Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ingeniería química
versión impresa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.11 no.3 Ciudad de México dic. 2012
Biotecnología
Zymogram patterns of extracellular laccases of Pleurotus species grown on non-inducer agar medium
Patrón zimográfico de lacasas extracelulares de especies de Pleurotus crecidos en un medio no-inductor
M. Téllez-Téllez, C. Sánchez, R. Díaz and G. Díaz-Godínez*
Laboratory of Biotechnology, Research Centre for Biological Sciences, Universidad Autónoma de Tlaxcala, Tlaxcala CP 90000, México. * Corresponding author. E-mail: diazgdo@hotmail.com Tel. & fax: +52-248-48-15482
Received 24 of April 2012
Accepted 20 of September 2012
Abstract
Zymogram patterns of extracellular laccases of ten strains of Pleurotus grown on agar without addition of inducers, using 2.6-dimethoxyphenol, ρ-anisidine and o-tolidine as substrates, were obtained. Zymogram patterns were only similar for strains within same species, independently of the substrate used, six strains op P. octreatus and one strain of P. ostreatus var. florida showed three isoenzymes, two strains of P. pulmonarius showed two isoenzymes and one strain of P. cornucopiae also showed two isoenzymes but in a different position in comparison to the other strains. These results showed that Pleurotus species produce a basal level of laccase activity and the number of extracellular laccase isoenzymes is species dependent.
Keywords: Pleurotus, isoenzyme, enzymatic activity, zymogram, laccases.
Resumen
Se obtuvo el patrón zimográfico de lacasas extracelulares de diez cepas de Pleurotus crecido sobre agar sin la adición de inductor, usando 2.6-dimethoxifenol, ρ-anisidina y o-tolidina como sustratos. Los patrones zimográficos fueron similares para las cepas de la misma especie, independientemente del sustrato utilizado, seis cepas de P. ostreatus y una cepa de P. ostrectus var. florida mostraron tres isoenzimas, dos cepas de P. pulmonarius mostraron dos isoenzimas y una cepa P. cornucopiae también mostró dos isoenzimas pero en diferente posición en comparación con las otras cepas. Estos resultados mostraron que las especies de Pleurotus producen un nivel basal de actividad de lacasa y que el número de isoenzimas de lacasas extracelulares dependen de la especie.
Palabras clave: Pleurotus, isoenzima, actividad enzimatica, zimograma, lacasas.
1 Introduction
White rot fungi produce several isoenzymes of extracellular oxidases and peroxidases, which are involved in the degradation of lignin in their natural environments (Palmieri et al., 2000). Pleurotus ostreatus belongs to a subclass of lignin-degrading microorganisms that produce laccases, manganese peroxidases and veratryl alcohol oxidases but no lignin peroxidases (Palmieri et al., 1997). Laccases (benzendiol:oxygen oxidoreductases, EC 1.10.3.2) are Cu-containing glycoproteins which require O2 to oxidize phenols, polyphenols, and aromatic amines as well as non-phenolic organic substrates by one-electron abstractions resulting in the formation of H2O and reactive radicals undergoing further depolymerization, repolymerization, demethylation, dehalogenation, or quinine formation (Claus, 2004).
Although their specific physiological functions are not completely understood, there are several indications that laccases are involved in the morphogenesis of microorganisms (e.g., fungal spore development, melanization) and in the formation and/or degradation of complex organic substances such as lignin or humic matter. Due the catalytic action of laccases, these enzymes can be used for various biotechnological and environmental applications such as textile dye decolouration, delignification, pulp bleaching, effluent detoxification, biosensing, and bioremediation (Thurston, 1994; Hublik and Schinner, 2000; Mayer and Staples, 2002). Solis-Oba et al. (2007), produced an active, stable and oxidized form of the chemical mediator ABTS (2,2'-azino-bis-(3- ethylbenzothiazoline-6-sulphonic acid)) and observed that oxidation of some compounds by a mixture of ABTS and laccase can be done in a cyclic manner, such a way the oxidation rate was 94 times higher for indigo, 17 times for brilliant blue G, 34 times for orange 7 and 5 times higher for p-cresol compared with using the mediator or the laccase alone.
