Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ingeniería química
versão impressa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.11 no.3 Ciudad de México Dez. 2012
Catálisis, cinética y reactores
Self-assembly of size-selected clusters and nanoparticles of Cu, Ag and Au in mordenite matrix
Auto-ensamble de cúmulos y nanoparticulas de Cu, Ag y Au de tamaños selectos en matrices de mordenita
E. Smolentseva1*, V. Gurin2 and V. Petranovskii1
1 Universidad Nacional Autónoma de México, Centro de Nanociencias y Nanotecnología, Km. 107 Carretera Tijuana a Ensenada, C.P. 22860, Ensenada, Baja California, México. * Corresponding author. E-mail: elena@cnyn.unam.mx Tel. 646-174-4602 ext. 456, Fax 646-174-4603
2 Physico-Chemical Research Institute, Belarusian State University, Minsk, 220080, Belarus.
Received 20 of February 2012
Accepted 31 of October 20 12
Abstract
In the present work, the self-assembly of Cu, Ag and Au clusters and nanoparticles in mordenite channels was considered. It was revealed that Si/Al molar ratio and the experimental conditions are the key factors in nanospecies formation. Ag8 clusters can be easily assembled in the mordenite; copper demonstrates multiples reduced forms, while gold preferably forms nanoparticles with average diameter 7.5 nm and plasma resonance peak observed even in freshly prepared samples. The temperature increase up to 500°C leads to aggregation of gold particles.
Keywords: mordenite, nanoparticles, clusters, copper, silver, gold.
Resumen
En el trabajo presente discutimos el auto-ensamble de cúmulos y nanopartículas de Cu, Ag y Au en canales de mordenita. Se encontró que la relación molar Si/Al y las condiciones experimentales son los factores claves en la formación de estas nanoespecies. Los cúmulos de plata Ag8 pueden ser fácilmente ensamblados en la mordenita; el cobre muestra múltiples formas reducidas, mientras el oro preferentemente forma nanopartículas con diámetro promedio de 7.5 nm y el pico de plasma resonancia, observado desde las primeras etapas de su preparación. El aumento de la temperatura hasta 500°C promueve la agregación de las partículas de oro.
Palabras clave: mordenita, nanopartículas, cúmulos, cobre, plata, oro.
1 Introduction
The study of small metal particles is interesting for at least two reasons: firstly, they appear in many materials of practical importance (catalysts, colored glasses, metalopolymers, etc.) Fendler (1998) and secondly, they result in nontrivial features in many phenomena due to unusual size-dependent electronic properties Schmid et al. (1998). Coinage metals (Cu, Ag, Au) are of great interest due to the importance of their catalytic properties. For example, gold and silver nanoparticles supported on TiO2, demonstrated great catalytic properties in the oxidesulfurization of organosulfur compounds Zanella et al. (2007). Nanoparticles of iron oxyhydroxides supported on a mesoporous material SBA-15 permitted to eliminate organic pollutants from the water by advanced oxidation processes Vergara-Sanchez et al. (2012).
Zeolites, which are nanoporous materials possessing cages or channels of 2.5 to 12 Å in size, are well-suited starting materials for purposeful assembly of clusters and nanoparticles and connected to this design electronic, magnetic and optical properties of obtained composites. Zeolite matrices, with their excellent ion-exchange properties and multi-porous structure have been used in the fabrication of metal-containing systems, mainly with the purpose of preparing special catalysts, and in general, zeolites open the possibility of the regulation both the concentration of exchanged ions and the strength of the metal ion bonding with the matrix Breck (1974). The zeolitic nanopores can be filled with inorganic guest molecules; the arrangement of the guest molecules is geometrically confined by the host pore system. Metal-containing zeolites are of great importance as challenging new catalysts for de NOx reactions Parvulescu et al. (1998), Van der Waal et al. (1998), Putluru et al. (2011).
