1: 3053 (s), 2914 (m), 1611 (s), 1521 (s), 1380 (s), 1183 (s), 1105 (s), 1104 (s), 851 (s), 683 (s). 13C NMR spectrum (CDCl3, 75 MHz), δ, ppm: 176.42, 173.69, 150.77, 133.27, 129.15, 128.03, 123.92, 121.03. Found,%: C 66.20; H 3.22; N 14.31. C22H14N4O4. Calculated,%: C 66.33; H 3.54; N 14.06. (m/e) +: 398 (%65).
Bis 1,3,4-oxadiazole (VIIIb). In a round bottom 25 mL flask equipped with magnetic stirrer bis-tetrazole (VIb) (1.48 g, 6.0 mmol), and 3,5-dinitrobenzoyl chloride (VIIb) (3.69 g, 16.0 mmol) were refluxed in 1.5 mL of pyridine for 4 hours. Nitrogen cessation was occurred at 61-63 °C. The mixture was then poured in%5 NaOH, stirred at room temperature for 30 minutes, washed with distilled water, dried at 50-60 °C under reduced pressure, and chromatographed on silica gel column using 40:60 ethyl acetate:chloroform as eluant.
Yield 25%, mp 178-180 °C. IR (KBr), cm-1: 3063 (s), 3005 (m), 1606 (s), 1541 (s), 1342 (s), 1243 (s), 1137 (s), 1073 (s), 921 (s). 13C NMR spectrum (DMSO-d6, 100 MHz), δ, ppm: 110.58, 116.19, 121.12, 125.57, 126.38, 129.71, 147.84, 152.31, 171.06, 172.17. Found,%: C 45.89; H 1.85; N 18.65. C22H10N8O12 Calculated,%: C 45.69; H 1.74; N 19.37. Fragment (m/e)+: 344 (%52).
Bis 1,3,4-oxadiazole (VIIIc). In a round bottom 25 mL flask equipped with magnetic stirrer bis-tetrazole (VIc) (1.56 g, 6.0 mmol), and 3,5-dinitrobenzoyl chloride (VIIb) (3.69 g, 16.0 mmol) were refluxed in 1.5 mL of pyridine for 4 hours. Nitrogen cessation was occurred at 59-61 °C. The mixture was then poured in%5 NaOH, stirred at room temperature for 30 minutes, washed with distilled water, dried at 50-60 °C under reduced pressure, and chromatographed on silica gel column using 5:95 ethyl acetate:chloroform as eluant.
Yield 26%, mp 196-198 °C. IR (KBr), cm-1: 3083 (s), 2950 (m), 2866 (m), 1744 (m), 1545 (s), 1455 (s), 1354 (s), 1256 (s), 1177 (s), 1033 (s), 912 (s). 13C NMR spectrum (DMSO-d6, 100 MHz), δ, ppm: 14.92, 117.20, 120.21, 121.98, 121.33, 125.55, 126.28, 128.52, 130.43, 147.73, 149.36, 170.01, 172.48. Found%: C 47.03; H 2.84; N 18.59. C23H12N8O12 Calculated,%: C 46.63; H 2.04; H 3.83; N 18.91, Fragment (m/e) +: 358 (%24).
Bis 1,3,4-oxadiazole (VIIId). In a round bottom 25 mL flask equipped with magnetic stirrer bis-tetrazole (VId) (2.18 g, 6.0 mmol), and 3,5-dinitrobenzoyl chloride (VIIb) (3.69 g, 16.0 mmol) were refluxed in 1.5 mL of pyridine for 4 hours. Nitrogen cessation was occurred at 64-66 °C. The mixture was then poured in%5 NaOH, stirred at room temperature for 30 minutes, washed with distilled water, dried at 50-60 °C under reduced pressure, and chromatographed on silica gel column using 25:75 ethyl acetate:hexane as eluant.
Yield 33%, mp 154-156 °C. IR (KBr), cm-1: 3083 (s), 2950 (s), 2860 (m), 1593 (s), 1525 (s), 1455 (s), 1357 (s), 1207 (s), 1165 (s), 1081 (s), 918 (s), 845 (s). 13C NMR spectrum (DMSO-d6, 100 MHz), δ, ppm: 29.86, 41.61, 118.14, 121.21, 126.06, 126.60, 127.31, 147.32, 148.08, 150.07, 171.05, 172.96. Found,%: C 53.86; H 2.83; N 16.38. C31H20N8O12 Calculated,%: C 53.46; H 2.89; N 16.08. (m/e) +: Fragment (m/e) +: 355 (%13).
