Introduction
Phytoestrogens are chemical estrogen-mimicking compounds, among which isoflavones, coumestans and lignins are best known, being ginestein, coumestrol and enterolactone the most representative examples of each group.1,2,3 These compounds act either as agonists or antagonists of steroidal hormones, depending on the dose used.4,5 This apparently contradictory action is due to their capacity to bind with less affinity to alpha estrogen receptors in the uterus, the mammary gland, the cardiovascular system and bones; and with more affinity to beta estrogen receptors in the prostate, ovaries, testes, urinary tract, lymphoid tissue and some regions of the brain, like the hypothalamus.6
Phytoestrogens constitute one of the most important groups of natural endocrine disruptors since they are substances capable of altering the normal homeostasis of the endocrine system, causing imbalance among estrogens, androgens, progestogens and thyroid hormones. These compounds may interfere with the production, release, transportation, metabolism, specific receptors binding, biological action, or removal of the hormones responsible for the homeostasis and development regulation.7
Neonatal exposure to phytoestrogens such as genistein and coumestrol causes alteration in weaning time, anogenital distance, puberty initiation, preoptic dimorphic nucleus size in the hypothalamus, pituitary response to the gonadotropin-releasing hormone (GnRH), reproductive hormone concentration and reproductive organs mass.8,9,10 In addition, exposure to these compounds during pregnancy and lactation, both in mice and male rats, was found to produce the above mentioned effects in relation to dose and exposure time.11,12,13,14,15
In previous works,16 the authors showed that intake and subcutaneous administration of Prosopis torquata fruit powder and extract, respectively, in the neonatal period, caused morphological and histological changes in the ovary, and an earlier initiation of the reproductive stage in female Wistar rats. These results led us to suggest that the methanolic extract used contains potential estrogen-mimicking compounds.
The purpose of this work was to study the effects of methanolic extract of Prosopis torquata fruit in the reproductive biology of male Wistar rats. To achieve this purpose, the extract was administered subcutaneously in the neonatal period, and the reproductive performance indicators were analysed in the puberty period.
Materials and methods
Prosopis torquata fruits were collected from the ground during the dry season (winter). The collection site was Sierra de las Quijadas National Park, located in the northwest of the province of San Luis, Argentina (32 ° 20 'and 32 ° 47' S and 67 ° 10 'and 66 ° 58' W). This region is an ecotone between the Chaco and Monte phytogeographic provinces,17,18 and it is one of the driest areas of the province, with an annual rainfall regime of 250 mm.19 A specimen of the plant is stored as # 6021 in the Herbarium of Universidad Nacional de San Luis.
The fruits were placed in an oven at 40 oC until a constant dry weight was achieved and were pulverized to fine powder in a ring mill. The powder was subjected to an extraction method by cold maceration with solvents of increasing polarity (petroleum ether, dichloromethane, ethyl acetate and methanol). The methanolic extract with the highest flavonoid glycoside concentration was used in the assay.20,21,22
Every experiment was carried out according to the protocol revised and approved by CICUA (Res. No 1126/08 and 1127/08). Thirty female Wistar rats from the Bioterio of the Universidad Nacional de San Luis were used, and daily exudates were carried out during until the estrous cycle was stable. The proestrus rats mated during the night, and the presence or absence of spermatozoids was verified the following morning. The sperm presence in the exudates was considered day 0 of pregnancy. Pregnant rats were distributed in two groups: I) those fed with a commercial diet containing phytoestrogens from soy bean flour, and II) those fed with phytoestrogen-free diet. Immediately after birth, mothers and male offspring were distributed in four groups: animals in group I continued with the commercial food, were considered positive control of the phytoestrogen intake and were administered vehicle s.c. (Cf n=7). Animals in group II were subdivided in 3 groups: a) positive control (17ß-estradiol s.c. (E n=5)), b) negative control (Ve n=7) and c) treatment (methanolic extract s.c. (MEx n=6).
All the animals were kept under a light-controlled environment (12:12), controlled temperature (23-25 oC) and with food intake and water ad libitum. The phytoestrogens-free diet was carried out in agreement with "Nutrient Requirements of Laboratory Animals of Institute for Laboratory Animal Research". The plant protein was replaced by fish protein. The Cf diet was a commercial food for rats and mice, containing soy as protein content supplement. Soy-based food contains phytoestrogens in biologically active amounts.23,24,16
Methanolic extract, estradiol and vehicle were administered subcutaneously after the third day of birth and for five days (perinatal period).
