Introduction
Guillain-Barré syndrome (GBS) is classically presented by acute areflexic tetraparesis, which is potentially fatal because it compromises respiratory musculature and it is associated with autonomic dysfunction. The pathophysiology of GBS lies in the immune damage caused by autoantibodies production against myelin, and the axonal membrane of spinal roots and peripheral nerves, which, depending on the predominant pathophysiologic mechanism, classically cause two types of abnormalities: slowing of conduction speed and nerve conduction blockages, when primary demyelination occurs, or Wallerian degeneration, in relation to primary axonal damage1.
The neurophysiological diagnosis of GBS includes the application of criteria, aimed at demonstrating phenomena of primary demyelination in motor nerves, such as delayed distal latencies, slowing of conduction, and conduction blocks2,3.
The presence of some of these criteria, and the typical clinical picture, supports the diagnosis of GBS. For axonal forms, it is considered a diagnosis that does not meet criteria for primary demyelination and that the clinical picture is compatible. However, it has recently been shown that even in the axonal variants, antibodies directed against the components of the node and paranodal region can also generate conduction blocks, at axonal level4. However, alterations in sensory nerves are not commonly part of the established diagnostic criteria, despite the fact that sensory symptoms are prevalent in GBS.
The spinal roots and terminal segments of peripheral nerves are anatomical sites susceptible to autoimmune damage in GBS5.
In fact, it is not unusual to find in nerve conduction studies (NCSs) performed early during the evolution of the clinical picture of GBS, abnormality in sensory nerves in upper extremities with normality conduction in the lower extremities, a phenomenon commonly known as “sural nerve preservation” (SS, from the English sural sparing)3. It is possible explanation lies in two technical aspects related to the pathophysiology of GBS: first, the recording of the sensory action potentials (SAPs) of the sural nerve is performed in segments that are not as distal, as it is done in the upper extremities, where they are less susceptible to demyelinating damage and, second, in the longer time required in longer sensory nerves (for example, sural nerve) to observe reduction in the amplitude of the SAP when Wallerian degeneration occurs due to axonal damage in the spinal roots5.
Although the sensitivity reported for SS as a marker of GBS is low (~ 20%), its presence is usually considered to be highly specific for acute inflammatory demyelinating polyradiculoneuropathy (AIDP)6, although in axonal forms, cases have been reported exceptionally. The present study aims to describe the prevalence of SS among the classic forms of GBS in patients hospitalized in our institute.
Materials and methods
We retrospectively reviewed a total of 103 clinical files with diagnosis of presumptive discharge of GBS corresponding to the period 1999-2017 of the National Institute of Medical Sciences and Nutrition Salvador Zubirán (INCMNSZ), evaluating the presence of Hadden criteria for SGB, as well as the clinical presentation, such as the presence of neuropathy associated with another disease, data of carpal tunnel syndrome, dysesthesia, or dysautonomia, which preliminarily excluded 42 of these patients, in whom GBS was ruled out, or had serological alterations, that is, they presented uncontrolled diabetes mellitus, storage diseases, electrolyte alterations, use of neurotoxic drugs, and/or an alternative diagnosis was found.
On the other hand, a total of 61 patients with the diagnosis of GBS were included, according to the clinical picture, evolution, and the neurophysiological criteria.
Once the true cases of GBS were identified, we identified each of them according to the clinical presentation subtype, differentiating them from demyelinating (AIDP), axonal, pure motor (acute motor axonal neuropathy [AMAN]) or sensory motor (acute sensory axonal neuropathy [AMSAN]) forms, as well as Miller-Fisher (MF) variant. Subsequently, we analyzed the electrophysiological variants such as amplitude and speed of conduction of sural, ulnar, and median nerves, establishing values of normality according to the criteria of Hadden and Rajabally (Table 1).
