Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Journal of the Mexican Chemical Society
versión impresa ISSN 1870-249X
J. Mex. Chem. Soc vol.56 no.2 Ciudad de México abr./jun. 2012
Article
Efficient and Convenient Reduction of Organic Carbonyl Compounds to their Corresponding Alcohols by Zn(BH4)2/Charcoal in THF
Davood Setamdideh* and Mehdi Rahmatollahzadeh
Department of Chemistry, Faculty of Sciences, Mahabad Branch, Islamic Azad University, Mahabad, 59135–443, Iran. *davood.setamdideh@gmail.com
Received November 25, 2011.
Accepted March 28, 2012.
Abstract
Zn(BH4)2 (0.5–1.5 mmol) in the presence of charcoal (1 mmol) reduces a variety of organic carbonyl compounds such as aldehydes, ketones, acyloins, α–diketones and α,β–unsaturated carbonyl compounds to their corresponding alcohols. Reduction reactions were carried out in THF at room temperature in high to excellent yields. The chemoselective reduction of aldehydes over ketones was successfully accomplished with this reducing system. In addition, regioselectivity and exclusive 1,2–reduction of conjugated carbonyl compounds to their corresponding allylic alcohols was also performed in in high to excellent yields with this reducing system.
Key words: Zn(BH4)2, reduction, carbonyl compounds, charcoal, chemoselectivity, regioselectivity.
Resumen
Los compuestos carbonílicos como aldehidos, cetonas, aciloínas, α–dicetonas y compuestos carbonílicos α,β–insaturados se reducen con Zn(BH4)2 (0.5–1.5 mmol) conteniendo carbón (1 mmol) a los alcoholes correspondientes. Este sistema reductor permite la reducción quimioselectiva de aldehidos en presencia de cetonas y la reducción regioselectiva de compuestos carbonílicos insaturados a los alcohols alílicos correspondientes con rendimientos de buenos a excelentes.
Palabras clave: Zn(BH4)2, reducción, compuestos carbonílicos, carbón, quimioselectividad, regioselectividad.
Introduction
Reduction is one of the most fundamental and useful reactions in organic synthesis. During the past decades, sodium borohydride is always playing an important role in modern organic synthesis [1]. This reagent has gained commercial status, in spite of its poor solubility in organic solvents and lesser reactivity, the reagent is inevitably used in excess quantities [2]. So, controlling the reducing power of this reagent has been one of the main interests for organic chemists in many years. To overcome these drawbacks, soluble metal borohy–drides have been realized such as variation of alkali–metal cation and metal cation in the hydride complex, e.g., Li[BH4] [3], K[BH4] [4], Ca[BH4]2 [5], Cu[BH4]2 [6], Zn[BH4]2 [7], Ti[BH4]3 [8], Zr[BH4]4 and others [8b] have been developed. LiBH4, Ca(BH4)2 and Zn(BH4)2 are the modified borohydride agents which have a better solubility in aprotic solvents, so their uses and applications are of the interest in organic synthesis. Among these reagents, zinc borohydride is unique because of (a) Zn2+ is a soft Lewis acid in comparison to Ca2+, Li+ and Na+ which are hard acids and (b) better coordination ability of Zn2+ which imparts selectivity in hydride transferring reactions. Zinc borohydride is moderately stable in ethereal solution and found more applications in organic synthesis [9].
