Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ingeniería química
versão impressa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.11 no.3 Ciudad de México Dez. 2012
Catálisis, cinética y reactores
Ni-mordenite system: influence of SiO2/Al2O3 molar ratio on the catalytic activity in no reduction
Sistema Ni-mordenita: influencia de la relación molar SiO2/Al2O3 en la actividad catalítica para la reducción de no
R. Obeso-Estrella1, A. Simakov2, M. Avalos-Borja2'3, F. Castillón2, E. Lugo4 and V. Petranovskii2*
1 Posgrado en Ciencia e Ingeniería de Materiales, Universidad Nacional Autónoma de México, Centro de Nanociencias y Nanotecnología (PCeIM, CNyN-UNAM), Apdo. Postal 14, C.P. 22800, Ensenada, B.C., México.
2 Universidad Nacional Autónoma de México, Centro de Nanociencias y Nanotecnología, Departamento de Nanocatálisis, Apdo. Postal 14, C.P. 22800, Ensenada, B.C., México. *Corresponding author. E-mail: vitalii@cnyn.unam.mx
3 On leave at IPICyT, San Luis Potosí, S.L.P., México.
4 Departamento de Ingeniería Química, Instituto Tecnológico de Los Mochis, Blvd. Juan de Dios Bátiz y 20 de Noviembre, 81250 Los Mochis, Sinaloa, México.
Received 20 of Februaay 2012
Accepted 3a of October 2012
Resumen
Se intercambió con Ni2+ una serie de mordenitas con la relación molar (RM) SiO2/AI2O3 de 13, 20 y 90. Las muestras se caracterizaron por DRX, EDE, ERD y MET. Los espectros de UV-Vis de todas las muestras son típicos para iones coordinados octaedricamente con moléculas de agua de Ni(H2O)62+. En el rango de UV, las muestras con RM de 20 y 90 mostraron la absorción a 250-300 nm efecto de la deshidratación parcial de iones Ni2+ y su coordinación con O2- de la matriz zeolítica. Se reveló que las especies de Ni formadas en la muestra etiquetada como NiNaMor13 con la RM baja están débilmente ligadas a la estructura de mordanita; como una consecuencia se observa una alta actividad catalítica en la reducción de NO. La transformación sencilla de la reducción-oxidacion de tales especies causa la activacion de la pirólisis de propeno con la formación de nanoestructuras de carbono.
Palabras clave: Ni-mordenita, relacion molar SiO2/Al2O3, nanotubos de carbon, reduction de NO, UV-Vis.
Abstract
Mordenites with SiO2/Al2O3molar ratio (MR) of 13, 20 and 90 were exchanged with a Ni2+. The samples were characterized byXRD. EDS, DRS and TEM. UV-Vis spectra of all Ni-exchanged samples were typical for a Ni(H2O)62+ ions octahedrally coordinated by water molecules. In the UV range, samples with MR of 20 and 90 showed absorption around 250-300 nm due to partial dehydration on Ni2+ ions and their coordination with O2- of the zeolite framework. It was revealed that Ni species formed on mordenite NiNaMor13 with low molar ratio are weakly? bound to the mordenite framework; as a consequence, high catalytic activity in NO reduction is observed. Easy red-on transformation of such a species rasults in the activation of propene pyrolysis with formation of carbon nanostructures.
Keywords: Ni-mordenite, SiO2/Al2O3 molar ratio, carbon nanotubes, NO reduction, UV-Vis.
1 Introduction
Selective catalytic reduction (SCR) of NO by hydrocarbons is one of the most powerful methods for the effective control of NO emission Hu et al., (2009), Johnson (2009). Between other materials, metal-modified zeolites are extensively studied as catalysts for this process Rebrov et al. (1999), Rebrov et al. (2000), Yahiro and Iwamoto (2001). The metals most frequently used for zeolite modification are Co, Fe, and Cu Parvulescu et al. (1998). Zeolites exchanged with Ni are less studied in this reaction.
There are the different methods of metal incorporation into zeolite frameworks, such as traditional wet impregnation or more complicated ion exchange techniques Martinez and Corma (2011). It is known that the highest content of metals was achieved by solid state ion exchange of protonic zeolites with metal chloride precursors Sazonova et al. (1997), Abu-Zied et al. (2008).
