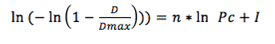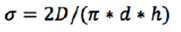Introduction
The tablet is the preferred pharmaceutical dosage form because of its advantages for the patient, a precise dosing, easy mass production and cost effectiveness. The pharmaceutical industry has shifted toward direct compression to produce tablets. This method provides advantages such as less number of processing steps, a simplified validation, removes heat and moisture from the process and improves the stability of the drug. However, the success of the direct compression process is especially affected by material properties such as fluidity and compactability.1
All medicines depend on excipients to stabilize and deliver their active ingredients. The quality and effectiveness of the medicine depends greatly on how the excipient performs. It is desirably good flowability, high compactibility, good recompactability for dry granulation, good blending properties without drug segregation as well as physical and chemical stability, including drug chemical compatibility.2
Pharmaceutical lactose is one of the most versatile excipients and is available in numerous physical forms. Lactose in solid form can be either in a crystalline state or in an amorphous state. Crystalline lactose can exist in a number of distinct forms. Most well known are α-lactose monohydrate and β-lactose. In addition, two crystalline anhydrous α-lactose types are known, a stable and an unstable (hygroscopic) form.3
Spray dried lactose exhibits very good flow and compaction properties but is sensitive to the effect of relative humidity. Increasing the relative humidity can cause an increase in the water content of amorphous lactose and with this, an increase in plasticity and compactibility. Granulated lactose is a very good alternative to spray dried lactose because it contains no amorphous component. It is essentially non-hygroscopic and is therefore extremely robust in direct compression.4
A limitation of lactose is the propensity of the material to react with primary and secondary amine drugs via a Maillard reaction to produce colored products.5 It has been observed that the melting peaks in DSC curves of some drugs such as rifampicin, isoniazid, ethambutol and pyrazinamide, change when mixed with lactose. This suggests physicochemical interactions. With this technique, it was also observed the classical interaction of a reducing sugar such as lactose with isoniazid and pyrazinamide.6 On the other hand; the DSC and TG curves of the physical mixtures of captopril and lactose monohydrate and SuperTab demonstrated there is no alterations in the thermoanalytical profiles of the drug.7
From the four solid forms of lactose: α lactose monohydrate, anhydrous α-lactose, anhydrous β-lactose and amorphous lactose, anhydrous lactose is preferred for direct compression because of its brittle nature, the presence of more spherical particles and rougher surfaces, being relatively insensitive to over lubrication effects of magnesium stearate.8
It has been observed that the batch-to-batch variability of different types of lactose from different vendors is low in terms of compactibility, however, some differences were observed between the suppliers. This has been partially attributed to a different content in fines. A higher content in fines filling up inter-particle voids, reducing particle rearrangement during compression and therefore, requiring higher pressures to produce particle deformation.2
Compared to some other direct compression excipients, lactose and microcrystalline cellulose (MCC) display moderate flowability while dicalcium phosphate (DCP) flows remarkably. On the other hand, MCC shows extremely good compact hardness, lactose moderate tablet hardness and DCP poor tablet hardness.9
Pharmatose® DCL-11, a spray-dried grade of lactose monohydrate and Lactose NF 312, a granulation grade of lactose monohydrate are materials compacting by a combination of plastic deformation and brittle fracture. The deformation properties are estimated medium for spray-dried lactose and low for granulated lactose. Both types of lactose display a trend to an increasing elasticity with increasing compression pressure.10
Spray dried lactose possesses bad compactibility and a low dilution potential when compared to other dry binders. However, it possesses very good flow properties, which are attributed to a larger particle size and sphericity. It is assumed that this type of lactose compacts through fragmentation and plastic deformation, depending on the particle size. It is thought that above 45 μm fragmentation occurs while smaller particles undergo plastic deformation, therefore showing certain sensitivity to lubricants.11
SuperTab 24AN is an agglomerated anhydrous lactose obtained from milled samples processed by fluid bed to give agglomerates of anhydrous lactose (patent EP1851344, US2009/0081308). SuperTab 24AN is supposed to combine the benefits of granulated and anhydrous lactose: excellent flow, quick disintegration, superior compaction properties, minimum water content, low hygroscopicity and low lubricant sensitivity.12
The SuperTab 24AN has been used to improve the powder flowability of other excipients and formulations. The coarse grades of MCC have been reported to exhibit the minimum flow consistent with high speed tableting whereas SuperTab 24AN, as a direct compression lactose, exhibits better flow than MCC.13
A pharmaceutical tablet is a large cluster of particles, held together by bonds active between external particle surfaces. In this sense, the formation of a tablet is based on materials properties that make possible the formation of these bonds between particles.