This reagent as a nonconventional hydride transferring agent has been reported to effect very efficient chemo–, regio–and stereoselective reductions in several complex substrates. This reducing agent is neutral and can be used in a range of aprotic solvents such as ether, THF and DME [10]. The literature review shows that the combination systems of H2 or HCOONH4 with Pd, Pt, Ru and Rh supported on carbon (catalytic hydrogenation or transfer hydrogenation) have been widely used for the reduction of functional groups in organic synthesis [11]. The reducing abilities of zinc tetrahydroborate have been reviewed [9]. In addition to using zinc tetrahydroborate alone as a mild reducing agent, its combination systems e.g., Zn(BH4)2/TMEDA [12a], Zn(BH4)2/Me3SiCl [12b] and Zn(BH4)2/TFA/DME [12c] are of interest and have been used for different reduction purposes. Although in the reported methods, showed an activity towards reduction, the literature did not show any application for the use of carbon alone as a promoter in combination with Zn(BH4)2. This subject and our continuous efforts to explore the synthetic utilities of modified borohydride agents [12d–h] encouraged us to investigate reduction of functional groups by the combination system of Zn(BH4)2/charcoal in dry media. Herein, we wish to introduce an efficient and convenient method for reduction of a variety of carbonyl compounds such as aldehydes, ketones, acyloins, α–diketones and α,β–unsaturated carbonyl compounds to the corresponding alcohols with Zn(B4)2/charcoal system in dry–THF at room temperature.
Results and Discussion
To investigate the influence of charcoal on the rate of reductions, we performed a set of experiments on the reduction of benzaldehyde as a model compound by Zn(BH4)2 as shown in Table 1. Our experiments showed that the reduction of benzaldehyde with 1 molar equivalents of Zn(BH4)2 in the absence of charcoal in THF (the more efficient aprotic solvent) was not completed after 1 hour at room temperature, because Zn(BH4)2 is the active specie and easily decomposed. However, we found that by adding 1 molar equivalents of charcoal to the reaction mixture, the rate of reaction was extremely accelerated with the evolution of hydrogen gas. The reaction was completed in 1 min and benzyl alcohol was obtained in 94% yield (Scheme 1).
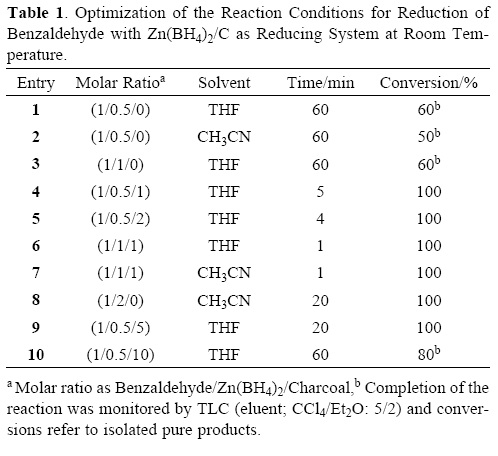
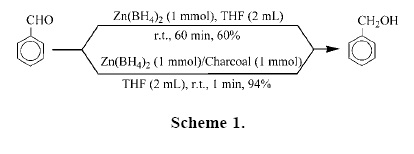
The reaction conditions were optimized and they resulted in using the molar ratio of PhCHO/Zn(BH4)2/charcoal (1:0.5:1) in THF at room temperature is optimal for the complete conversion (Table 1, entry 4). We applied the optimal conditions for the reduction of a variety of aromatic and aliphatic aldehydes to their corresponding primary alcohols. As shown in Table 3, the product alcohols were obtained in excellent yields within 2–8 min.
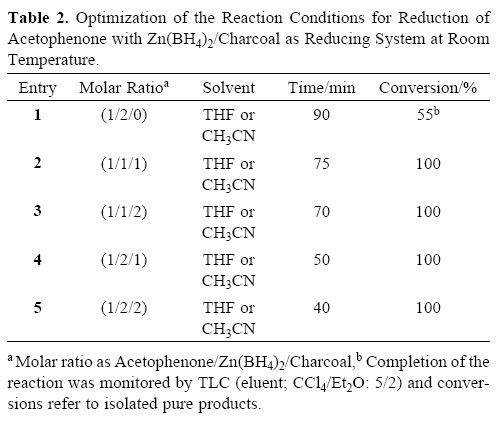
At the next attempt, we turned our attention into the reduction of ketones by the Zn(BH4)2/charcoal system with aceto–phenone as a model compound. The inherent low reactivity of ketones relative to aldehydes led us to perform the reduction with 1 molar equivalents of Zn(BH4)2 and charcoal (1 mmol) in THF at room temperature (Table 2, entry 2). The reaction was completed within 75 min and 1–phenylethanol was obtained in 95% yield. The synthetic utility of the Zn(BH4)2/C system in THF was further examined with the reduction of a variety of aliphatic and aromatic ketones. All ketones were reduced effectively to their corresponding secondary alcohols within 30–240 min at room temperature, as shown in Table 4.