Cu, Ag and Au can be easily incorporated into zeolites by the ion-exchange from solutions and reduced under heat treatment in reducing gaseous media. A final state of reduced species depends on the type of zeolite, their SiO2/Al2O3 molar ratio (MR) and processing conditions. A number of species of the ionic nature was detected in the prepared samples; neutral small metal particles and few atomic clusters maybe possible products of ion transformations. We have already shown that under certain conditions the reduction of silver in zeolites can lead to the selfassembly and stabilization of Ag8 clusters Gurin et al. (2001) and that the reduction of copper supported in zeolites can lead to a variety of different Cu species Gurin et al. (2002), Petranovskii et al. (2005), Lashkul et al. (2011), López-Bastidas et al. (2012).
It's known that the spacing between the clusters may be varied, depending on the size and spacing of the zeolite voids and the electronic properties can be changed vastly due to the clusters' interactions. In fact, such research suggests that zeolites may have a role in "bootstrapping" of more sophisticated molecular assemblers.
There are several important features of zeolites that permit governing the properties of such composite materials. Those features are monosized voids in the crystal lattice, variable Si/Al ratio of zeolite matrix, the amount of metal ions tolerated by the Si/Al ratio, nature of counter-ion, amount and the particle size of introduced material, as well as the nature of the introduced material (metal, semiconductor, insulator, etc.). The inertness of the Si-O-Si framework is especially important in the applications, as there is minimal interaction of the framework with the guests; they are free to interact with each other or with diffusing through zeolite lattice reagents.
2 Experimental
Protonated forms of mordenites (HM) with 10 ≤ MR ≤ 206 were supplied by the TOSOH Corporation, Japan. Copper and silver ion exchange was carried out from 0.1 M aqueous solutions of corresponding nitrates, and prepared samples (CuM and AgM) were filtered, washed with deionized water and dried under ambient conditions followed by heating in a H2 flow at temperatures from 150 to 450°C for 4 h. Both copper and silver content measured by energy dispersive spectroscopy (EDS) was approximately 1 wt. %.
Gold was ion-exchanged in zeolites from aqueous solution of [Au(NH3)4](NO3)3 complex synthesized from HAuCl4 (Alfa-Aesar) and NH4NO3 according to procedure described by Bogdanchikova et al. (2008). Gold exchanged samples were washed with deionized water and dried at 60 °C for 6 h in air. Then, AuM samples were calcined at selected temperatures in the range of 100-500 °C for 1 h. The gold loading measured by EDS was ~ 2.7 wt. %.
Samples were studied by UV-Visible diffuse reflectance spectroscopy (DRS) using a CARY 300 SCAN (Varian) spectrometer. Transmission electron microscopy (TEM) was performed with a JEOL 2010 microscope. The sample was dispersed in isopropanol and dropped on a copper grid coated with carbon film. To determine the mean diameter of gold particles more than 150 particles were chosen.
The obtained copper, silver or gold samples are abbreviated as (Cu/Ag/Au)M followed by MR of mordenite and temperature of reduction.
3 Results
DRS spectra of CuM and AgM samples with different MR reduced at different temperatures are shown at Fig. 1. After reduction treatment the DRS spectra of AgM10 and AgM20 demonstrate the development of pronounced absorption peaks at 285-290 nm and 320323 nm (curves 1 and 2, Fig. 1B). These peaks were assigned to the Ag8 cluster in the correspondence with the data on its optical absorption in solutions according to Ershov et al. (1993) and frozen rare gases according to Fedrigo et al. (1993). Those clusters may be formed within the mordenite intracrystalline channels with cross section 0.65 x 0.7 nm as reported by Bogdanchikova et al. (1999), Bogdanchikova et al. (2000). In line with cluster peaks, wide band of plasma resonance from silver nanoparticles at ~ 400 nm appears at increasing of reduction temperature, while cluster peaks gradually disappear. The absorption at λ > 350 nm is usual for ultrafine silver species and associated with the plasma resonance in silver nanoparticles bigger than 10 nm according to Kreibig et al. (1995). A broadening of this band shows that particles have wide size distribution. Because of their size, exceeding diameters of regular mordenite channels, these particles may be localized at the outer surface of mordenite crystals or at the specific sites inside of the mesoporous volume of zeolite matrix like cleaved areas, defects, uneven surface, etc.