The compaction properties of pharmaceutical powders are characterized by their compressibility and compactibility.
Compressibility is the powder's ability to deform under pressure, and compactibility is defined as the capability of a material to form coherent agglomerates or mechanically strong compacts after compression. Compactibility has been quantified in several different ways; most often, it is expressed as the slope of the curve of the tensile strength versus compression pressure (compactibility profile).14-15
Powder compression is an attractive method of analysis; however, variations in loading conditions during compression as well as in the data handling may affect the value of the derived compression parameters.
Calculated parameters to define the compaction process allow the reduction of compactibility to a numerical value that characterizes the materials. However, Compactibility defined by different parameters such as the maximal attained tablet hardness (Dmax), the tablet tensile strength (σ), the specific crushing strength (SCS) and a parameter derived from SCS, Cp or a dimensionless compactibility parameter do not allow the identification of an all-purpose compactibility magnitude.16
This article explores eventual advantages of SuperTab 24AN over some other types of lactose used for direct compression. The excipients are evaluated as individual powders, in mixtures with a lubricant and in mixtures with model drugs, amoxicillin and captopril. This article does not aim the discussion of compaction models but the use of two of them. Compactibility data examined with an empiric model derived from fitting the experimental points in a sigmoid mathematical model based on the Weibull distribution and with a model based on a physical parameter such as the compacts density.
Materials and methods
Lactose anhydrous NF, batch 1320014992, Lactopress anhydrous, batch: 636524; SuperTab 21AN, batch: 10444192; SuperTab 24AN, batch: 10605538; microcrystalline cellulose type 102, Helmcel 200, batch 20535; dicalcium phosphate, batch: 3156; stearic acid, batch: K32003061; magnesium stearate, batch: D00252. All obtained from Helm-México. The drugs amoxicillin trihydrate batch: MB2433 and captopril batch: 1001934001 were obtained from Química Alkano. The drug and the excipients were used as received.
Methods
The drug and the excipients were evaluated as individual powders and as mixtures with a lubricant. The different types of lactose were mixed for 30 min in a tween shell blender with stearic acid in proportions of 1% and 2%. Corresponding amounts of the materials were weighed to obtain 60 g of mixtures of amoxicillin with an equal proportion of every excipient, adding 1.0% magnesium stearate. The drug and the lubricant were mixed gently in a mortar for 20 min. Thereafter, the different types of lactose were added, mixing in a twin shell blender or V-blender for 30 min. The same was made with captopril. However, before making tablets a premix (lactose-captopril-stearate) was completed and passed through a sieve number 20, then the mixture was further mixed in a tween shell blender or V-blender for 30 minutes.
Compactibility
Tablets weighing 200 mg were compacted for 10 s in a hydraulic press, at a series of compaction pressures from 27 MPa to 272 MPa, using 8 mm circular flat-shaped punch and die. Tablet crushing strength was measured in triplicate, registering the results as an average. For this purpose, a tablet hardness tester Erweka TBH30 was used. The procedure involved placing each tablet diametrically between two flat surfaces and applying pressure until the tablet breaks down.
Results and discussion
Compactibility of direct compression excipients
Compactibility in the pharmaceutical literature has been usually referred as tablet hardness. Hardness tests have been widely used in the pharmaceutical industry to examine solid dosage forms since the 1970's.