Synthetic utilities of vicinal diols are well known and their preparations from the reduction of acyloins or α–diketones have attracted a great deal of attention. Reduction of α–diketones usually gives a mixture of α–hydroxy ketones and vicinal diols. Selective reduction of α–diketones to acyloins [13] or vicinal diols [14] can be undergone with some chemical or biochemical reagents. Reduction of α–diketones to vicinal diols with modified hydroborate agents is also a subject of interest [12, 15] and this goal was easily achieved by using 1.5 molar amounts of Zn(BH4)2 in the presence of 1 molar amounts of charcoal in THF at room temperature. Reduction reactions were performed efficiently in shorter reaction times (40–50 min) (92–95%) (Table 5, entry 1, 3 and 5). Under different conditions, our attempts to reduce α–diketones into acyloins were unsatisfactory and only vicinal diols were identified as the sole products. In addition, reduction of acyloins to vicinal diols is also a subject of interest in organic synthesis. The applications of non–hydridic reductants [16] and modified hydroborate [12, 15] have also been reported for such reduction. Using Zn(BH4)2 (1 molar amounts) in the presence of charcoal (1 molar amounts) in THF also easily provided this transformation at room temperature. Acyloin compounds were reduced to their corresponding vicinal diols in high to excellent yields with this reducing system (93–94%) (Table 5, entry 2, 4).
The chemoselective reduction of one functional group without affecting the other one is a well–known strategy for preparing molecules with ever–increasing complexity in organic synthesis. Since under the defined conditions, reduction of aldehydes and ketones with zinc borohydride in the presence of charcoal is molar ratio of Zn(BH4)2 dependent, therefore, we thought that this system can have a chemoselectivity towards reduction of aldehydes over ketones. This fact was demonstrated with the selective reduction of benzaldehyde in the presence of acetophenone using 0.5 molar amounts of Zn(BH4)2 in the presence of 1 molar amounts of charcoal at room temperature in THF (Table 6, entry 3) (Scheme 2).
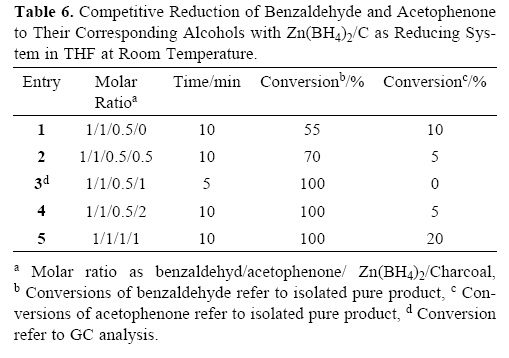

The usefulness of this chemoselectivity of the reduction was further examined with the reduction of benzaldehyde in the presence of other ketones such as benzophenone, cyclo–hexanone or etc, as shown in Table 7.
On the other hand, reduction of unsaturated carbonyl compounds with sodium borohydride, one of the most widely utilized reducing agents, is highly solvent dependent and generally does not result in a useful regioselectivity [17a–b]. To control the reducing potential and selectivity of NaBH4 in the 1,2–reduction of conjugated enones, numerous hydroborate agents have been developed in the following ways: a) by the replacement of hydride(s) with sterically bulky substituents or electron–withdrawing/releasing groups in order to discriminate between the structural and electronic environments of carbonyl groups[17c–f]; b) combination with Lewis acids [17g–i] such as Luche reduction [17j–k] and mixed solvent systems [17a]; c) use of transition metal hydroborates and their new modifications [17l], d) use of quaternary ammonium and phosphonium tetra–hydroborates [17m–n] and finally e) immobilization on an anion exchange resin [17o]. The usefulness of the Zn(BH4)2/C reducing system was further investigated with the regioselective 1,2–reduction of a,β–unsaturated carbonyl compounds. We first examined reduction of cinnamaldehyde as a model compound. The reduction reaction took place with 0.5 molar amounts of Zn(BH4)2 in the presence of 1 molar amounts of charcoal in THF at room temperature. The reaction was completed in 8 min with a complete regioselectivity. The product, cinnamyl alcohol, was obtained in high yield (Table 8, entry 1). This procedure was also applied for the reduction of citral and geraniol was obtained regioselectively in 97% yield. In the next attempt, we examined the reductions of conjugated enones with the Zn(BH4)2/C reducing system. The results showed that our procedure was also regioselective and efficient, but reduction reactions were performed by using 1 molar amounts of Zn(BH4)2 in the presence of 1 molar amounts of charcoal in THF at room temperature. Regioselective 1,2–reductions of benzalacetone, benzalacetophenone and β–ionone were achieved successfully, with high to excellent yields of the corresponding allylic alcohols (Table 8).