There are more than 200 zeolite types with different structure of channels and cavities Baerlocher et al. (2007). The most applicable zeolites for SCR of NO are ZSM-5, Y, Beta, and Mordenite. It was shown that the SiO2/Al2O3 molar ratio (MR) is one of the important parameters managing acidity, ionexchange capacity, and metal coordination in zeolites Bogdanchikova (1999), Bogdanchikova (2000), Gurin (2002), Gurin (2005).
The aim of the present work was to study influence of MR on the formation of different Ni species in mordenite, and evaluation of their catalytic activity in SCR of NO in the presence of propene and CO.
2 Experimental
2.1 Catalyst preparation
Mordenites with SiO2/Al2O3 molar ratio of 13, 20 and 90 in sodium, ammonium and proton forms, respectively, were supplied by Zeolist International. Ni was incorporated by liquid phase ion exchange of zeolites using an aqueous solution of Ni(NO3)2 at 60 °C under continuous stirring for 24 h. Subsequently, the zeolites were washed thoroughly with deionized water and dried at 110 °C overnight. Through the text and figures samples are denoted as "Ni" if materials were exchanged, followed by exchangeable cations (Na, NH4, H) and "Mor" for mordenite, finished with value of SiO2/Al2O3molar ratio; for example NiNaMor13.
2.2 Catalyst characterization
The crystallinity of samples before and after ion exchange was tested by XRD measurements using Philips X'Pert diffractometer with Cu K α radiation. To evaluate electronic state of Ni species UV-Vis diffuse reflectance spectra were recorded by a Varian Cary 300 spectrophotometer equipped with an integrated sphere assembly. The spectra were recorded at room temperature using Teflon (HALON) as reference and plotted in terms of absorption. In order to show clearly a presence of nickel species, the spectra of Ni exchanged zeolites presented below were obtained by subtraction of spectrum of corresponding zeolite. The nickel content was measured by energy dispersive spectroscopy (EDS) on a JEOL 5300 Scanning Electron Microscope.
The catalytic activity of the samples in NO reduction in the presence of CO, propene and oxygen was studied in a continuous flow reactor with fixed bed of a catalyst. The mixture (NO-0.09 vol%, C3H6 -0.22 vol.%, CO - 1.18 vol.%, O2 - 0.46 vol.%, N2 -balance) flow was 55 mL/min. 0.1 g of catalyst as a fine powder was supported on quartz wool. The activity characterized by light-off curves of NO conversion vs temperature was measured within 30-550° C temperature interval with a heating ramp of 1 °C/min. Before contact with the reaction mixture samples were pretreated in situ in oxygen within 25-500°C temperature range with a ramp rate of 10 oC/min. NO and NO2 content was monitored by means of AO2000 gas analyzer.
The high resolution transmission electron microscopy (HRTEM) images of the samples after the catalytic tests were carried out in a FEI Tecnai F30 transmission electron microscope.
3 Results and discussion
The chemical composition of the samples is presented in the Table 1. Ni content obtained experimentally was compared with theoretical exchange capacity of the mordenite samples, taking into account their MR value. It means, that as each atom of Al isomorphously substituting Si atom in zeolite framework generate one negatively charged tetrahedra, which have to be equilibrated by positively charged ion. Starting zeolites materials studied in this paper were equilibrated by sodium, ammonium and proton cations. In addition, applied zeolites are characterized with different SiO2/Al2O3 molar ratio, that is, with different amount of cations to be exchanged. Therefore, amounts of Ni2+ substituted cations in elementary cells of Mor13, Mor20, and Mor90 has to be different.
Indeed, NiNaMor13 is characterized with higher content of Ni than two other samples (NiNH4Mor20 and NiHMor90). Therefore, increasing of MR decreases the amount of exchanged Ni. The variation of percent of Ni exchange degree and the lowest observed value for NiMor20 probably is due to difference in cation selectivity for Ni2+ → Na+, Ni2+ → NH4+ and Ni2+ → H+ equilibria, complicated by MR dependence. But detailed study of thermodynamic of ion exchange of nickel as a function of exchangeable cations and molar ratio in this set of samples is out of scope of present work.