The MEx group (n=6) was administered 1.7 mg/100g weight/ day of the methanolic extract, and the E group (n=5) was administered 2 mg of 17ß-estradiol/100g weight/day (Fluka AG, CH-9470 Buchs) suspended in the vehicle (0.020 ml of sesame oil and 0.175 ml DMSO). In this group, only three animals (n=3) survived. The Cf and Ve groups were administered only vehicle.
The experiment ended after 72 days of age. All the animals were sacrificed by cardiac puncture under anesthesia. The animal weight and the weight of the reproductive organs: testes and epididymis were registered. The right-side organs of the epididymis were kept for histological studies, and the left- side organs were immediately placed on phosphate-buffered saline (PBS) for conservation and later sperm release. Serum was extracted and stored at -20 oC until testosterone and androstenedione determination.
The serum concentrations of androstenedione and testosterone were determined by specific RIA kit (radioimmuno assay). The testis and the epididymis cauda (mature sperm storage site) were used for the histological study of the reproductive organs. The tissues were immersed in Bouin's fixative followed by dehydration in increasing ethanol levels. After that, the different organs were included in paraffin, and 5 μm cuts were done in a sled microtome (Reichert Jung Hn 40). Finally, they were stained with hematoxylin-eosin in order to examine the histological characteristics of the different tissues and to identify the different cell types in the optical microscope.
Sperm was collected in the epididymis cauda and was kept in PBS for its conservation and subsequent processing. Appropriate dilutions were done from this suspension according to the sample quantity obtained for the sperm quantification in Neubauer chamber. Sperm motility degree was determined and then classified into rapid progressive motile sperm, moderate progressive motile sperm, non progressive motile sperm and non motile sperm. Sperm morphology in the different groups was analyzed using hematoxylin- brilliant green, and sperm vitality was assessed using the eosin test.
The treatment effects (E, MEx, Cf, Ve) on body, testes and epididymis weight, and on testosterone and androstenedione serum concentrations were assessed using one way analysis of variance (ANOVA) on day 72. ANOVA was used to determine the differences on the same variables on days 49, 55 and 72. Tukey's test was used to determine differences among treatments or days. P values lower or equal to 0.05 were considered significant.
Results and discussion
Body, testes and epididymis weight increased on days 49, 55 and 72 for all the treatments (F2,19=5,94 p= 0,010; F2,19=6,3 p= 0,008 and F2,19=17,07 p= 0,001, respectively). These differences are due to the mass increase on days 49 and 72. Body mass was affected by estrogen (Figure 1A). The difference of testes weight between treatments disappeared when adjusted by body weight (F2,19=0,264 p= 0,77), but it remained for the adjusted epididymis weight (F2,19=15,14 p= 0,001). Treatments had an effect on testes weight on day 72 ((E) not included) (F2,8=7,44 p= 0,016), due exclusively to a difference between Cf and MEx (Tukey's test; p<0,013) because Ve does not differ with the other treatments (Figure 1B) There were no differences between treatments for epididymis weight (F2,8=3,43 p= 0,08) (Figure 1C), nor when this variable was adjusted to body mass (F2,8=1,13 p= 0,36). The statistical analysis of the results obtained for group E was not done because, as previously mentioned, only three animals survived, and they were sacrificed on days 49, 55 and 72. However, we decided to show these results because the tendency of the estrogens disrupting effect on the reproductive organs allowed us to compare them with the results obtained for the MEx and Cf groups.
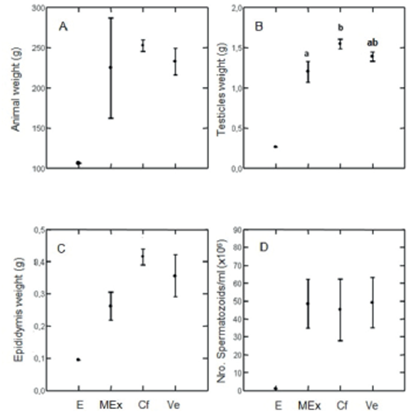
Figure 1 A) Animal body weight, B) Testicles weight (g), C) Epididimys weight (g) y D) N° spermatozoids/ml (x 106), of animals 72 days of age in experimental groups Ve, Cf, MEx y E. Tukey Test was used. Values of p, equal or lower tan 0,05 were considered significant. Letters a y b mean significant differences between groups. Values are expressed as mean ± one standard error.