Bis 1,3,4-oxadiazole (VIIIe). In a round bottom 25 mL flask equipped with magnetic stirrer bis-tetrazole (VIe) (2.12 g, 6.0 mmol), benzoyl chloride (VIIa) (2.25 g, 16.0 mmol), and pyridine (1.27 g, 16 mmol) were refluxed in 5 mL of sodium dried toluene for 4 hours. Solvent was removed under reduced pressure and the residue was poured into 20 mL of%5 NaOH. The mixture was stirred for 30 minutes, filtered, and washed with distilled water. The precipitates was chromatographed on silica gel column using 40:60 ethyl acetate:hexane as eluant (%28 yield), and then re-crystallized from mixture of dichloromethane and hexane.
Yield 28%, mp 68-69 °C. IR (KBr), cm-1: 3077 (s), 2956 (s), 2913 (m), 2871 (m), 1746 (s), 1551 (s), 1484 (s), 1352 (s), 1253 (s), 1192 (s), 1156 (s), 1034 (s), 986 (s), 803 (s). 13C NMR spectrum (CDCl3, 75 MHz), δ, ppm: 176.40, 173.50, 152.82, 133.26, 132.36, 129.14, 128.02, 123.91, 122.71, 120.59. Found,%: C 66.73; H 3.22; N 10.65. C28H18SN4O4. Calculated,%: C 66.39; H 3.58; N 11.06. (m/e) +: 506 (%70)
Bis 1,3,4-oxadiazole (VIIIf). In a round bottom 25 mL flask equipped with magnetic stirrer bis-tetrazole (VIf) (1.78 g, 6.0 mmol) and 3,5-dinitrobenzoyl chloride (VIIb) (3.69 g, 16.0 mmol) were refluxed in 1.5 mL of pyridine for 4 hours. Nitrogen cessation was occurred at 73-75 °C. The mixture was then poured in%5 NaOH, stirred at room temperature for 30 minutes, washed with distilled water, dried at 50-60 °C under reduced pressure, and then re-crystallized from mixture of dichloromethane and hexane.
Yield 24%, mp 214-216 °C. IR (KBr), cm-1: 3077 (s), 2917 (w), 1744 (s), 1623 (s), 1551 (s), 1346 (s), 1256 (m), 1135 (m), 1069 (m), 912 (s), 813 (m). 13C NMR spectrum (CDCl3+DMSO-d6, 100 MHz), δ, ppm: 115.83, 118.93, 121.78, 125.90, 126.27, 127.08, 129.75, 148.49, 151.10, 152.48, 172.20, 173.21. Found,%: C 49.40; H 1.74; N 17.53. C26H12N8O12. Calculated,%: C 49.69; H 1.92; N 17.83. Fragment (m/e) +: 507 (%22).
Bis 1,3,4-oxadiazole (VIIIg). In a round bottom 25 mL flask equipped with magnetic stirrer bis-tetrazole (VIg) (1.50 g, 6.0 mmol), benzoyl chloride (VIIa) (2.25 g, 16.0 mmol) and pyridine (1.27 g, 16 mmol) were refluxed in 5 mL of sodium dried toluene for 4 hours. Nitrogen cessation was occurred at 70-72 °C. Solvent was removed under reduced pressure and the residue was poured into 20 mL of%5 NaOH. The mixture was stirred for 30 minutes, filtered, and washed with distilled water. The precipitates was chromatographed on silica gel column using 40:60 ethyl acetate:hexane as eluant, and re-crystallized from mixture of methanol and water.
Yield 20%, mp 122-123 °C. IR (KBr), cm-1: 3053 (m), 3017 (m), 1525 (s), 1404 (s), 767 (w), 708 (s). (m/e) +: 374 (%12).
Results and Discussion
As a part of a continuous research project for preparation of thermally stable polymers containing 1,3,4-oxadiazole moieties [22,24], in this work, the preparation and characterization of new polynuclear nonfused bis(1,3,4-oxadiazole)s (VIIIa-VIIIg) by Huisgen reaction between bis-(5-oxy-(1H)-tetrazole)s (VIa-VIg) and acid chlorides (VIIa-VIIb) has been reported. The rearrangement takes place between (1H)-tetrazoles and acid chlorides through a mechanism shown in Scheme 2 [25].
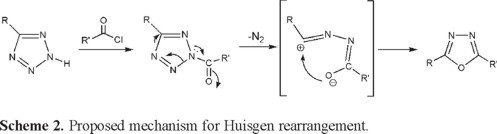
Compounds (VIa-VIf) have been obtained from the corresponding bis-phenols, which converted into bis-cyanoester and then bis-tetrazoles upon reaction with cyanogen bromide and sodium azide, respectively [21-23]. Bis-tetrazole VIg was synthesized from the reaction of suberonitrile with sodium azide in the presence of ammonium hydrobromide in DMF as reaction medium [24].