Table 1 Electrophysiological values found in our patients, and proposed by Hadden classification, in brackets
| Sural nerve | Ulnar nerve | Median nerve | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amp uV | VdeC m/s | NR n (%) | Amp uV | VdeC m/s | NR n (%) | Amp uV | NR n (%) | Amp uV | NR n (%) | Amp uV | NR n (%) | Amp uV | NR n (%) | |
| Right | Left | Right | Left | Right | Left | |||||||||
| All | 11.0 (9.6) | 47.8 (7.5) | 10 (29) | 10.8 (10.1) | 47.2 (8.1) | 12 (35) | 20.8 (17.8) | 8 (23) | 20.2 (17.1) | 8 (23) | 25.7 (27.4) | 10 (29) | 26.4 (26.4) | 10 (29) |
| AIDP | 9.9 (8.7) | 48.4 (8.5) | 6 (35) | 9.5 (9.4) | 46.0 (9.6) | 7 (41) | 16.6 (15.4) | 5 (29) | 16.0 (14.1) | 5 (29) | 20.3 (26.1) | 6 (35) | 20.9 (25.8) | 7 (41) |
| AMAN | 19.3 (8.3) | 48.9 (7.2) | 0 (0) | 19.3 (8.7) | 49.3 (6.9) | 0 (0) | 38.7 (14.4) | 0 (0) | 37.7 (15.4) | 0 (0) | 49.9 (24.9) | 0 (0) | 48.5 (22.8) | 0 (0) |
| AMSAN | 1.9 (2.6) | 45.0 (7.1) | 3 (66) | 2.6 (5.8) | 24.0 (33.9) | 4 (88) | 7.0 (9.0) | 2 (44) | 7.3 (7.5) | 2 (44) | 5.9 (10.0) | 3 (66) | 8.6 (12.5) | 2 (44) |
SS was defined as normality in the amplitude or relative preservation of the sensory nerve action potential (SNAP) of the sural nerve with abnormality of the SNAPs of the median and/or ulnar nerve5, excluding from the final analysis, the cases according to previously defined criteria1 (Fig. 1).
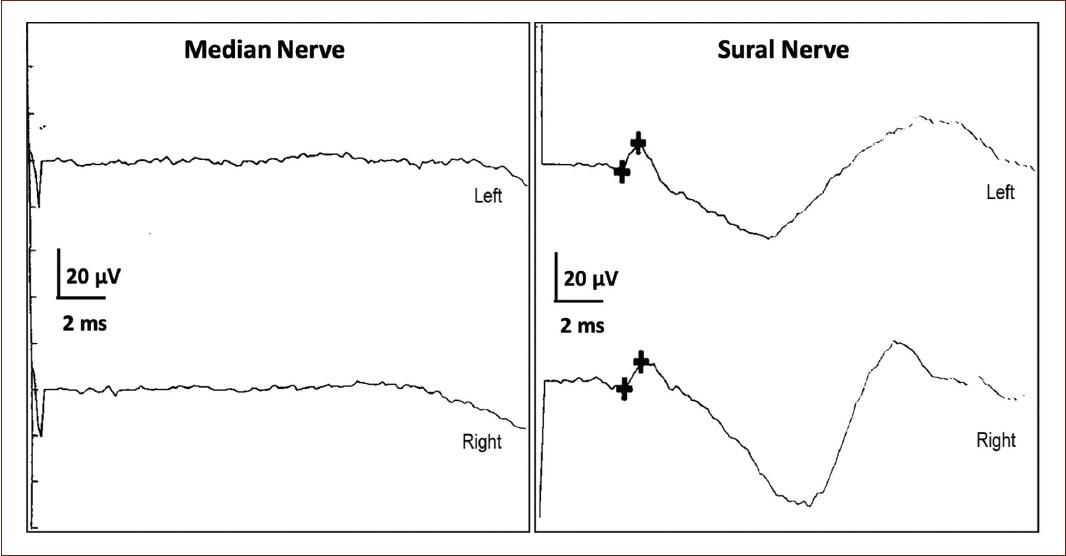
Figure 1 Presentation of the sural sparing phenomenon in the sural nerve, compared to normal median nerve.
We performed the statistical analysis with the statistical package SPSS 20, considering statistically significant differences with p < 0.05.
Results
Thirty-four patients met the selection criteria for SGB, which presented the following characteristics. Neurophysiological parameters showed a lower amplitude of the PANS and a high frequency of NR in AIDP and AMSAN compared to AMAN (Table 2).
