Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ingeniería química
versão impressa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.10 no.3 Ciudad de México Dez. 2011
Biotecnología
Survivability of entrapped Lactobacillus rhamnosus in liquid– and gel–core alginate beads during storage and simulated gastrointestinal conditions
Sobrevivencia de Lactobacillus rhamnosus entrampados en cápsulas de alginato con centro líquido y gelificado durante el almacenamiento y en condiciones gastrointestinales simuladas
M.E. Rodríguez–Huezo1, C. Lobato–Calleros2, J.G. Reyes–Ocampo3, O. Sandoval–Castilla3, C. Perez–Alonso4 and D.J. Pimentel–Gonzalez5*
1 Departamento de Ingeniería Química y Bioquímica, Tecnológico de Estudios Superiores de Ecatepec, Ecatepec, Edo. México, México.
2 Departamento de Preparatoria Agrícola, Universidad Autónoma Chapingo, Estado de México, México.
3 Departamento de Biotecnología, Universidad Autónoma Metropolitana Unidad–Iztapalapa, México D.F.
4 Departamento de Ingeniería Química, Facultad de Química, Universidad Autónoma del Estado de México, Toluca, Estado de México, México.
5 Instituto de Ciencias Agropecuarias, Universidad Autónoma del Estado de Hidalgo, Av. Rancho Universitario S/N Km. 1, CP 43600 Tulancingo, Hgo., México. *Corresponding author. E–mail: pimentel@uaeh.edu.mx Tel. + (52) 7717172000 Fax: + (52) 7717172125.
Received 7 of June 2011.
Accepted 12 of October 2011.
Abstract
L. rhamnosus cells were encapsulated in liquid–core (LCBR) and gel–core (GCBR) calcium alginate beads, and cell survivability under storage conditions and simulated gastrointestinal conditions were evaluated, and compared with that of non–encapsulated cells. The average external diameters of both beads (1.37 ± 0. 25 mm) were non–significantly different, and the average thickness of alginate gelled layer in LCBR was of 0.27 ± 0.01 mm. The bacteria entrapped into LCBR tended to gather together forming clusters in the bulk of the liquid phase of the bead, whereas the bacteria entrapped into GCBR were compartmentalized in the gelled bead biopolymer matrix. LCBR showed significant lower hardness and chewiness, higher cohesiveness, and comparable springiness values than GCBR. Cells survivability under storage and simulated gastrointestinal conditions was significantly higher in LCBR than in GCBR and for the non–encapsulated free cells.
Keywords: encapsulation, gelation, sequestration, Lactobacillus rhamnosus, liquid core beads, survivability.
Resumen
Células de L. rhamnosus fueron entrampadas en cápsulas de alginato de calcio con centro líquido (LCBR) y centro gelificado (GCBR), evaluando la sobrevivencia de las células bajo condiciones de almacenamiento y condiciones gastrointestinales simuladas en comparación con las de células sin entrampar. No hubo diferencias significativas entre los diametros externos promedio (1.337 ± 0.25 mm) de ambas cápsulas, mientras que el grosor promedio de la capa gelificada en LCBR fue de 0.27 ± 0.01 mm. Las bacterias entrampadas en LCBR tendieron a agregarle en el seno de la fase líquida de la cápsula, mientras que las bacterias entrampadas en GCBR se distribuyeron homogéneamente en la matriz biopolimérica de la cápsula. LCBR mostro valores de dureza y masticabilidad significativamente menores, una cohesividad significativamente mayor y una elasticidad comparable a los exhibidos por GCBR. La sobrevivencia de las células bajo condiciones de almacenamiento y condiciones gastrointestinales simuladas fue sigaificativamente mayor en LCBR que en GCBR y para las células libres sin encapsular.
Palabras clave: entrampamiento, gelación, agente quelante, Lactobacillus rhamnosus, cápsula de centro líquido, sobrevivencia.
1 Introduction
The lactic acid bacteria (LAB) Lactobacillus rhamnosus is considered as probiotic microorganism as it inhibits pathogens growth and has the capability to adhere to intestine cells (Figueroa–Gonzalez et al., 2010). However, probiotic bacteria can only exert positive health effects if they reach their site of action in adequate numbers (Sultana et al., 2000). Many studies have shown that probiotics viable cells number is considerably reduced when they are incorporated into dairy products, during storage, and in transit through the gastrointestinal tract (Rao et al., 1989). Thus, an ongoing research topic is to develop inexpensive and readily available methods that may help improve the survivability and achieve high viable cell numbers of probiotic bacteria. Microencapsulation techniques have been successfully used to improve the survivability of probiotic microorganisms in dairy products (Picot and Lacroix, 2004) or/and under artificial gastrointestinal juice (Chandramouli et al., 2004). Food–grade polymers such as alginate, chitosan, carboxymethylcellulose, carrageenan, gelatin and pectin have been used as microcapsules forming materials using various microencapsulation technologies (Anal and Singh, 2007). In particular, calcium alginate gel is widely used to encapsulate probiotic bacteria because of its biocompatibility and because the gelling procedure is very simple and occurs under mild conditions (Wang et al., 1995). However, probiotic cells entrapped in alginate beads tend to grow at the periphery of the calcium alginate beads, resulting in an increased cells leakage release caused by gel surface breakage (Seki et al., 1990). Moreover, as cell density increases in alginate gels, the strength of polymeric matrices decreases (Nussinovitch et al., 1996b); an expansion of alginate beads may occur with cell growth (Koyama and Seki, 2004); and apparent diffusivities of substrates and products within the gel decrease. Diffusion of substrates and products through beads occurs and is determined by pore size of the gel, reflected by the viscosity of the carrier, due to the size of the molecule, and/or its concentration can affect the diffusion of the substrates or products and limit the reaction rates of the encapsulated cells (Tanaka et al., 1984).