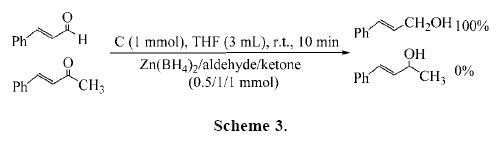
The chemo– and regioselectivity of this procedure were demonstrated by a competitive reduction of cinnamaldehyde over benzalacetone (Scheme 3).
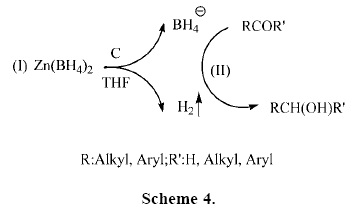
It is notable that due to the low solubility of charcoal in THF, the reaction takes place under heterogeneous conditions. The mechanism for the influence of charcoal is not clear, however, we think that the following factors may play a role in the reduction process: a) as shown in Scheme 4 (step I), we observed that with the addition of charcoal to the reaction mixture (substrate and Zn(BH4)2 in THF), hydrogen gas is slowly liberated in situ. The synergistically generated molecular hydrogen combines with the hydride attack from the borohydride and thus accelerates the rate of reduction reaction (Scheme 4, step II).
Hydrogen gas generation seems to be directly related to the amount of charcoal. In the presence of large amounts of charcoal (5–10 mmol vs 0.5 mmol of Zn(BH4)2, hydrogen gas generation increase dramatically. The use of large amounts of charcoal are not beneficial, because it causes the Zn(BH4)2 rapidly decomposes before the reduction reactions are complete (Table 1, entry 9–10). Also, the chemoselectivity of Zn(BH4)2/C system decreases and the regioselectivity is reduced. b) charcoal is a very fine porous material. Consequently, zinc borohydride, generated hydrogen gas in situ and substrates finely disperse on the surface of charcoal and therefore more interaction produces the efficiency in the reductions.
Conclusions
In this investigation, we have shown that the combination system of Zn(BH4)2/charcoal in THF reduces a variety of carbonyl compounds to their corresponding alcohols in high to excellent yields. Reduction reactions were carried out with 0.5–1.5 molar equivalents of Zn(BH4)2 in the presence of 1 molar equivalents of charcoal in THF. Reduction of acyloins and α–diketones by the Zn(BH4)2/charcoal system also produced efficiently the corresponding vicinal diols in THF. The chemoselective reduction of aldehydes over ketones was accomplished successfully with this reducing system. In addition, regioselectivity of this system was also investigated with exclusive 1,2–reduction of conjugated carbonyl compounds to their corresponding allylic alcohols in high to excellent yields. All reductions were accomplished at room temperature with high efficiency, shorter reaction times and easy work–up procedure. Therefore, this new protocol for Zn(BH4)2 reduction of carbonyl compounds could be a useful addition to the currently available present methodologies.
Experimental
General
All substrates and reagents were purchased from commercially sources and used without further purification. Charcoal was used in activated form (Merck, art. 102186). IR and 1H NMR spectra were recorded on PerkinElmer FT–IR RXI and 300 MHz Bruker spectrometers, respectively. Agilent 6890N gas chromatograph equipped with a FID detector was used for this study. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). Organic layers were dried over anhydrous sodium sulfate. All yields refer to isolated pure products.