Sperm count increased from day 49 to 72 from 0 to 48,7 ± 20,0 sperm/ml x 106 in all treatments. In E, sperm count was only 1 sperm/ml x 106. There were no differences between treatments at day 72. (F2,8=0,10 p<0.9) (Figure 3D). These values correspond to all the groups except group E. The testosterone levels increased from day 49 to day 72 in all the treatments (F2,19=3.61 p= 0,046) (Figure 2A). No significant differences were observed for androstenedione on the days mentioned (F2,19=0,59 p= 0,56) (Figure 2B). On day 72 no significant differences were observed between treatments for any of the studied hormones: testosterone (F2,8=0.17 p= 0,84) and androstenedione (F2,8=1.93 p= 0,20) (Figure 2 and B respectively).
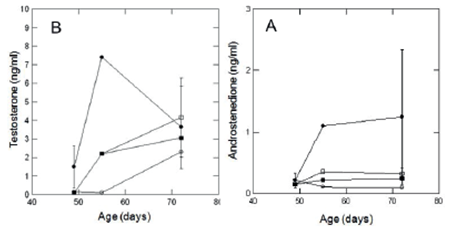
Figure 2 Testosterone levels (A) and Adrostenedione levels (B) in ng/ml of rats of 49, 55 and 72 days of age. Emptied circle - Estradiol (E), Filled circle Methanolic Extract (MEx), Emptied square Comercial diet with phytoestrogens (Cf) and Filled square Vehicle (Ve). Values are expressed as mean ± one standard error.
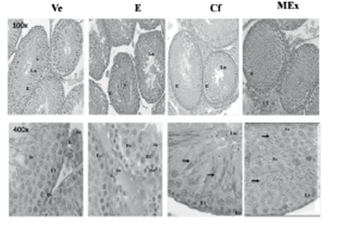
Figure 3 Testicular histology at 72 days of age. H & E 100x and 400x. E: germinal epithelium; SC: Sertoli cell; Eo: spermatogonia; E1: primary spermatocytes; Sr: round spermatid; L: Leydig cell; arrow: elongate spermatids; bv: blood vessel; Lu: lumen; *: desquamated epithelial cell; CT: connective tissue.
The testes histological cuts on day 72 showed a complete spermatogenesis with elongated spermatids in every group, except in group E. Group Ve and group Cf exhibited well- developed seminiferous tubules, appropriate to the analyzed age (Figure 3). The animals treated with E and MEx exhibited disorganization of the germinal epithelium, and the animals treated with MEx presented elongated spermatids (Figure 3).
In previous works in our laboratory, a potential estrogen- mimicking effect of the methanolic extract was observed in female Wistar rats in the proliferative phase (precocious puberty) and in the secretory phase (corpora lutea hypertrophy). In this work, only the male rats treated16 with estradiol (group E) and methanolic extract (group MEx) for five days on the perinatal stage exhibited disorganization in the testicular germinal epithelium on day 72 (Figure 3).
These results suggest that the extract has compounds with a potential disrupting effect comparable to the estradiol dose used. The disorganization of the testicular epithelium might be explained by the functionality of the Sertoli cells, which proliferate on the perinatal stage and respond to the follicle stimulating hormone (FSH), thus regulating spermatogenesis. The presence of exogenous estradiol or any other compound with potential estrogen-mimicking effect, depending on concentration and exposure time, might inhibit FSH secretion or vary its pulse frequency during the embryonic or perinatal stage, thus modifying the Sertoli cells activity,25,26,15 expressed by the disorganization of the germinal epithelium of the seminiferous tubules. However, disorganization in the testicular germinal epithelium is not observed in group Cf, as observed in group MEx, probably because the phytoestrogens incorporated by the diet circulate as conjugates, and as they are less biologically active, they minimize the feedback inhibition of FSH.27
The weight of the animals and of the studied reproductive organs decreased in group E in relation to the other groups. This result might be due to the estradiol disrupting effect on the nervous system, which causes an anorexic effect made evidentin the sensibility decrease of the adipose tissue to insulin.28 The phytoestrogens concentrations of the commercial diet (Cf) and of the methanolic extract compounds (MEx) did not cause any variation on the animal weight and hormone values.