As well as other enzymes, including tannases, pectinases, chitinases, etc., the laccase expression is affected by culture and environmental conditions, mainly the type and concentration of carbon sources (Belmares-Cerda et al., 2003; Díaz-Godínez et al., 2001; Sastoque-Cala et al., 2007). On the other hand, it has been reported that laccases are secreted in multiple isoforms (Mansur et al., 1998; Téllez-Téllez et al., 2005; Téllez-Téllez et al., 2008). Additionally, the expression of different patterns of laccase isoenzymes coded by gene families is differentially regulated, depending on the growth conditions (Bollag and Leonowicz, 1984; Rogalski and Leonowicz, 1992) and physiological states (Rogalski et al., 1993; Mansur et al., 1998).
There are several reports about the effect of inducer or the cultural composition and environmental conditions on the laccase production. In recent years, there has been a renewed interest in solid-state fermentation processes due to its high productivity of bioactive compounds in comparison to submerged fermentation (Ruiz-Leza et al., 2007). Téllez-Téllez et al. (2008), reported an atypical behavior of Pleurotus ostreatus, they observed that the isoenzymes laccase number and the laccases activity were higher in submerged than in solid-state fermentation. On the other hand, there are few reports about the laccases production without inducer addition. In this research, the extracellular laccase activity on three substrates and the isoenzymes number, of ten strains of Pleurotus sp. grown on agar medium without addition of any inducer were evaluated.
2 Methodology
2.1 Strain, media, and growth conditions
Ten strains of Pleurotus were studied; P. ostreatus 32783 (Po-83), P. ostreatus 38537 (Po-37), P. ostreatus 58052 (Po-52), P. ostreatus 201218 (Po-7) and P. ostreatus 201216 (Po-3) from the ATCC collection, P ostreatus 3526 (Po-26) from the NRRL, P. pulmonarius Pp-134 and P. pulmonarius Pp-127 from the Chinese University of Hong Kong Collection, P. ostreatus var. florida (Pfl) and P cornucopiae (Pcc) from the Mushroom Experimental Station, Horst, The Netherland.
Inoculum was taken from the periphery of colonies growing on potato dextrose agar at 28ºC for 7 days, by using a sterile cork borer (4 mm diam.). The mycelial plug was placed (mycelium facing-down) on the center of the culture medium. In all experiments, well-developed colonies grown on Petri dishes were used. The composition of culture medium was (in g/L): starch, 10.5; (NH4)2SO4, 1.0; KH2PO4, 0.5; MgSO4 • 7H2O, 0.5; Ca(H2PO4)2- H2O, 0.3; FeSO4 • 7H2O, 0.02; ZnSO4 • 7 H2O, 0.02; MnSO4• H2O, 0.02 (Sanchez and Viniegra-Gonzalez, 1996). All the strains were grown at 25ºC, except the strains of P pulmonarius, which were grown at 28ºC.
2.2 Extracellular extract
The extracellular enzymatic extract (EE) was obtained from the agar with deionized water after removing the mycelium from the surface of each colony for every strain. All the EE were centrifuged at 20 000 X g for 10 min at 2ºC.
2.3 Laccase activity assay
In EE, laccases activity was assayed using 2,6-dimethoxyphenol (DMP), p-anisidine and o-tolidine. For DMP the assay mixture contained 950 μl of substrate (2 mM of DMP in 0.1M phosphate buffer, pH 6.0) and 50 μl of EE, which were incubated at 39ºC for 15 min. Oxidation of DMP was followed by absorbance increase at 468 nm. For those assays with p-anisidine, reaction mixture contained 950 μl of substrate (10 mM of p-anisidine in 0.1M phosphate buffer, pH 6.0) and 50 μl of EE, which were incubated at 39ºC for 15 min. Oxidation of p-anisidine was followed by absorbance increase at 460 nm. With o-tolidine as a substrate, the assay mixture contained 950 μl of substrate (2 mM of o-tolidine in 0.1M acetate buffer, pH 3.7) and 50 μl of EE, which were incubated at 30ºC for 15 min. Oxidation of o-tolidine was followed by absorbance increase at 627 nm.