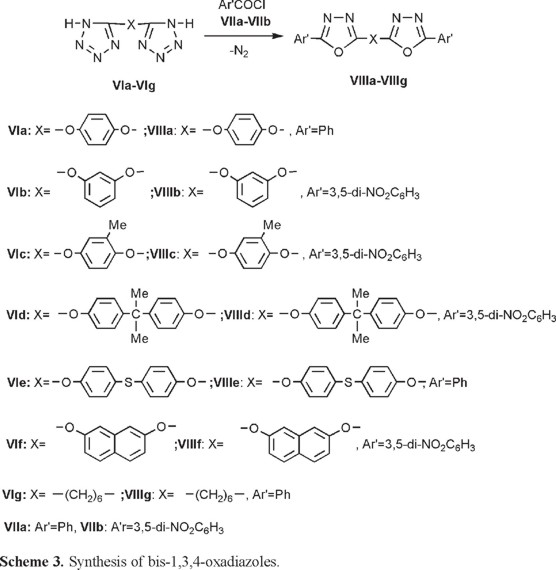
The prepared compounds can be considered as models for polymerization of bis(1H)-tetrazoles with bis-acid chlorides [22]. These polymers are interesting materials due to their high thermal stabilities and optical behaviors. The reaction sequences have been shown in the Scheme 3. As clear from the experimental section, the reaction conditions were not uniform. Compounds VIIIa, VIIIe, and VIIIg were prepared in toluene as solvent and in the presence of pyridine as base, while the best results for the syntheses of compounds VIIIb, VIIIc, VIIId, and VIIIf were obtained in pyridine as solvent.
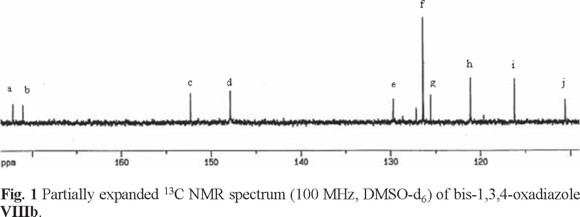
The synthesized compounds were characterized by conventional methods (IR, 13C NMR, elemental analysis, and mass spectrometry). In the IR spectra of the bis-1,3,4-oxadiazole compounds significant changes were observed. Tetrazole ring vibrations which observe at 3000-2480 cm-1 as a complex and strong pattern is completely disappeared, and the -C=N-stretching of oxadiazole ring observes at 1535-1550 cm-1. Fig. 1 shows the typical assigned 13C NMR spectrum of bis-1,3,4-oxadiazole VIIIb. Two oxadiazole ring carbon atoms appear at lower fields (172 ppm and 171 ppm).
Molecular ion peak was only observed in the mass spectra of bis-1,3,4-oxadiazoles VIIIa and VIIIg. In the Fig. 2 and Fig. 3 the mass spectra of the compound VIIIa and VIIIg are shown.
For the other higher molecular weight investigated compounds, molecular ion fragments can be distinguished in the mass spectra. The fragmentation patterns for compounds VIIIa, VIIIb, VIIIc, VIIId, VIIIe, VIIIf, and VIIIg are shown in Scheme 4.
Conclusion
A series of new polynuclear nonfused bis(1,3,4-oxadiazole)s have been synthesized by Huisgen reaction of bis-tetrazoles and aromatic acid chloride, (Scheme 3). Bis-tetrazoles VIa-VIg have been prepared according to previously reported method through two step reaction of bis-phenols with cyanogen bromide and sodium azide. The prepared bis(1,3,4-oxadiazole)s can be considered as model compounds in the syntheses of new thermally stable polymers containing 1,3,4-oxadiazole groups in the main chain.
Acknowledgement
The Research Council of University of Mohaghegh Ardabili (Iran) is gratefully acknowledged for their financial support.