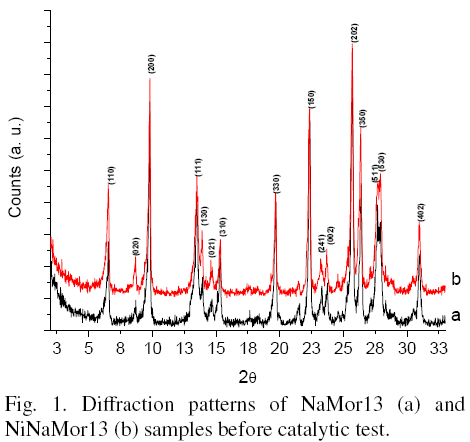
XRD analysis of catalysts was carried out to confirm stability of the zeolites in initial, exchanged, and spent materials. All samples before and after treatments showed patterns typical for mordenite structure. As an example, Fig. 1 shows the diffraction patterns of starting NaMor13 and exchanged NiNaMor13 samples. No change in the structure and crystallinity of samples was observed after ion exchange. Therefore, one can conclude that no distortion of the mordenite structure during exchange treatment at slightly elevated temperature was observed.
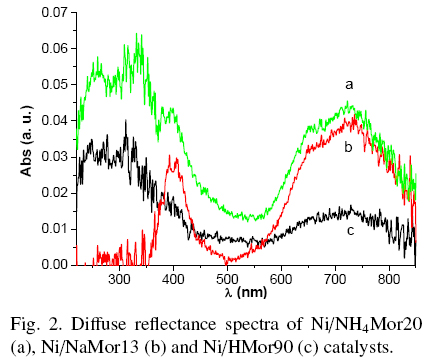
In the UV-Vis spectra of dried Ni-exchanged samples a band at 390-400 nm and a doublet with a maxima at 650 and 720 nm were observed (see Fig. 2), those are representative for Ni2+ ions in an octahedral oxygen environment, typically Ni(H2O)2+6 due to a transition A2g → T1g Lever (1984). Also, spectra of NiNH4Mor20 and NiHMor90 showed absorption around 250-300 nm that can be due to partial dehydration on Ni2+ ions and their coordination with O2- of zeolite framework, forming in this way the electron donor-acceptor complex. Therefore, a band of charge-transfer complex can be expected in this region Lever (1984). In contrast, the NiNaMor13 sample shows no absorbance in the ultraviolet region (λ ~ 200-350 nm) unlike the other two samples. From this observation, one can deduce that in NiNH4Mor20 and NiHMor90 samples nickel species are more strongly linked to the zeolite lattice, in contrary to those in NiNaMor13.
The curves of NO conversion on prepared catalysts are presented in the Fig. 3. NiHMor90 sample shows low activity within studied temperature interval. The value of NO conversion does not exceed 3%.
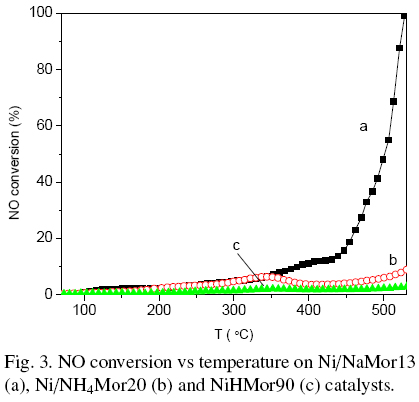
Catalytic activity of other two samples up to the temperature of 350 oC is also low and comparable to each other. However, further temperature increase leads to drastic increment of catalytic activity only for NiNaMor13 sample up to 100%. The catalytic activity for NiNH4Mor20 sample with increasing of temperature first drops down and than increased, but only up to 8 % at 530 °C.
The similar performance of NiNH4Mor20 and NiHMor90 catalysts seems to be due to partial thermal transformation of ammonium mordenite into protonic form that is carried out at temperatures higher than 350 ° C.
Similar tendencies were observed by Mosqueda-Jimenez et al. (2003) for Ni catalysts supported on mordenite in sodium and protonic forms. They found that the catalytic activity in the SCR of NO with propene and propane was higher for the sodium form of the zeolite. The activity of samples in NO reduction with propene seems to be decreased with increasing of concentration of acid sites due to propene oligomerization and coking Vergne et al. (1998). The later causes blocking of active sites and pores Satsuma et al. (1995).