One enzymatic unit (U) of laccases activity was defined as the amount of enzyme, which gave an increase of 1.0 unit of absorbance per min in the reaction mixture. The laccases activity was expressed in U per g of dry biomass (U/g X).
2.4 Laccase zymograms
The extracellular laccases activity was also detected through zymograms (Téllez-Téllez et al., 2005; Téllez-Téllez et al., 2008). The running gel contained 100 g acrylamide/L and 27 g bis-acrylamide/L. The stacking gel contained 40 g acrylamide/L and 27 g bis-acrylamide/L. Each EE (30 μL aprox.) was mixed with sample buffer without a reducing agent the disulfide bonds. Without heating, the samples were placed in gels (thickness 0.75 mm) of Mini-Protean III electrophoresis system (BioRad) and then 150 V was applied for 1-1.25 h. After the electrophoresis, gels were washed with deionized water on an orbital shaker (20-30 rpm) for 1 h, water was changed every 15 min to remove SDS. Finally, the gels were incubated at room temperature in substrate solutions (either 2 mM of DMP, 10 mM of p-anisidine or 2 mM of o-tolidine). Laccase activity bands appeared on the gel by the oxidation of the substrate after approx. 1 h.
3 Results and discussion
The extracellular laccase activity of ten strains of Pleurotus, were evaluated on different substrates (Table 1). In general, the strains Po-7, Po-26 and Pcc showed the highest laccase activity in the three substrates. Pfl, Po-3 and Po-52 showed the lowest laccase activity in all the substrates. The highest laccase activity was observed with DMP followed by o-tolidine and p-anisidine for all the strains. The laccase activity was result of the action of the all isoenzymes present in each EE, and the same isoenzymes oxidized the three substrates used.
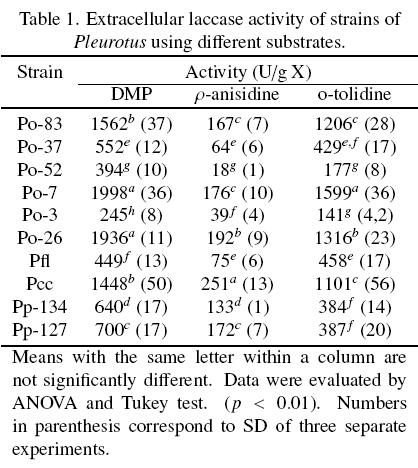
The Fig. 1 shows the zymogram patterns of extracellular laccase activity using DMP, o-tolidine and p-anisidine as substrates. The patterns of each strain were independent of the substrate used and were similar between species. The strains of P. ostreatus showed three isoenzymes. Li and Eger (1979) isolated a strain of Pleurotus from Florida U.S.A., that was called P. florida and later classified as P. ostreatus "var. florida". The two strains of P pulmonarius and the strain of Pcc showed two isoenzymes to different position on the gel.
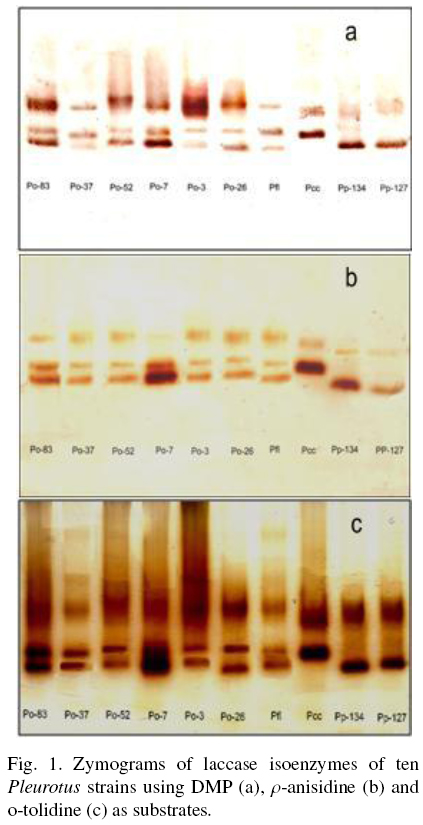
It has been reported that the laccases expression exhibit differential regulation, then, laccases activity and number of isoenzymes are influenced by environmental conditions such as pH, temperature, inductors, and culture medium conditions (Giardina et al., 1999; Téllez-Téllez et al., 2008). Téllez-Téllez et al. (2005) reported a basal intracellular laccases activity in Pleurotus species, with at least two isoenzymes for P. ostreatus and P. pulmonarius and one isoenzyme for P cornucopiae. Téllez-Téllez et al. (2008) reported that Po-83 produced three and four extracellular isoenzymes using Cu as inducer in solid-state fermentation (SSF) and in submerged fermentation (SMF) conditions, respectively. In this study, the basal extracellular laccase activity was higher than that previously reported for the intracellular laccase activity of the same strains grown under the same fermentation conditions (Téllez-Téllez et al., 2005). On the other hand, the enzymatic activity using DMP as substrate was around twice and eight times lower than those observed in other study by the same strain in SSF and SMF, respectively (Téllez-Téllez et al., 2008). It was also found that the presence of Cu.