Probiotic cell entrapment in liquid–core capsules constitutes an alternative cell immobilization procedure that may overcome drawbacks encountered in gel–core capsules entrapment. In liquid core beads, the probiotic cells are suspended in a liquid medium, but on turn surrounded by a thin layer of alginate gel, which allows for a uniform cell growth and reduces mass transfer limitations. At the same time, the semi–permeable membrane layer acts as a barrier to cell release and minimizes phage contamination (Dembczynski and Jankowski, 2002). In order to obtain a liquid–core capsule, a calcium sequestering agent must be used for controlling the calcium alginate gelling rate (Bennett, 1989). The calcium binding competition between the sequestering agent and sodium alginate results in a slowing down of the rate of alginate gelling, and thereby leading to a successful liquid–core calcium alginate bead formation (Monshipouri and Price, 1995).
While a lot of information is available regarding the obtention and properties of gel–core beads, little is known about liquid–core beads including their mechanical and diffusive properties (Nussinovitch et al., 1996a).
The aim of this work was to compare the survivability of entrapped L. rhamnosus in liquid–core and gel–core alginate beads, during storage and after exposure to simulated gastrointestinal conditions.
2 Materials and methods
2.1 Materials
Low molecular weight sodium alginate (SA), with 60.5% guluronic acid content was purchased from Danisco Mexico, S.A. of C.V. (Grindsted alginate FD 175). Sodium citrate (SC), calcium chloride (CaCl2), and analytical grade methanol (MeOH) were obtained from J.T. Baker Chemical Co., Phillipsburg, NJ. Bacto peptone, de Man Rogosa Sharpe (MRS) lactobacilli broth and agar were purchased from BD DIFCO Becton Dickinson and Company (Detroit, Mi).
2.2 Bacterial strain and culture
A lyophilized culture of L. rhamnosus LC705 (DANISCO, Niebull, Germany) was activated by inoculating twice in MRS broth (1 g 100 ml–1) for 18 h at 37 °C (Pimentel–Gonzalez et al., 2009). The cells were harvested in the late exponential growth phase (18 h, 37 °C) by centrifugation at 15800 g for 10 min using a minispin plus centrifuge (Eppendorf Centrifuge Type 22331, Eppendorf AG, Hamburg, Germany). The supernatant was discarded and the cells were re–suspended in fresh and sterile MRS broth in order to obtain a cell concentrate (CC) containing a load of 11.34 log10 cfu ml–1.
2.3 Beads forming procedure
A preliminary study was carried out in order to formulate and prepare mechanically stable liquid–core beads (LCB) using an aqueous dispersion of SA (Fiszman et al., 2002). To this end, the initial attempt was to determine the concentration of SC that would slow down the crosslinking between SA and CaCl2 in aqueous dispersion, but at the same time would permit the formation of stable beads with a liquid–core by the extrusion technique used in this work. Beads with liquid–core volumes larger than 58% presented a weak structure prone to rupture and were discarded. It was found that an aqueous dispersion formed by 1g 100 ml–1 of SA and 0.2 g 100 ml–1 SC (SASC), and an aqueous dispersion containing 0.08 g 100 ml–1 CaCl2 yielded mechanically stable beads having a liquid–core that represented from 40–57 % of the total volume of the beads, and these conditions were used for obtaining the LCB containing L. rhamnosus (LCBR). Thus, the detailed method for obtaining the LCBR beads was as follows: CC containing a load of 11.34 log10 cfu ml–1 was blended in a volume ratio of 1:9 with SASC aqueous dispersion (sterilized at 121 °C, 15 min) obtaining CC–SASC suspension. The beads were formed by Muthukumarasamy et al. (2006) modified method. Fifty milliliters of CC–SASC suspension were dripped into one hundred milliliters of sterile CaCl2 solution (0.08 g 100 ml–1) using a peristaltic pump with a tubing discharge of 1 mm of diameter and a flow of 3 ml min–1 (Cole–Parmer, Chicago Il) applying continuous agitation at 100 rpm. After the CC–SASC suspension addition to CaCl2 solution was ended, the formed capsules were left to stand in this calcium chloride solution for 10 min, then they were filtered through a stainless steel mesh of 0.19 mm, rinsed with sterile physiological saline solution (0.08 g of NaCl 100 ml–1 for 3 min) and subsequently kept in sterile physiological saline solution at 4 °C.