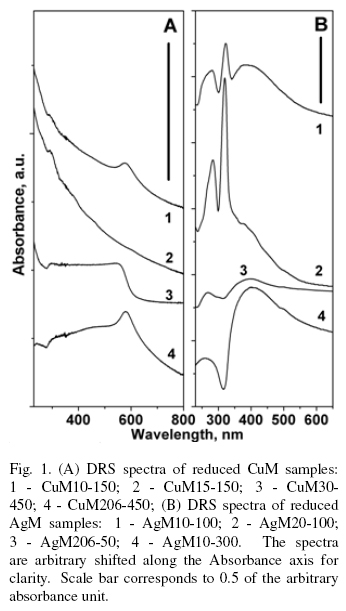
Copper is similar to silver in chemical behavior, but copper chemistry is more complicated due to the two main valence states, Cu(I) and Cu(II), occurring in different environment. An unambiguous detection of copper clusters of the low nuclearity is more problematic than analogous silver species. Probably, weak peak at ~ 290 nm for the CuM10-150 and CuM15-150 (curves 1 and 2, Fig. 1A) may be due to some amount of unidentified copper clusters. With increasing of temperature this peak disappears, while plasma resonance band (λ ~ 550-600 nm) due to copper nanoparticles starts growing. The corresponding plasmon peak appearance is governed by the same logic as the silver band - high temperatures favor the efficient copper nanoparticle formation (Fig. ). The shape of this band is sensitive to MR and reduction temperature.
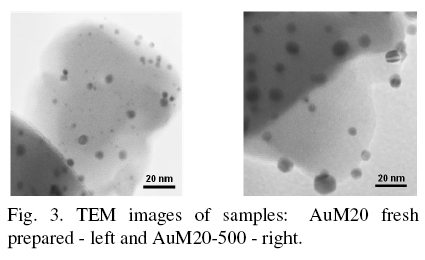
UV-visible spectra of AuM samples (Fig. 2) present three pronounced signals at around 220, 280320 and 540 nm. The signal at 540 nm was assigned to the plasma resonance of the metallic gold particles as mentioned by Salama et al. (1996), Kuge et al. (2003), Chen et al. (2003), Smolentseva et al. (2012). Gold metal particles with size around 7.5 nm were presented in freshly prepared samples (Fig. 3, left). The blue shift was observed in UV-Visible spectra in the region of 540-520 nm. It may be caused by the difference of gold particles size. TEM images showed that gold particles were growing up to 20 nm with increase of samples calcination temperature (see Fig. 3, right). It is well known, that the majority of zeolites are characterized with existence of channels with radii around 2 nm, Hernandez et al. (2005). Thus, the main portion of gold nanoparticles was located on the external surface of zeolite and in the mesoporous voids of zeolite matrix.
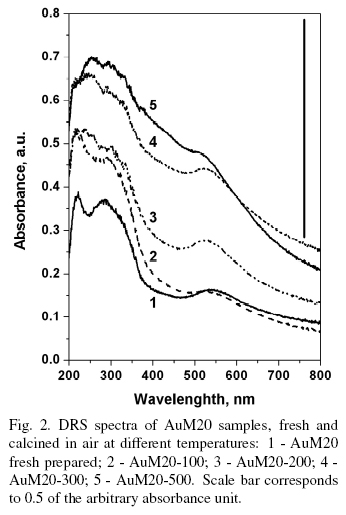
According to Pestryakov et al. (2005), Smolentseva et al. (2007) peaks at 220 and 280-320 nm were assigned to the gold charged clusters Aun+ with different size and effective charge. These sta2es were formed as results of partial reduction of gold oxides. Subsequent temperature increase leads to the increment of spectra intensity and band at around 220 nm was almost overlapped with band at 280 nm.