Tablets should have sufficient strength to resist wear and breakage during handling or processing, particularly during packing. For this reason, the hardness of the tablets is important and with practical relevance.17
Under the current regulatory environment and the quality by design (QbD) paradigm, the functionality of an excipient emphasizes performance. It seeks to identify, assess and control the critical attributes of materials, to ensure consistent product performance.18 Compactibility curves describing the relationship between hardness or tensile strength of the tablets and the compaction pressure used to obtain them can be considered to be in conformity with this purpose.
The compactibility of different pharmaceutical materials or a different physical presentation of the same has been defined with regression parameters of Eq. 1.19-20
Where: D is the hardness or crushing strength of the tablets, Dmax is the greatest tablet hardness attained, Pc is the compaction pressure, n is the slope of the curve and I is the intercept of the curve.
The physical parameters considered in this model are the separation between the particles and the surface of the particles used effectively to form inter-particle bonds. At the beginning, the particles are separated without possibilities to form permanent bonds. As the compaction pressure increases some particle are close enough to establish binding points. Progressively the number of particles and the surface used to forms bonds increases up to a limit given by the available surface area and visco-elastic behavior of the particles. Thereafter the tablet mechanical strength level out.
Figure 1 shows the experimental data and the calculated compactibility curve for 8 mm flat faced tablets weighing 200 mg of SuperTab 24AN. The same figure shows, as a reference, the experimental data and the calculated compactibility curves of microcrystalline cellulose (MCC) and dicalcium phosphate (DCP). The drop in tablet hardness of SuperTab 24AN, at intermediate compaction pressures, is attributed to compaction without the necessary lubricant.
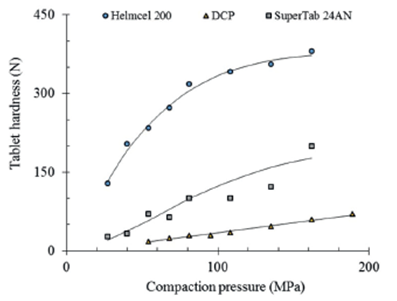
Figure 1 Comparative compactibility of SuperTab 24AN against microcrystalline cellulose (MCC) and dicalcium phosphate (DCP), considering the tablet hardness or crushing strength.
As can be seen, the compactibility of SuperTab 24AN is in between of the well-known direct compression excipients MCC and DCP. Considering a compaction pressure of 135 MPa, the calculated tablet hardness of SuperTab 24AN (150 N) is about three times greater than that of DCP (48 N) and about a half (43%) of that of MCC (349 N).
The above-mentioned data are similar to that observed by bilayer tablets. That study showed a compactibility of SuperTab 24AN lubricated with 0.5% magnesium stearate of about 35% of that of MCC. In this publication, SuperTab 24AN was observed to compact better than other grades of lactose.21
Another approach to study the consolidation of powders is to observe the compactibility of materials by determination of the tablet hardness and relating the compact strength with the relative density or porosity. In this method, the parameter used to characterize the mechanical behavior of pharmaceutical tablets is the tensile strength. The tensile strength of the tablet (σ) as expressed by equation 2:22
Where: D is the hardness or crushing strength of the tablets, d is the diameter and h is the height or thickness of the tablet. The logarithm of the tensile strength against the relative density of the tablet would produce a linear relationship. This treatment of the experimental data allows calculating the compactibility as the tensile strength of tablets with a porosity of zero or a relative density of one (σ' or σmax).
Figure 2 shows the experimental data and the calculated regressions for the relationship between the tensile strength of tablets and their relative density or the relative specific gravity of those materials displayed on figure 1.
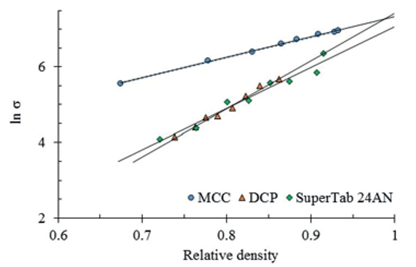
Figure 2 Comparative compactibility of Supertab 24AN against microcrystalline cellulose (MCC) and dicalcium phosphate (DCP), considering the tablet tensile strength (σ).