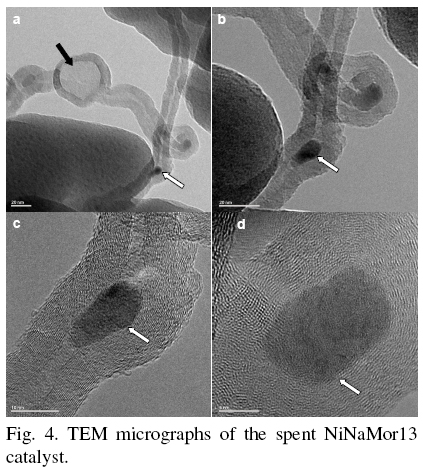
Indeed, analysis of spent catalysts by TEM reveals carbon deposit formation on the surface of NiNaMor13 catalyst (see Fig. 4). Formation of carbon nanotubes containing encapsulated nickel species (marked with white arrows) is observed. Such structures are well known to be formed during the pyrolysis of hydrocarbons over Ni metal particles Komova et al. (2007), Ying et al. (2011). The process of nanotube formation include adsorption of hydrocarbon over Ni surface, decomposition of hydrocarbon with formation of carbon atoms, their diffusion through Ni particles with final assembly of carbon nanotubes on the bottom position of nickel particles Liu et al. (2005). Isotropic carbon diffusion leads to partial Ni particles encapsulation MacKenzie et al. (2010). The shape of the carbon shows that it was formed by jumping of the metal tip at regular time intervals over similar distances (Fig. 4). This effect was explained by supposing that the metal is in a quasi-liquid state with high surface energy and poor wetting ability towards graphite carbon Li et al. (1999) and that the compartments on the inside of the nanotubes might be a kind of mark of the periodic condensation of graphite layers through the catalyst metal Woo Lee et al. (2004) (Fig. 3c). Fig. 3a shows amorphous carbon encapsulated particle (marked with black arrow), which suggests a large growth rates of carbon nanospecies Merkulov et al. (2005).
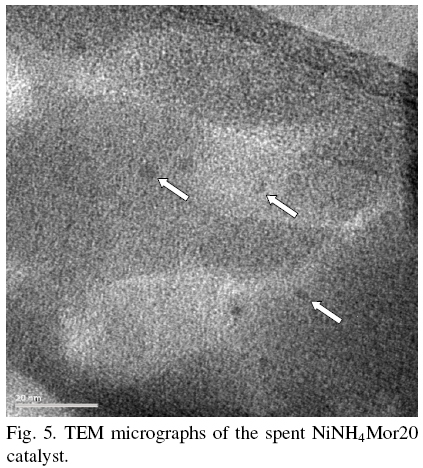
TEM images of NiNH4Mor20 catalyst (Fig. 5), unlike the sample NiNaMOR13, do not shown formation of any carbon nanostructures. Only smaller dark Ni particles (marked with white arrows) are observed. No carbon nanostructures development is observed on the smaller Ni particles.
The results show that the high catalytic activity was found for NiNaMor13 sample only, which is also characterized with carbon nanotube formation. These two facts could be related with the nature of nickel species stabilized in mordenite framework of NiNaMor13 catalyst. The decrease of SiO2/Al2O3 ratio leads to the increase of amount of exchangeable sites (Al-O-(Si-O)N-Al, with N ≤ 3), that is reflected in the increase of Ni exchanged in a NaMor13 sample. However, not only amount of nickel but the strength of Ni interaction with the silica-alumina structure depends on the arrangement of Al-centered tetrahedra in framework Bogdanchikova et al (2000).
Only NiNaMor13 sample is characterized with the presence of Ni species weakly bonded with zeolite framework according to the UV-Vis spectra. The easiness of red-ox transformations of Ni species is directly related with catalytic activity in de-NOx process, as it was shown by Garbowski et al. (1983). In contrary with NiNaMor13 catalyst other two samples manifest strong metal-support interaction by appearance of the charge transfer absorption band in UV-Vis spectra (Fig. 2). Strong interaction with zeolite matrix leads to the difficulties in red-ox transformation of nickel species and as a consequence to the decrease of the activity in NO reduction.
On the other hand, easiness of red-ox transformation of Ni species can provoke easy formation of Ni metal particles in the presence of such reducing agents as CO and propene in the reaction mixture, which is crucial for the formation and growth of carbon nanotubes Li et al. (1999), Height et al. (2005), Liu et al. (2005), MacKenzie et al (2010). Both propene and CO could be considered as precursors for carbon nanotube formation.