Thus, the whole series of AgM, CuM and AuM samples allow formation of the two types of reduced products: few atomic clusters and nanoparticles. The effect of long-term stabilization of few atomic silver clusters for Ag-mordenite can be associated with geometrical matching of the cluster size (in particular Ag8) and the size of intracrystalline cavities. Cu and Au atoms have different size referring to Ag atoms; so, for long-term stabilization of copper and gold clusters inside intracrystalline cavities and/or channels it is necessary to search for zeolites with slightly different size of voids.
4 Discussion
Gold, silver and copper belongs to so-called coinage metals those are distinguished from other metals by ability to have noticeable color in visible range. In the case of small particles this ability becomes much more expressed and under appropriate conditions the particles can cover all colors. The mentioned above is due to the absorption maxima lying in visible range those are commonly accepted to associate with plasmon resonance in small particles, which is characteristic for particles of any metal. Position of the band varies from metal to metal depending on characteristics of conductivity electrons in them. A theory of light absorption by metal particles (Mie theory and it developments) is based on analysis of classical interaction of electromagnetic waves with dipole oscillators. In general, absorption on particles with size less than wavelength does not contain a size-dependency explicitly. The main dependence consists in the term with combination of dielectric constants ε1 and ε2 (these two values determine any dielectric response of a matter with respect to variable electromagnetic field rather than one ε in the static limit) and εm, dielectric function of a medium. ε1 and ε2 are phenomenological values and tabulated for many compounds, they both include contributions from different light absorption mechanisms.
he criterion of appearance of a maximum in absorption is very simple and looks as ε1 = ε2 * εm, or in the case of medium with εm = 1, => ε1 = ε2. That means that the function of ε(ω), where ω is frequency, determines the fact of absorption maximum of small particles and, it has universal character, but in most cases a maximum enters IR (semiconductors) or UV (most of other metals) range. In the visible range this criterion obeys the above mentioned set of "coinage" metals. The existing difference in spectra of Ag, Au and Cu originates from contribution of many factors into the dielectric function. The plasmon resonance (excitation of oscillating charges) is the main factor. The plasmon frequency depends on the concentration of charges (N), and the effective mass (m), according to the formula (1):
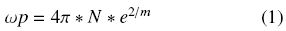
The plasmon oscillations themselves are not specific for small metal particles, surface and bulk ones exist in metals, but the Mie theory shows that the resonance oscillations in small particles are associated with the plasmon oscillations. In other words, an absorbed light excites surface plasmon in particles, which raise this contribution due to large part of surface in any ensemble of small particles.
The most "pure" form of the plasmon resonance in small metal particle among coinage metals occurs for silver. Silver is most close to so-called simple metals (the typical ones are alkali), which atoms have a single electron and remaining core in the electronic shell is completely occupied (5s-electron). That provides the high intensity of the plasmon resonance. Different situation occurs with copper and gold. They both scarcely could be assigned to "simple" metals, their conductivity electrons have less concentration than in silver, and the plasmon resonance is red-shifted from that in Ag. Also, d-electrons contribute more noticeably into the electronic spectrum of Cu and Au. The inter-band transitions in these metals are distinguished in energies: from ~ 4 eV in Ag (again, the reason of the more value is "closed" d-shell) to 22.5 eV in Cu and Au. So, plasmon peaks for Cu and Au are overlapped with inter-band (d-s) transitions. The latter provides more complex outlook of an absorption spectrum for copper and gold particles.