Considering the tensile strength, the relative compactibility among these three excipients is different as that determined using the tablet hardness. The highest tensile strength corresponds to DCP (σmax=1693 N/cm2), followed by MCC (σmax=1537 N/cm2) and thereafter by SuperTab 24AN (σmax=1167 N/cm2). Although these results are physically related to tablets characteristics through the compacts porosity, they do not allow the inference of the current excipients functionality. The results do not correlate with the tablets mechanical strength expressed as tablet hardness.
Another way to use the model expressed by equation 2 is the calculation of the tensile strength at a given tablet porosity. In this manner, the by regression calculated values are supported by the entire experimental data. At a relative density of 0.8, MCC displays the highest tensile strength (σ0.8=520 N/cm2) followed by DCP and SuperTab 24AN (σ0.8=134 N/cm2), both with the same value. These results still do not correlate with and display different values and proportions than those showed by the tablet hardness.
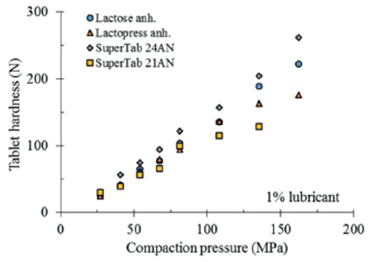
The compactibility of four different types of lactose is depicted in figure 3. As can be seen, the results obtained from pure excipients do not allow the perception of a different compactibility among anhydrous lactose NF, Lactopress anhydrous, SuperTab 21AN and SuperTab 24AN.
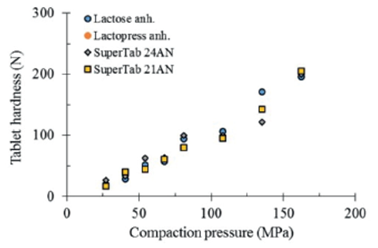
Figure 3 Comparative compactibility of different types of lactose, tablets of pure excipients compacted without lubrication.
Effect of lubrication on compactibility of direct compression excipients
Lubrication is a key component in manufacturing pharmaceutical solid dosage forms. Lubricants are essential to achieve robust formulations. The optimal concentration and mixing of the lubricants are to be taken into consideration because both parameters influence the performance of pharmaceutical products and processes. High lubricant concentrations and over-mixing often result in adverse effects on products and processes, including the reduction of tablet hardness.23
Figure 4 displays the effect of two different proportions of a lubricant, stearic acid, on the tablet hardness of different direct compression excipients. MCC still displays the maximal tablet hardness after addition of 2% stearic acid, followed by SuperTab 24AN and DCP. Although the comparative compactibility keeps the same order as that of unlubricated materials, the individual compactibility underwent some changes. While MCC decreased its compactibility in about 10%, SuperTab and DCP increased their compactibility in about 30% and 20% respectively.
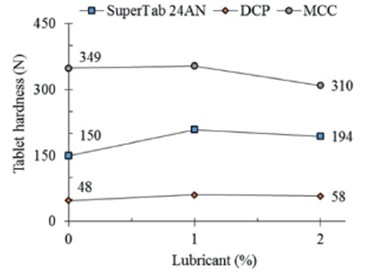
Figure 4 Effect of lubrication with stearic acid on compactibility of different direct compression excipients. Calculated values for tablets compacted at 135 MPa.
Figure 5 depicts the comparative compactibility of the different types of lactose lubricated with 1% stearic acid. Contrarily as described in figure 3, the different types of lactose display different compactibilities. This is graphically more evident as the compaction pressure increases.
Figure 6 depicts the compactibility of the different types of lactose containing 2% stearic acid. The regression curves were calculated according to equation 1. It is evident a greater compactibility of SuperTab 24AN over other types of lactose. Considering a compaction pressure of 162 MPa, SuperTab 24AN (D162=231 N) shows an about 90% greater compactibility than SuperTab 21AN (D162=123 N) while Anhydrous Lactopress (D162=171 N) and anhydrous lactose NF (D162=201 N) are in between. Tablets compacted at 81 MPa display a lesser difference in compactibility between SuperTab 24AN and SuperTab 21AN (75%).