References
1. Comprehensive Heterocyclic Chemistry, Katritzky, A. R., and Ress, C. W., Eds. Oxford: Pergamon, 1984, 6, 427. [ Links ]
2. Saiz, P. G.; Tojal, J. G.; Maestro, M. A.; Arnaiz, F. J.; Rojo, T. F. Inorg. Chem. 2002, 41, 1345-1347. [ Links ]
3. Khan, K. M.; Ullaha, Z.; Rani, P. M., Haider, S. M.; Choudhary, M. I.; Rahman, A.; Voelter, W. Lett. Org. Chem. 2004, 1, 50-52. [ Links ]
4. Vereshchagin, L. I.; Petrov, A. V.; Proidakov, A. G.; Pokatilov, F. A.; Smirnov, A. I.; Kizhnyaev, V. N.; Russ. J. Org. Chem. 2003, 39, 912-917. [ Links ]
5. Adachi, C.; Tsutsui, T.; Saito, S. Appl. Phys. Lett. 1990, 56, 799-801. [ Links ]
6. Adachi, C.; Tsutsui, T.; Saito, S. Appl. Phys. Lett. 1990, 56, 531-533. [ Links ]
7. Mochizuki, H.; Hasui, T.; Kawamoto, M.; Shiono, T.; Ikeda, T.; Adachi, C.; Taniguchi,Y.; Shirota, Y. Chem. Commun. 2000, 1923-1924. [ Links ]
8. Wang, J.; Wang, R.; Yang, J.; Zheng, Z.; Carducci, M. D.; Cayou, T.; Peyghambarian, N.; Jabbour, G. E. J. Am. Chem. Soc. 2001, 123, 6179-6180. [ Links ]
9. Lee, R.-H.; Chen, C.-T.; Yeh, H.-C.; Chan, L.-H. 2002, US Pat. 20020102433A1.
10. Chen, L.; Yang, C.; Qin, J.; Gao, J.; You, H.; Ma, D. J. Organomet. Chem. 2006, 691, 3519-3530. [ Links ]
11. Li, H.-Y.; Li, T.-Y.; Liu, Q.; Xu, Q.-L.; Wang, C.-C.; Zhang, S.; Lin, C.; Huang, W.; Zheng, Y.-X.; Wang, X.-Q. J. Organomet. Chem. 2013, 743, 37-43. [ Links ]
12. Lu, H.; He D. Spectrochim. Acta Part A, 2014, 123, 336-341. [ Links ]
13. Ge, Y. Q.; Jia, J.; Wang, T.; Sun, H. W.; Duan, G. Y.; Wang, J. W. Spectrochim. Acta Part A, 2014, 124, 91-96. [ Links ]
14. Vereshchagin, L. I.; Petrov, A. V.; Proidakov, A. G.; Pokatilov, A. F.; Smirnov, A. I. Russ. J. Org. Chem. 2006, 42, 1049-1055. [ Links ]
15. Maslat, A. O.; Abussaud, M.; Tashtoush, H.; AL-Talib, M. Pol. J. Pharmacol. 2002, 54, 55-59. [ Links ]
16. Maxwell, J. R.; Wasdahl, D. A.; Wolfson, A. C.; Stenberg, V. I. J. Med. Chem. 1984, 27, 1565-1570. [ Links ]
17. Sava, I.; Iosip, M.-D.; Bruma, M.; Hamciuc, C.; Robison, J.; Okrasa, L.; Pakula, T. Eur. Polym. J. 2003, 39, 725-738. [ Links ]
18. Yang N. C.; Lee C. I.; Kim J. K.; Suh D. H. J. App. Polym. Sci. 2004, 92, 3112-3118. [ Links ]
19. Deng L.; Furuta, P. T.; Garon, S.; Li, J.; Kavulak, D.; Thompson, M. E.; Frechet, J. M. J. Chem. Mater. 2006, 18, 386-395. [ Links ]
20. Pradeep, K. M.; Venugopala Reddy, K. R.; Harish, M. N. K.; Chidananda, B.; Madhud B. J.; Mruthyunjayachari, C. D.; Ganesh, S. D. Synth. Met. 2013, 185-186, 79-88. [ Links ]
21. Mansoori, Y.; Sarvari, R.; Zamanloo, M. R.; Imanzadeh, Gh. Russ. J. Org. Chem. 2009, 49, 154-157. [ Links ]
22. Mansoori, Y.; Sarvari, R.; Zamanloo, M. R.; Imanzadeh, Gh. Chin. J. Polym. Sci. 2010, 28, 21-28. [ Links ]
23. Muehlbauer, E.; Grigat, E.; Puetter, R. 1965, DE Pat. 1216880.
24. Mansoori, Y.; Barghian, G.; Koohi-Zargar, B.; Imanzadeh, Gh.; Zamanloo, M.R. Chin. J. Polym. Sci. 2012, 30, 36-44. [ Links ]
25. Huisgen, R.; Sauer, J.; Sturm, H. Angew. Chem. 1958, 70, 272-273. [ Links ]