Another important factor that distorts the Cu plasmon resonance is the higher chemical activity of copper that results in appearance of some surface compounds on particles and the more particle-matrix interaction. The chemical effects lead to absorption from Cu2+ and Cu+ states those enter the ranges from low-energy side and higher-energy side from the plasmon resonance, respectively. Ag, and, in particular, Au are subjected much less to these effects, and ions like Ag+, Au+ absorb in the UV-range far from the plasmon resonance.
The above mentioned peak of Cu plasmon resonance is subjected to variations in the position and shape as well as the plasmon resonance peaks of Ag and Au nanoparticles. They are affected by size, shape, environment medium, and aggregation of particles. The different factors changing the plasmon resonance acts as some counterpart of an ideal resonance of perfect sphere of small radius (with respect to wavelength) in vacuum. Some observations are known in the case of Ag particles when a little blue-shift appears due to peculiar charge transfer between surface and interior of a particle, and they are possible as well for Cu, however, this effect cannot be very high. Thus, one of the possible ways of interpretations of short wavelength absorption in the case of Cu (as well as of Ag and Au) is clusters with size too small to develop a natural plasmon resonance but with allowed inter-level transitions similar to the absorption in molecular systems.
Conclusions
In spite of notable difference in chemical properties of three coinage metals, process of their reduction and followed self-assembling of metal atoms in the process of aggregation shows some similarity. A first step of the assembling, and then chemistry of ion reduction and neutral atoms formation occurs inside of zeolite channels, and it is very different for studied CuM, AgM and AuM systems. Clusters stability in the case of AgM is high due to "cluster sieving effect" of matching of mordenite channel and Ag8 cluster sizes. Under increased temperature the diffusion of reduced species from the channels of zeolite to mesoporous voids, and on the outer surface of the mordenite crystals equalize the differences between the metal properties, and nanoparticles characterized with plasma resonance bands are formed as the final step of self-assembling.
Acknowledgements
The authors thank E. Flores, I. Gradilla, E. Aparicio, F. Ruiz, P. Casillas, J. Peralta and J. Palomares, for the precious technical support. This research was supported by CONACYT through grant # 102907.
References
Bogdanchikova N., Petranovskii V., Machorro R., Sugi Y., Soto V.M. and Fuentes S. (1999). Stability of silver clusters in mordenites with different SiO2/Al2O3 molar ratio. Applied Surface Science 150, 58-64. [ Links ]
Bogdanchikova N., Petranovskii V., Fuentes S., Paukshtis E., Sugi Y. and Licea-Claverie A. (2000). Role of mordenite acid properties in silver cluster stabilization. Materials Science and Engineering A 276, 236-242. [ Links ]
Bogdanchikova N., Simakov A., Smolentseva E., Pestryakov A., Farias M., Díaz A., Tompos A. and Avalos M. (2008). Stabilization of catalytically active gold species in Fe-modified zeolites. Applied Surface Science 254, 40754083. [ Links ]
Breck D. (1974). Zeolite Molecular Sieves. J. Wiley & Sons, Inc., New York. [ Links ]
Chen W., Zhang J. and Cai W. (2003). Sonochemical preparation of Au, Ag, Pd/SiO2 mesoporous nanocomposites. Scripta Materialia 48, 1061-1066. [ Links ]
Ershov B.G., Janata E. and Henglein A. (1993). Growth of Silver Particles in Aqueous Solution: Long-Lived "Magic" Clusters and Ionic Strength Effects. Journal of Physical Chemistry 97, 339-343. [ Links ]