In SMF enhanced the laccase activity around 37, 6, 13, 14 and 60 times which was higher than those previously reported for the strains Po-83, Po-3, Po-7, Po-37 and Po-52, respectively. (Díaz et al., 2011). In addition, the constitutive basal activity enhances when the growing conditions of the fungus are changed. The extracellular laccase activity (1936 U/g X) of the strain Po-26 was approximately 2.4 and 194 times higher than the reported when the same strain was grown on lignocellulosic substrates and in Petri dishes (803 U/g X) respectively (Sainos et al., 2006).
The biological role of the laccases is not fully understood; it appears to vary depending on the type of organism. It has been suggested that fungi have a biodegradation role (degradation of lignin or elimination of those toxic phenols generated by such process), participate in cellular processes, morphogenesis, pigments production, pathogenesis and virulence. However, only few of these functions have been experimentally validated (Eggert et al., 1998). It is known that the extracellular isoenzymes are responsible of the biodegradation role and the intracellular of the rest of the suggested functions, however, some isoenzymes might have both functions (Das et al., 2011).
Conclusion
These results show that the number of extracellular laccase isoenzymes depends on the species, however, the activity is different even between strains from the same species. It could be due to factors related with the habitat conditions of the strain. The zymogram patterns in each strain were similar in all the substrates, showing that all the produced isoenzymes have the ability to catalyze the same substrates. The extracellular laccase activity and the isoenzymes number were higher than those previously reported for the EE intracellular of the same strains.
Acknowledgements
We thank the Mexican Council of Science and Technology (CONACYT) for supporting this research (Project No. 156406).
References
Belmares-Cerda, R., Contreras-Esquivel, J.C., Rodríguez, R., Ramírez-Coronel, A., Aguilar, C.N. (2004). Microbial production of tannase: An enzyme with potential use in food industry. Lebensmittel Wissenschaft und Technologie 37, 857-864. [ Links ]
Bollag, J. and Leonowicz, A. (1984). Comparatives studies of extracellular fungal laccases. Applied and Environmental Microbiology 48, 849-854. [ Links ]
Claus, H. (2004). Laccases: structure, reactions, distribution. Micron 35, 93-96. [ Links ]
Das, N., Naskar, S., Chowdhury, P., Pasman, B. and Adhikari, D. (2011). Experimental Evidence for Presence of a Growth Regulating Extracellular Laccase in Some Pleurotus Species. Research Journal of Microbiology 6,496-502. [ Links ]
Díaz, R., Alonso, S., Sánchez, C., Tomasini, A., Bibbins-Martínez, M. and Díaz-Godínez, G. (2011). Characterization of the growth and laccase activity of strains of Pleurotus ostreatus in submerged fermentation. BioResources 6, 282-290. [ Links ]
Díaz-Godínez, G., Soriano, J., Augur, C., and Viniegra-González, G. (2001). Exopectinases produced by Aspergillus niger in solid-state and submerged fermentation: A comparative study. Journal Industrial Microbiology Biotechnology 26, 271-275. [ Links ]
Eggert, C., LaFayette, P.R., Temp, U., Eriksson, K-E. and Dean, J. (1998). Molecular analysis of a laccase gene from the white rot fungus Pycnoporus cinnabarius. Applied and Environmental Microbiology 64, 1766-1772. [ Links ]