A typical procedure for reduction of aldehyedes with Zn(BH4)2/charcoal in THF
Zn(BH4)2 was prepared from ZnCl2 and NaBH4 according to an established procedure [7]. In a round–bottomed flask (10 ml) equipped with a magnetic stirrer, a solution of benzaldehyde (0.106 g, 1 mmol) in THF (2 ml) was prepared. To this solution, Zn(BH4)2 (0.048 g, 0.5 mmol) and charcoal (0.012 g, 1 mmol) were added and the mixture was stirred at room temperature for 5 min. Completion of the reaction was monitored by TLC (eluent; CCl4/Et2O: 5/2). Water (5 ml) was then added to the reaction mixture and it was stirred for another 5 min. The mixture was extracted with CH2Cl2 (3 x 6 ml) and the organic layer was dried over anhydrous Na2SO4. Evaporation of the solvent and purification in a short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Et2O: 5/3) afforded benzyl alcohol (0.102 g, 94%, Table 3, entry 1).
A typical procedure for reduction of ketones to alcohols with Zn(BH4)2/charcoal in THF
In a round–bottomed flask (10 ml) equipped with a magnetic stirrer, a solution of acetophenone (0.12 g, l mmol) in THF (2 ml) was prepared and Zn(BH4)2 (0.095 g, 1 mmol) and charcoal (0.012 g, 1 mmol) were added. The mixture was stirred at room temperature for 70 min. TLC monitored the progress of the reaction (eluent; CCl4/Et2O: 5/2). After completion of the reaction distilled water (5 ml) was added to the reaction mixture and it was then stirred for an additional 5 min. The mixture was extracted with CH2Cl2 (3x8 ml) and the organic layer was dried over anhydrous sodium sulfate. Evaporation of the solvent and purification in a short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Et2O: 5/2) afforded 1–phenylethanol (0.l1 g, 96% yield, Table 4, entry 2).
A typical procedure for reduction of a–diketones and acyloins with Zn(BH4)2/charcoal in THF
In a round–bottomed flask (10 mL) equipped with a magnetic stirrer and charged with a solution of benzil (0.21 g, l mmol) in THF (3 ml), Zn(BH4)2 (0.14 g, 1.5 mmol) and charcoal (0.012 g, 1 mmol) were added. The resulting mixture was stirred at room temperature for 40 min. TLC monitored the progress of the reaction (eluent; CCl4/ Et2O: 5/2). After completion of the reaction, distilled water (5 ml) was added to the reaction mixture and this combination was then stirred for an additional 5 min. The mixture was extracted with CH2Cl2 (3x8 ml) and the organic layer was dried over anhydrous sodium sulfate. Evaporation of the solvent and purification in a short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Et2O: 5/3) afforded hydrobenzoin (0.20 g, 95% yield, Table 5, entry 1).
A typical procedure for competitive reduction of aldehydes and ketones with Zn(BH4)/charcoal in THF
In a round–bottomed flask (10 ml) equipped with a magnetic stirrer, a solution of benzaldehyde (0.106 g, l mmol) and acetophenone (0.12 g, 1 mmol) in THF (3 mL) was prepared. To this solution Zn(BH4)2 (0.048 g, 0.5 mmol) and charcoal (0.012 g, 1 mmol) were added. The resulting mixture was stirred at room temperature for 5 min. TLC monitored the progress of reaction. After 5 min, the reaction mixture was quenched by addition of distilled water (5 ml) and this mixture was then stirred for an additional 5 min. The mixture was extracted with CH2Cl2 (5x8 ml) and the organic layer was dried over anhydrous sodium sulfate. The solvent was evaporated and conversions were determined by GC analysis. (Table 6, entry 3).