For the samples characterized with high strength of Ni-mordenite interaction the formation of carbon nanotubes is not observed. Fig. 5 demonstrates only small Ni particles on the surface of NiNH4Mor20, without any detectable carbon nanostructures.
On the other hand, formed carbon nanotubes could act as active centers, thus allowing the NO reduction to continue at high temperatures Serp et al. (2003). As can be seen from the TEM images (Fig. 4), carbon nanostructures are characterized with a presence of structural defects on their external walls. These defects can serve both as centers for adsorption of reaction mixture components and as reducing reactant. Note that sharp increase in catalytic activity of NiNaMor13 sample begins at the high temperature, characteristic for carbon nanotubes formation over Ni metal particles due to pyrolysis of hydrocarbons Esconjauregui et al. (2009).
Conclusions
It was shown that SiO2/Al2O3 molar ratio is important parameter influencing the interaction of Ni species with mordenite framework. Ni species formed on mordenite NiNaMor13 with low molar ratio are characterized with low strengths of metal-support interaction, as a consequence high catalytic activity in NO reduction. In the same time easy redox transformation of such a species results in the formation of carbon nanostructures, those can be responsible for catalytic activity improvement as well.
Acknowledgements
The authors acknowledge E. Aparicio, I. Gradilla, J. Peralta, V. Gradilla and E. Flores for technical assistance. This research was supported by PAPIIT IN207511, PAPIIT IN110608, PAPIIT IN224510 and CONACYT 102907 grants.
References
Abu-Zied, B.M., Schwieger, W. and Unger A. (2008). Nitrous oxide decomposition over transition metal exchanged ZSM-5 zeolites prepared by the solid-state ion-exchange method. Applied Catalysis B-Environmental 84, 277-288. [ Links ]
Baerlocher, Ch., McCusker, L.B. and Olson D.H. (2007). Atlas of zeolite framework types, 6th edition, Amsterdam: Elsevier. v [ Links ]
Bogdanchikova, N., Petranovskii, V., Machorro Mejia, R., Sugi, Y., Soto, V.M. and Fuentes, S. (1999). Stability of silver clusters in mordenites with different SiO2/Al2O3 molar ratio. Applied Surface Science 150, 58-64. [ Links ]
Bogdanchikova, N., Petranovskii, V., Fuentes, S., Paukshtis, E., Sugi, Y. and Licea-Claverie, A. (2000). Role of mordenite acid properties in silver cluster stabilization. Materials Science and Engineering A 276, 236-242. [ Links ]
Esconjauregui, S., Whelan, C.M. and Maex, K. (2009). The reasons why metals catalyze the nucleation and growth of carbon nanotubes and other carbon nanomorphologies. Carbon 47, 659-669. [ Links ]
Gurin, V.S., Petranovskii, V.P. and Bogdanchikova, N.E. (2002). Metal clusters and nanoparticles assembled in zeolites: an example of stable materials with controllable particle size. Materials Science and Engineering C 19, 327331. [ Links ]
Gurin, V., Petranovskii, V., Hernandez, M.-A., Bogdanchikova, N. and Alexeenko, A.A. (2005). Silver and copper clusters and small particles stabilized within nanoporous silicate-based materials. Materials Science and Engineering A 391, 71-76. [ Links ]
Height, M.J., Howard, J.B., Tester, J.W. and Vander Sande, J.B. (2005). Carbon nanotube formation and growth via particle-particle interaction. The Journal of Physical Chemistry B 109, 12337-12346. [ Links ]
Hu, Y., Griffiths, K. and Norton, PR. (2009). Surface science studies of selective catalytic reduction of NO: Progress in the last ten years. Surface Science 603, 1740-1750. [ Links ]
Johnson T.V. (2009). Review of diesel emissions and control. International Journal of Engine Research 10, 275-285. [ Links ]