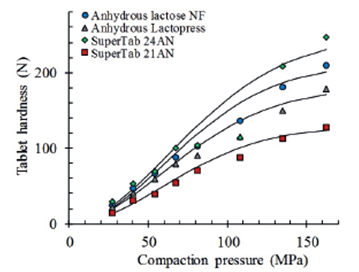
Figure 6 Comparative compactibility of tablets of different types of lactose containing 2% magnesium stearate. Regressions calculated according to equation 1.
Bilayer tablets have observed a similar smaller tablet crushing strength for SuperTab 21AN tablets, compared to SuperTab 24AN tablets. SuperTab 24AN tablets displayed an about 40- 50% higher compactibility than SuperTab 21AN tablets. This occurred in tablets obtained in a range of compaction pressures from 10 kN to 15 kN and with formulations containing 95.5% of different types of lactose, 4% croscarmellose sodium (Primellose) and 0.5% magnesium stearate.21
Differences in compactibility of different types of lactose have been also observed when comparing the tablet hardness of compacts prepared through dry granulation. Tablets containing 84% of crystalline sieved monohydrated lactose products such as Pharmatose 110M, 125M and 200M showed almost double higher compactibilities than tablets containing SuperTab 21AN in the same proportion.24
The effect of lubrication on compactibility of different types of lactose is depicted in figure 7. It is considered that lactose compaction occurs through a mixture of mechanisms, particularly fragmentation and plastic deformation, depending on the particle size and aggregation state of the matter. The more fragmenting presentations being less sensitive to presence of greasy lubricants and the opposite for lactose presentations compacting more through plastic deformation.
As can be seen in figure 7, considering a compaction pressure of 135 MPa, SuperTab 24AN displays the higher compactibility after lubrication. Lubrication increases its compactibility in about 30% from 146 N to 194 N. On the other hand, SuperTab 21AN exhibits the lowest compactibility. Lubrication decreases the compactibility of SuperTab 21AN in about 35% from 150 N to 110 N. Lactopress is not affected by lubrication, maintaining its compactibility in 149-150 N while lactose anhydrous NF increases its compactibility in about 10% from 159 N to 176 N.
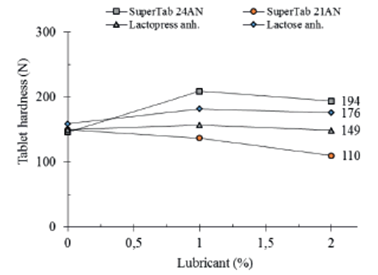
Figure 7 Effect of magnesium stearate on compactibility of different types of lactose for direct compression. Calculated values at a compaction pressure of 135 MPa.
The effect of lubrication on compactibility of different types of lactose has been also observed in spray-dried lactose. An increase in concentration of the lubricant, magnesium stearate, in mixtures with SuperTab 14SD and Flowlac 100 does not produce a decrease in strength of their tablets. Even if SuperTab 14SD showed stronger tablets than Flowlac 100.11
In contrast, in a different study, the spray-dried lactose monohydrate SuperTab with a particle size of 168 μm displayed a decrease in tablet hardness after increasing the lubrication. In the same way, an increased lubricant level showed a decrease in tablet hardness by Fast Flo lactose, a spray-dried lactose monohydrate with a particle of size of 103 μm.25
As observed before, the compaction behavior of lactose depend on the particle size and the state of aggregation. The compactibility of fragmenting materials is favored by lubrication while that of materials deforming plastically deteriorates after lubrication. SuperTab 24AN is a fluid bed granulated material, which fragments easier than individual crystals, allowing the appearance of new surfaces. This circumstance avoid the lubricant negative effects on compactibility. Moreover, the presence of the greasy lubricant allows also an easier densification, improving the compaction properties. On the other hand, materials that exhibit characteristics that are more plastic undergo a deleterious effect on compactibility due to an undisturbed coat of lubricants.