Fedrigo S., Harbich W. and Buttet J. (1993). Collective dipole oscillations in small silver clusters embedded in rare-gas matrices. Physical Review B 47, 10706-10715. [ Links ]
Fendler J.H. (1998). Nanoparticles and nanostructured films: preparation, characterization and application. Wiley-VCH: Weinheim, Germany. [ Links ]
Gurin V., Bogdanchikova N. and Petranovskii V. (2001). Self-assembling of silver and copper small clusters within the zeolite cavities: prediction of geometry. Materials Science and Engineering C18, 37-44. [ Links ]
Gurin V., Bogdanchikova N. and Petranovskii V. (2002). Metal clusters and nanoparticles assembled in zeolites: an example of stable materials with controllable particle size. Materials Science and Engineering C 19, 327-331. [ Links ]
Hernández M.A., Rojas F., Corona L., Lara V.H., Portillo R., Salgado M.A. and Petranovskii V. (2005). Evaluación de la porosidad de zeolitas naturales por medio de curvas diferenciales de adsorción. Revista Internacional de Contaminación Ambiental 21, 71-81. [ Links ]
Kreibig U., Vollmer M. (1995). Optical Properties of Metal Clusters. Springer-Verlag, Berlin. [ Links ]
Kuge K. and Calzaferry G. (2003) Gold-loaded zeolite A. Microporous and Mesoporous Materials 66, 15-20. [ Links ]
Lashkul A., Pleshakov I.V., Glebova N.V., Nechitailov A.A., Kuzmin Yu.I., Matveev V.V., Pyatyshev E.N., Kazakin A.N. and Glukhovskoi A.V. (2011). Investigation of composite structure of magnetically ordered material-semiconductor composite based on cobalt and porous silicon. Technical Physics Letters 37, 664-666. [ Links ]
López-Bastidas C., Petranovskii V. and Machorro R. (2012). Optical response of Cu clusters in zeolite template. Journal of Colloid and Interface Science 375, 60-64. [ Links ]
Pârvulescu V., Grange P. and Delmon B. (1998). Catalytic removal of NO. Catalysis Today 46, 233-316. [ Links ]
Pestryakov A., Tuzovskaya I., Smolentseva E., Bogdanchikova N., Jentoft F.C. and Knop-Gericke A. (2005). Formation of gold nanoparticles in zeolites. International Journal of Modern Physics B 19, 2321-2326. [ Links ]
Petranovskii V., Gurin V. and Machorro R. (2005). Spectroscopic observation and ab-initio simulation of copper clusters in zeolites. Catalysis Today 107-108, 892-900. [ Links ]
Putluru R.S.S., Riisager A. and Fehrmann R. (2011). Alkali resistant Cu/zeolite deNOx catalysts for flue gas cleaning in biomass fired applications. Applied Catalysis B: Environmental 101, 183-188. [ Links ]
Schmid G. and Chi L.F. (1998). Metal clasters and colloids. Advanced Materials 10, 515-526. [ Links ]
Smolentseva E., Simakov A., Beloshapkin S., Estrada M., Vargas E., Sobolev V., Kenzhin R. and Fuentes S. (2011). Gold catalysts supported on nanostructured Ce-Al-O mixed oxides prepared by organic sol-gel. Applied Catalysis B: Environmental 115-116, 117-128. [ Links ]
Smolentseva E., Bogdanchikova N., Simakov A., Pestryakov A., Gurin V., Avalos M., Farias M., Díaz A. and Tompos A. (2007). Catalytic activity of gold nanoparticles incorporated into modified zeolites. Journal of Nanoscience and Nanotechnology 7, 1882-1886. [ Links ]
Van der Waal J.C. and Van Bekkum H. (1998). Molecular sieves, multifunctional microporous materials in organic synthesis. Journal of Porous Materials 5, 289-303. [ Links ]
Vergara-Sánchez J., Pérez-Orozco J.P., Suárez-Parra R. and Hernández-Pérez I. (2012). Degradation of reactive red 120 azo dye in aqueous solutions using homogeneous/heterogeneous iron systems. Revista Mexicana de Ingeniería Química 11, 121-131. [ Links ]
Zanella R., Cedeno-Caero L., Viveros O. and Mireles E. (2007). Oxidesulfurization of organosulfur compounds with gold and silver catalysts supported on titania. Revista Mexicana de Ingeniería Química 6, 147-156. [ Links ]