Komova, O.V., Simakov, A.V., Kovalenko, G.A., Rudina, N.A., Chuenko, T.V. and Kulikovskaya, N.A. (2007). Formation of a nickel catalyst on the surface of aluminosilicate supports for the synthesis of catalytic fibrous carbon. Kinetics and Catalysis 48, 803-811. [ Links ]
Lever, A.B.P. (1984). Inorganic electronic spectroscopy, 2nd edition, Elsevier, Amsterdam - New York. [ Links ]
Li, Y., Chen, J., Ma, Y., Zhao, J., Qin, Y. and Chang, L. (1999). Formation of bamboo-like nanocarbon and evidence for the quasi-liquid state of nanosized metal particles at moderate temperatures. Chemical Communications 12, 1141-1142. [ Links ]
Liu, H., Cheng, G., Zheng, R. and Zhao, Y. (2005). Controlled growth of Ni particles on carbon nanotubes for fabrication of carbon nanotubes. Journal of Molecular Catalysis A: Chemical 225, 233-237. [ Links ]
MacKenzie, K.J., Dunens, O.M. and Harris, A.T. (2010). An updated review of synthesis parameters and growth mechanisms for carbon nanotubes in fluidized beds. Industrial and Engineering Chemistry Research 49, 5323-5338. [ Links ]
Martinez, C. and Corma, A. (2011). Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coordination Chemistry Reviews 255, 1558-1580. [ Links ]
Merkulov, I.A., Meleshko, A.V., Wells, J.C., Cui, H., Merkulov, V.I., Simpson, M.L. and Lowndes, D.H. (2005). Two growth modes of graphitic carbon nanofibers with herring-bone structure. Physical Review B 72, 045409. [ Links ]
Mosqueda-Jiménez, B.I., Jentys, A., Seshan, K. and Lercher, J.A. (2003). Reduction of nitric oxide by propene and propane on Ni-exchanged mordenite. Applied Catalysis B: Environmental 43, 105-115. [ Links ]
Parvulescu V.I., Grange P. and Delmon B. (1998). Catalytic removal of NO. Catalysis Today 46, 233-316. [ Links ]
Rebrov, E.V., Simakov, A.V., Sazonova, N.N. and Stoyanov, E.S. (1999). Dinitrogen formation over low-exchanged Cu-ZSM-5 in the selective reduction of NO by propane. Catalysis Letters 58, 107-118. [ Links ]
Rebrov, E.V., Simakov, A.V., Sazonova, N.N. and Stoyanov, E.S. (2000). Rate-determining stage in NO SCR with propane on low-exchanged Cu-ZSM-5 catalyst. Catalysis Letters 64, 129-134. [ Links ]
Satsuma, A., Yamada, K., Mori, T., Niwa, M., Hattori, T. and Murakami, Y. (1995). Dependence of selective reduction of NO with C3H6 on acid properties of ion-exchanged zeolites. Catalysis Letters 31, 367-375. [ Links ]
Sazonova, N.N., Komova, O.V., Rebrov, E.V., Simakov, A.V., Kulikovskaya, N.A., Rogov, V.A., Olikhov, R.V. and Barannik, G.V. (1997). Catalytic and adsorptive properties of Cu-ZSM-5 catalyst synthesized by solid phase method. Reaction Kinetics and Catalysis Letters 60, 313-321. [ Links ]
Serp, P., Corrias, M. and Kalck P. (2003). Review. Carbon nanotubes and nanofibers in catalysis. Applied Catalysis A: General 253, 337-358. [ Links ]
Vergne, S., Berreghis, A., Tantet, J., Canaff, C., Magnoux, P., Guisnet, M., Davias, N. and Noirot, R. (1998). Reduction of NO by propene over a Cu-MFI catalyst. Investigation of the mechanism from the composition of compounds trapped in the zeolite pores. Applied Catalysis B: Environmental 18, 37-50. [ Links ]
Woo Lee, G., Jurng, J. and Hwang, J. (2004). Formation of Ni-catalyzed multiwalled carbon nanotubes and nanofibers on a substrate using an ethylene inverse diffusion flame. Combustion and Flame 139, 167-175. [ Links ]
Yahiro H. and Iwamoto M. (2001). Copper ion-exchanged zeolite catalysts in de-NO(x) reaction. Applied Catalysis A-General 222, 163-181. [ Links ]
Ying, L.S., Salleh, M.A. B., Yusoff, H.B. M., Rashid, S.B. A. and Abd Razak, J.B. (2011). Continuous production of carbon nanotubes - A review. Journal of Industrial and Engineering Chemistry 17, 367-376. [ Links ]