Effect of SuperTab 24 AN on compactibility of a drug product To determine the performance of SuperTab 24AN to improve the compactibility of a pharmaceutical product or its explicit functionality as a direct compression excipient, amoxicillin was the first of the chosen model drugs. The SuperTab 24AN functionality to improve the compactibility of amoxicillin is depicted in Figure 8, along with that of some other types of lactose. The figure describes the hardness or crushing strength of tablets obtained from 1:1 blends made of the excipients with the drug amoxicillin, added of 1% magnesium stearate.
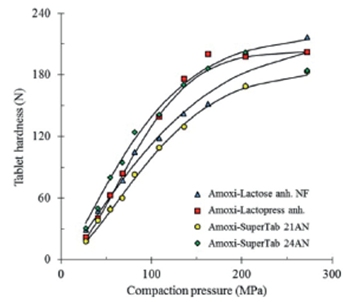
Figure 8 Compactibility of amoxicillin tablets containing 50% of different types of lactose and 1% magnesium stearate. Mortar blended.
In a first approach, the compactibility of lactose excipients, containing 1% magnesium stearate, decreases 27% after mixing with amoxicillin. Tablets of the lubricated excipients compacted at 135 MPa display an average tablet hardness of 177 N while that of formulations with amoxicillin decreases to 129 N. Secondly, the compactibility of formulations with amoxicillin maintain a similar order as observed before. The highest compactibility corresponds to SuperTab 24AN while the lesser corresponds to SuperTab 21AN.
Figure 9 depicts the effect of dilution with 50% amoxicillin on compactibility of different types of lactose tablets containing 1% magnesium stearate. The depicted values are the by regression calculated tablet hardness for a compaction pressure of 135 MPa.
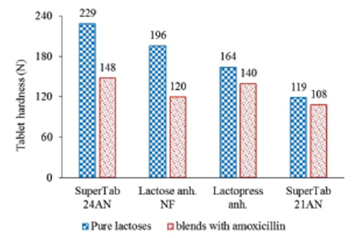
Figure 9 Effect of dilution with 50% amoxicillin on compactibility of different types of lactose tablets containing 1% magnesium stearate. Calculated values at a compaction pressure of 135 MPa.
It is noteworthy that the compactibility of the pure SuperTab 21AN is 52% of that of SuperTab 24AN while after mixing with amoxicillin the compactibility of SuperTab 21AN becomes 73% of that of SuperTab 24AN. The dilution with amoxicillin shows an equalizing effect in compactibility of these different types of lactose. This behavior seems to be related to the own compactibility of amoxicillin. The compactibility of a mixture is usually in between of compactibilities of the individual components.19-20
From another point of view, the use of curves of tensile strength (σ) against the relative density of tablets allow the calculation of the (σ') values or values of σ at a relative density of 1.0. The obtained results show a higher compactibility for tablets of Lactopress anhydrous (σ'=1589 N/cm2), followed by SuperTab 21AN (σ'=1195 N/cm2), SuperTab 24AN (σ'=1144 N/cm2) and Lactose anhydrous (σ'=827 N/cm2). These data do not correlate with data of tablet hardness and they neither correlate with data obtained with the different types of lactose containing 1% lubricant, where Lactopress shows the highest tensile strength (σ'=3008 N/cm2) followed by SuperTab 24AN (σ'=1557 N/cm2), lactose anhydrous NF (σ'=1476 N/cm2) and SuperTab 21AN (σ'=1080 N/cm2). In this sense, it is not possible to infer the behavior of these excipients in the formulation of amoxicillin from data of the lubricated different types of lactose.
A comparison of results obtained from compactibility curves and those of curves of tensile strength (σ) against the relative density of tablets can be observed in figure 10. In this case the comparison depicted is between the calculated tablet hardness of tablets obtained at a compaction pressure of 272 MPa against the calculated tensile strength at a relative density of 1.0 (σ'). Both sets of data show no correlation or equivalence between tablet hardness and tablet tensile strength.