Significant changes were observed on the testicular and epididymis weight (Figure 1B and C) in relation to the control group (Ve). These results are in agreement with the histological changes observed (Figure 3 and 4),29 and they indicate that the magnitude of the disrupting effect might depend on the dose,8 and the way of administration, orally for group Cf and subcutaneously for MEx. Numerous studies on the chronic exposure to phytoestrogens during gestation and lactation of mice, rats and male rabbits have demonstrated that these animals exhibit lower body weight and reduced anogenital distance in puberty when compared with the controls without phytoestrogens. Some phytoestrogens and estradiol itself have proven to have anorexigen effects on rats, dramatically altering body mass.11,8,28,30,31
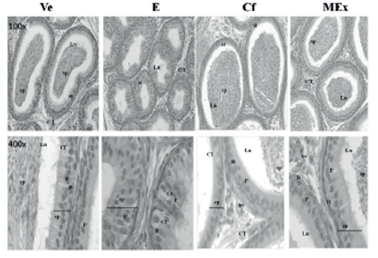
Figure 4 Histology of the epididymis at 72 days of age. H & E 100x and 400x. Lu: lumen, sp: sperm. *: desquamated epithelial cell. P: principal cell. B: basal cell. Cl: clear cell. ep: epithelium, CT: connective tissue; bv: blood vessel.
Similarly, no significant changes were observed in the hormone concentrations in the four experimental groups, which might be due to the fact that the disrupting effect was not strong enough to compromise the normal concentrations of testosterone and androstenedione in blood and in sperm production. However, the morphological changes observed when comparing the groups (Figures 3 and 4) indicate a disrupting effect of the extract which might involve spermatogenesis, regulated by FSH, as hypothesized at the beginning of the discussion.
Modifications involving the morphology of the reproductive organs of prepubertal rats exposed to Prosopis torquata fruits extract are observed in the testicular tissue during the perinatal period. In testes, these modifications are observed in the MEx and E groups. In both groups, the histological changes, disorganization of the seminiferous tubules germinal epithelium, the reduced lumen and a scarce presence of elongated spermatids might indicate that the fruit extract and estradiol exert a disrupting effect on the reproductive organs (Figure 3). The main histological change found is the epididymis epithelium height (Figure 4).32,33
These results are in agreement with those obtained in our previous studies with female Wistar rats,16 which show a marked effect on the tissue organization of the reproductive organs (corpora lutea hypertrophy, ovarian epithelium disorganization and early vaginal opening). No significant variations were found in the hormone concentrations in studies carried out with male and female rats. However, morphological changes were observed in the tissues receiving the action of FSH.
Although the mechanisms causing the previously mentioned effects are still unknown, the action of these compounds on the physiological mechanisms of endocrine regulation has been widely demonstrated. Thus, it is necessary to take them into account in investigations about hormonal processes which require animals fed with phytoestrogen compounds diets.
Conclusions
Male rats treated with methanolic extract of fruits of P. torquata (group MEx) exhibited disorganization in the testicular germinal epithelium, disorganization of the seminiferous tubules germinal epithelium, reduced lumen and a scarce presence of elongated spermatids. This effect is comparable to the effects caused by estradiol. Significant changes were observed on the epididymis weight in animals on MEx. There were no observable effects on hormones levels. We suggest that levels of phytoestrogens in the extract are high enough to produce a chronic effect after 72 days of exposure on the reproductive system of male rats, potentially altering reproductive function. However we do not known the bioavailability of the phytoextrogens in MEx, nor the specific concentration of each phytoestrogen in the extract. The methanolic extract of P. torquata fruits is a natural disruptor of the reproductive system of rats, with proven disrupting effects both in female and male Wistar rats.











 nueva página del texto (beta)
nueva página del texto (beta)