Giardina, P., Palmieri, G., Scaloni, A., Fontanella, B., Faraco, V., Cennamo, G. and Sannia, G. (1999). Protein and gene structure of a blue laccase from Pleurotus ostreatus. Biochemical Journal 34, 655-663. [ Links ]
Hublik, G., Schinner, F. (2000). Characterization and inmobilization of the laccase from Pleurotus ostreatus and its use for the continuous elimination of phenolic pollutants, Enzyme Microbiology Technology 27, 330-336. [ Links ]
Li, S.F. and Eger, G. (1979) Characteristics of some Pleurotus strains from Florida, their practical and taxonomic importance. Mushroom Science 10, 155-169. [ Links ]
Mansur, M., Suarez, T. and González, A.E. (1998). Differential gene expression in the laccase gene family from Basidiomycete I-62 (CECT 20197). Applied Environmental Microbiology 64, 771-774. [ Links ]
Mayer, A.M and Staples R.C. (2002). Laccase: new functions for an old enzyme. Phytochemistry 60, 551-565. [ Links ]
Palmieri, G., Giardina, P., Bianco, C., Fontanella, B. and Sannia, G. (2000). Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Applied Environmental Microbiology 66, 920-924. [ Links ]
Palmieri, G., Giardina, P., Bianco, C., Scaloni, A., Capasso, A. and Sannia, G. (1997). A novel white laccase from Pleurotus ostreatus. The Journal Biological Chemistry 272, 31301-31307. [ Links ]
Rogalski, J. and Leonowicz, A. (1992). Phlebia radiata lactase forms induced by veratric acid and xylidine in relation to lignin peroxidase and manganese-dependent peroxidase. Acta Biotechnological 12, 213-221. [ Links ]
Rogalski, J., Hatakka, A., Longa, B. and Wojtas-Wasilewska, M. (1993). Hemicellulolytic enzymes of the ligninolytic white-rot fungus Phlebia radiata: infuence of phenolic compounds on the synthesis of hemicellulolytic enzymes. Acta Biotechnologica 13, 53-57. [ Links ]
Ruíz-Leza, H. A., Rodríguez-Jasso, R. M., Rodríguez-Herrera, R., Contreras-Esquivel, J.C., and Aguilar, C. N. (2007). Bio-reactors desing for solid state fermentation. Revista Mexicana de Ingeniería Química 6, 33-40. [ Links ]
Sainos, E., Díaz-Godínez, G., Loera, O., Montiel-González, A.M. and Sánchez, C. (2006). Growth of Pleurotus ostreatus on wheat straw and wheat-grain-based media: biochemical aspects and preparation of mushroom inoculum. Applied Microbiology Biotechnology 72, 812-815. [ Links ]
Sánchez, C. and Viniegra-González, G. (1996). Detection of highly productive strains of Pleurotus ostreatus by their tolerance to 2-deoxy-D-glucose in starch-based media. Mycological Research 100, 455-461. [ Links ]
Sastoque-Cala, L., Mercado-Reyes, M. Martínez-Salgado, M.M., Quevedo-Hidalgo, B. and Pedroza-Rodríguez, A.M. (2007). Production of extracellular chitinases from alkalophilic moderately Halophilic Streptomyces sp. isolated of shrimp waste. Revista Mexicana de Ingeniería Química 6, 137-146. [ Links ]
Solís-Oba, M., Barzana, E., García-Garibay, M. Viniegra-González, G. (2007). The ABTS•+an oxidant agent of different chemical compounds and its recycling process between laccase and substrate. Revista Mexicana de Ingeniería Química 6, 275-281. [ Links ]
Téllez-Téllez, M., Fernández, J.F., Montiel-González, A.M., Sánchez, C. and Díaz-Godínez, G. (2008). Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Applied Microbial Biotechnology 81, 675-679. [ Links ]
Téllez-Téllez, M., Sánchez, C., Loera, O. and Díaz-Godínez, G. (2005). Differential patterns of constitutive intracellular laccases of the vegetative phase for Pleurotus species. Biotechnology Letters 27, 1391-1394. [ Links ]
Thurston, C.F. (1994). The structure and function of fungal laccases. Microbiology 140, 19-26. [ Links ]