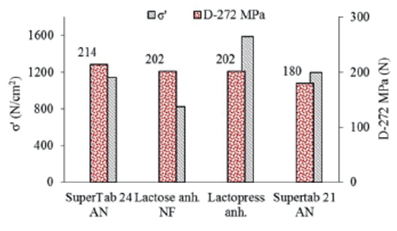
Figure 10 Compactibility of mixtures of amoxicillin containing 50% of different types of lactose and 1% magnesium stearate. Calculated tablet hardness at 272 MPa (D-272 MPa) and tensile strength at a relative density of 1.0 (σ').
A second drug selected to find out the explicit functionality of different types of lactose as direct compression excipients is captopril. Figure 11 depicts the by regression calculated compactibility curves of different types of lactose blended with 50% captopril and added of 1% magnesium stearate.
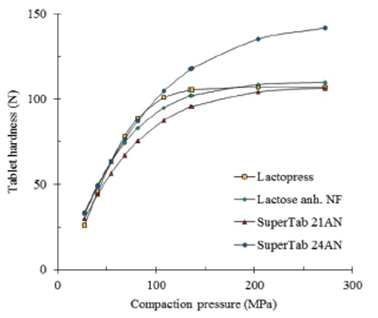
Figure 11 Calculated compactibility profiles of captopril tablets containing 50% of different types of lactose and 1% magnesium stearate. Mixed through manual sieving and V-blender.
As can be seen, the compactibility of the excipients containing 1% lubricant also decreases after mixing with captopril. Tablets of the lubricated excipients compacted at 135 MPa display an average tablet hardness of 177 N while that of the formulations with captopril decreases 41%, to 105 N. This percentage of missing compactibility is much higher than that observed by blends with amoxicillin (25%).
The compactibility of formulations with captopril maintain almost the same order observed before by the lubricated excipients. Considering tablets obtained at a compaction pressure of 135 MPa, the highest compactibility corresponds to SuperTab 24AN while the lesser corresponds to SuperTab 21AN (Figure 12).
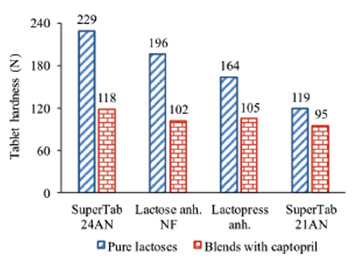
Figure 12 Effect of dilution with 50% captopril on compactibility of different types of lactose tablets containing 1% lubricant. Values calculated by regression for a compaction pressure of 135MPa.
The observed differences in compactibility displayed by different types of lubricated lactose is also equalized after dilution with captopril (Figure 12). The observed difference in compactibility of 48% between lubricated tablets of SuperTab 24AN and SuperTab 21AN decreases to 20% after mixing with 50% captopril.
Conclusion
In general, in the area of pharmaceutical excipients used for tablets obtained by direct compression, the compactibility of SuperTab 24 AN is about three times greater than that of DCP and something less than a half of that of MCC. Particularly, compactibility of different types of the unlubricated lactose does not allow the perception of a difference among them. However, the compactibility of different types of lactose containing 2% stearic acid shows a greater compactibility of SuperTab 24AN (D162=231 N) followed by anhydrous lactose NF (D162=201 N), Lactopress anhydrous (D162=171 N) and SuperTab 21AN (D162=123 N).
The compactibility of pure lactose excipients is reflected in their performance in a formulation, currently in formulations of amoxicillin and captopril. This means the performance of the excipient to modify the compactibility of a formulation could be inferred from the knowledge of the surrogate functionality or technological performance of the pure lubricated lactose excipients.
Dilution of lactose excipients with amoxicillin and captopril shows an equalizing effect in their compactibility. The advantage of one excipient over the other is less noticeable after dilution. This behavior seems to be related to the own compactibility of the drugs. The compactibility of a mixture is usually in between of compactibilities of the individual components.
From the viewpoint of compactibility, the above-mentioned results support the use of SuperTab 24AN as a first choice over other types of lactose, as an excipient for direct compression of tablets. Although this advantage was lesser after dilution of the excipients in the studied formulations.











 nueva página del texto (beta)
nueva página del texto (beta)