Table 2 Relationship between the clinical presentations of GBS and the variables of age, sex, time of evolution, and presence of diabetes
| All | AIDP | AMAN | AMSAN | |
|---|---|---|---|---|
| Age (years) | 44.2 ± 20.0 | 48.5 ± 21.8 | 35.8 ± 19.0 | 42.0 ± 10.1 |
| Gender (n, female, (%)) | 13 (28) | 7 (41) | 4 (44) | 1 (20) |
| Time evolution (days) | 10.4 ± 6.1 | 10.5 ± 6.5 | 8.9 ± 6.5 | 10.6 ± 5.0 |
| Diabetes (n, (%)) | 4 (10) | 2 (5) | 0 (0) | 1 (2) |
Seven patients with AIDP showed SS (17.6%) (which was not observed in the other forms of GBS), two of them corresponding to “extreme” SS (absent median nerve PANSs).
Discussion
Our study has the limitation that it is a retrospective study, in addition to using only the Hadden criteria for the diagnosis of GBS. Historically, neurophysiological criteria for the diagnosis of GBS are applied to motor nerves and not to sensory nerves. This is partly because the demonstration of the phenomena of primary demyelination in sensory nerves is technically more difficult, for example, mainly related to the high degrees of temporal dispersion of their potentials when stimulated in more than 1 point. However, some authors consider SS to be a useful tool in the diagnostic support of acquired demyelinating polyneuropathies, including GBS and chronic inflammatory demyelinating polyneuropathy5.
The previous studies have shown that SS has a sensitivity of 38% in the diagnosis of AIDP, with a specificity of 90.9% using the Hadden criteria, while a sensitivity of 36.8% and specificity of 69.6% have been found for AIDP with the Rajabally’s criteria7. It is necessary that the neurophysiological study be performed with some precocity during the evolution of the disease (typically before 21 days from the onset of symptoms), since in later stages, phenomena of secondary axonal degeneration of the sural nerve can obscure its presence.
On the other hand, the terminology “sural nerve preservation” (SS) in GBS depends on the definition that is used, for example, that normality is considered versus the preservation of the PANS of the sural or of the sensory nerves that are explored in upper extremities (median, ulnar, or radial nerve) to be confronted with the sural. Classically, the SS seems to be specific of the demyelinating forms that imply the absence of a median nerve response, independently of the criteria used for the classification of the GBS subtype. However, some authors consider that the use of the median nerve can increase the false positives of SS for GBS considering the high prevalence of compression of the median nerve in the carpal channel in the general population.
Histopathologically, in patients who have presented SS, generalized inflammation, demyelination, and axonal degeneration of the spinal nerves have been observed, although the sural nerve is usually relatively preserved, which correlates with the absence of electrophysiological alterations7. In addition, the SS can be explained based on the fact that the nerve is registered near the lateral malleolus, somewhere between the spinal roots and its distal end in the foot, where there seems to be a lower predisposition to focal demyelinating damage5.
The frequency of SS has been reported in different variants of GBS, being more frequent in the AIDP forms, followed by the MF syndrome (MFS) and occasionally found in patients with AMAN. It is not strange to observe this in the MFS that is mostly shown as a demyelinating form of GBS. However, due to the low prevalence found in SS and the high frequency of axonal forms described in our setting, it seems evident that SS is not applicable in electrophysiological screening to differentiate forms of GBS8.
Conclusion
In conjunction with the clinical picture and other typical neurophysiological findings, the preservation of the sural nerve in the GBS seems to be a diagnostic support tool, which also adds to the fact that it is easy to obtain in the practical electrodiagnostic environment. In an isolated way, it provides information on the physiopathology of the phenomenon in the classic forms of GBS, mostly demyelinating. In the Mexican population, where axonal forms prevail, it is assumed that routine SS screening should not provide diagnostic support related to the low frequency in which it has been found in this study (17.6% of cases), so it should be considered in populations with predominance of the axonal form as complementary diagnostic method.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.











 nueva página del texto (beta)
nueva página del texto (beta)