A typical procedure for regioselective 1,2–reduction of conjugated carbonyl compounds with Zn(BH4)2/charcoal in THF
In a round–bottomed flask (10 ml) equipped with a magnetic stirrer, a solution of benzylideneacetone (0.146 g, 1 mmol) in THF (2 ml) was prepared and Zn(BH4)2 (0.0.95 g, 1 mmol) and charcoal (0.012 g, 1 mmol) were added. The resulting mixture was stirred at room temperature. TLC monitored the progress of the reaction (eluent; CCl4/Et2O: 5/2). After completion of the reaction within 140 min, distilled water (5 mL) was added and the resulting mixture was then stirred for an additional 5 min. The mixture was extracted with CH2Cl2 (3x8 mL) and the organic layer was dried over anhydrous sodium sulfate. Evaporation of the solvent and purification in a short column chromatography of the resulting crude material over silica gel (eluent; CCl4/Et2O: 5/2) afforded 4–phenyl–3–buten–2–ol (0.l41 g, 95% yield, Table 8, entry 2).
Acknowledgments
The authors gratefully appreciated the financial support of this work by the research council of Islamic Azad University branch of Mahabad.
References
1. (a) House, H. O. In Modern Synthetic Reaction. Benjamine, Menlo Park, CA, 1972. [ Links ] (b) Burk, S. D.; Danheiser, R. L. Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents. Wiley–VCH, New York, 1999. [ Links ] (c) Seyden–Penne, J. Reductions by the Alumino– and Borohydrides in Organic Synthesis. Wiley–VCH, New York, 1997. [ Links ] (d) Hudlicky, M. Reductions in Organic Chemistry. Ellis Horwood, Chichester, 1984. [ Links ]
2. Brown, H. C.; Krishnamurthy, S. Tetrahedron, 1979, 35, 567–607. [ Links ]
3. (a) Brown, H. C.; Narasimhan, S.; Choi, Y. M. J. Org. Chem. 1982, 47, 4702–4708 (b) Oai, [ Links ] K.; Ookawa, A. J. Org. Chem. 1986, 51, 4000–4005. [ Links ] (c) Arase, A.; Nunokawa, Y.; Masuda, Y.; Hoshi, Y. M. J. Chem. Soc. Chem. Commun. 1991, 205–206. [ Links ]
4. Nishiwaki, I.; Fujiyama F. Synthesis 1972, 569–571. [ Links ]
5. Fujii, H.; Oshima, K.; Utimoto, K. Chem. Lett. 1991, 1847–1848. [ Links ]
6. (a) Osborn, M. E.; Pegues, J. F.; Paquette, L. A. J. Org. Chem. 1980, 45, 167–168. [ Links ] (b) Fleet, G. W. J.; Harding, P. J. C. Tetrahedron Lett. 1981, 22, 675–678. [ Links ] (c) Cowan, J. A. Tetrahedron Lett. 1986, 27, 1205–1208. [ Links ]
7. (a) Gensler, W. J.; Johnson, F.; Sloan, A. D. B. J. Am. Chem. Soc. 1960, 82, 6074–6081. [ Links ] (b) Crabbe, P. G.; Garcia, A.; Rius, C. J. Chem. Soc., Perkin Trans. 1, 1973, 810–816. [ Links ]
8. (a) Ravikumar, K.; Baskaran, S. S.; Chandrasekaran, S. Tetrahedron Lett. 1993, 34, 171–174. [ Links ] (b) Marks, T. J.; Kolb, J. R. Chem. Rev. 1977, 77, 263–293. [ Links ]
9. Narasimhan, S.; Balakumar, R. Aldrichim. Acta. 1998, 31, 1926. [ Links ]
10. Ranu, B. C. Synlett 1993, 885–892. [ Links ]
11. Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis, Wiley–VCH: New York, 2001. [ Links ]
12. (a) Kotsuki, H.; Ushio, Y.; Yoshimura, N.; Ochi, M. Bull. Chem. Soc. Jpn. 1988, 61, 2684–2686. [ Links ] (b) Kotsuki, H.; Ushio, Y.; Yoshimura, N.; Ochi, M. J. Org. Chem. 1987, 52, 2594–2596. [ Links ] (c) Ranu, B. C.; Das, A. R. J. Chem. Soc. Perkin Trans. 1 1992, 1561–1562. [ Links ] (d) Zeynizadeh, B.; Setamdideh, D. J. Chin. Chem. Soc. 2005, 52, 1179–1184. [ Links ] (e) Zeynizadeh, B.; Setamdideh, D.; Faraji, F. Bull. Korean Chem. Soc. 2008, 29, 76–90. [ Links ] (f) Setamdideh, D.; Khezri, B. Asian J. Chem. 2010, 22, 5575–5580. [ Links ] (g) Setamdideh, D.; Khezri, B. Asian J. Chem. 2010, 22, 5766–5772. [ Links ] (h) Setamdideh, D.; Khezri, B.; Mollapour, M. Orient. J. Chem. 2011, 27, 991–996. [ Links ]
13. (a) Kreiser, W. Ann. Chem. 1971, 745, 164–168. [ Links ] (b) Pechmann, V. H.; Dahl, F. Chem. Ber. 1890, 23, 2421–2427. [ Links ] (c) Ho, T. L.; Olah, G. A. Synthesis 1976, 815–816. [ Links ] (d) Mori, T.; Nakahara, T.; Nozaki, H. Can. J. Chem. 1969, 47, 3266–3269. [ Links ] (e) Mayer, R.; Hiller, G.; Nitzschke, M.; Jentzsch, J. Angew. Chem. 1963, 75, 370–373. [ Links ] (f). Rubin, M. B.; BenBassat, J. M. Tetrahedron Lett. 1971, 12, 3403–3406. [ Links ]
14. Imuta, M.; Ziffer, H. J. Org. Chem. 1978, 43, 3530–3532. [ Links ]
15. Zeynizadeh, B.; Shirini, F. J. Chem. Res. 2003, 335–339. [ Links ]
16. Blomquist, A. T.; Goldstein, A. Org. Synth. Coll. Vol. IV, 1963, 216–217. [ Links ]
17. a) Varma, R. S.; Kabalka, G. W. Synth. Commun. 1985, 15, 985–990. [ Links ] b) Nutaitis, C. F.; Bernardo, J. E. J. Org. Chem. 1989, 54, 5629–5630. [ Links ] c) Krishnamurthy, S.; Brown, H. C. J. Org. Chem.1975, 40, 1864–1865. [ Links ] d) Krishnamurthy, S.; Brown, H. C. J. Org. Chem. 1997, 42, 1197–1204. [ Links ] e) Kim, S.; Moon, Y. C.; Ahn, K. H. J. Org. Chem. 1982, 47, 3311–3315. [ Links ] f) Corey, E. J.; Becker, K. B.; Varma, R. K. J. Am. Chem. Soc. 1972, 94, 8616–8618. [ Links ] g) Fuller, J. C.; Stangeland, E. L.; Goralski, C. T.; Singaram, B. Tetrahedron Lett. 1993, 34, 257–260. [ Links ] h) Ganem, B. J. Org. Chem. 1975, 40, 146–147. [ Links ] i) Fortunato, J. M.; Ganem, B. J. Org. Chem. 1976, 41, 2194–2200. [ Links ] j) Luche, J. L. J. Am. Chem. Soc. 1978, 100, 2226–2227. [ Links ] k) Gemal, A. L.; Luche, J. L. J. Am. Chem. Soc. 1981, 103, 5454–5459. [ Links ] l) Ravikumar, K. S.; Baskaran, S.; Chandrasekaran, S. J. Org. Chem. 1993, 58, 5981–5982. [ Links ] m) Firouzabadi, H.; Adibi, M. Synth. Commun. 1996, 26, 2429–2441. [ Links ] n) Firouzabadi, H.; Adibi, M. Phosphorus, Sulfur, Silicon Relat. Elem. 1998, 142, 125–147. [ Links ] o) Sande, A. R.; Agadale, M. H.; Mane, R. B.; Salunkhe, M. M. Tetrahedron Lett. 1984, 25, 3501–3504. [ Links ]