The same general procedure was followed for obtaining the gel core beads (GCB) containing L. rhamnosus (GCBR), 50 ml of CC–SA suspension were dripped into 100 ml of a CaCl2 solution (0.08 g CaCl2 100 ml–1), but the GCBR were left to rest in the calcium chloride solution for 1 h for complete hardening.
2.4 Entrapped L. rhamnosus enumeration
In order to release the entrapped L. rhamnosus cells, 1 g of the beads variations were suspended in 9 ml of a SC solution (0.5 g 100 ml–1), followed by gentle shaking at room temperature (20 ± 2 °C) for 1 min (Nedovic et al., 2001). Numbers of viable cells in the suspensions were determined by plate counting using MRS agar with incubation at 37 ° C for 48 h (Pimentel–Gonzalez et al., 2009), and reported as log10 cfu ml–1.
2.5 Beads diameter and volume
The external diameter (De) of 30 freshly prepared LCBR and GCBR was measured with a digital micrometer caliper (Starrett model 436–1 IN, Leon Weill, Mexico City, Mexico), and the radius (Re) was calculated. The thickness of alginate gelled layer (GLt) of LCBR was estimated as follows: 30 beads were cut in half, the liquid core drained, and the thickness of the remaining bead shell was measured at least five times at different locations with the micrometer caliper. Liquid–core volumes (Vlc) of the LCBR were calculated by subtracting GLt from Re and considering a spherical morphology.
2.6 Beads microstructure
LCBR and GCBR were prepared for observing their structure under low and high vacuum conditions with a JEOL Scanning Electron Microscope JMS–035 (Jeol Ltd., Akishima, Japan), operated at 20 kV. The beads variations were cut in half, and for low vacuum observation, samples were mounted on scanning electron microscopy (SEM) stubs with the inner surface up, and observed at a magnification of 200×. For high vacuum inspection, the samples were fixed in 2 % v/v buffered glutaraldehyde (0.1 M phosphate buffer, pH 7.2) at room temperature for 6 h, washed with phosphate buffer, dehydrated by immersion in aqueous ethanol solutions of increasing concentration (50, 60, 70, 80, 90 and 100 % v/v, 30 min in each), and placed in acetone for 1 h (Lobato–Calleros et al., 2006). Samples were then critical point dried in a CPA II Techniques Critical Point Dryer (Tousimis, Rockville, MD), coated with a thin layer of gold in a Fine Coat Ion Sputter JFC 1100 (Jeol Ltd., Akishima, Japan), mounted on SEM stubs with their inner surface up, and observed at a magnification of 5000x. Representative micrographs were selected for presentation.
2.7 Textural properties ofthe capsules
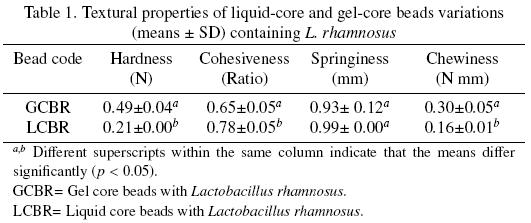
Textural properties of the LCBR and GCBR were determined by Texture Profile Analysis (TPA) (Bourne, 2002) using a Stable Micro Systems Texturometer model TA–XT2i (Texture Technologies Corp., White Plains, NY) equipped with a 25 kg load cell, and a cylindrical steel probe with a large contact area (35 mm in diameter). Each measurement was carried out at room temperature on 30 g of beads placed on a fixed bottom glass plate under the probe. The automatic detection of the contact by the probe with the beads was carried out with a contact force of 0.005 N (Artignan et al., 1997). In all cases the samples were compressed 30%, using two compression cycles at a constant crosshead velocity of 0.5 mm s–1 (Sandoval–Castilla et al., 2010). The primary textural properties of hardness (maximum force of the first peak), cohesiveness (ratio of the positive force areas under the second and first peaks), springiness (length of the second compression, from its beginning to its peak/ length of the first compression, from its beginning to its peak) (Artignan et al., 1997), and chewiness (hardness × cohesiveness × springiness) (Lobato–Calleros et al., 2007) were obtained using the equipment's Texture Expert Software for Windows Version 3.2.
2.8 Survivability of entrapped L. rhamnosus during storage
LCBR (8.85 ± 0.09 log10 cfu ml–1), GCBR (8.85 ± 0.13 log10 cfu ml–1), and non–encapsulated L. rhamnosus cells (8.64 ± 0.33 log10 cfu ml–1), the latter used as control, were poured into 50 ml of sterile physiological solution, followed by gentle stirring. All the samples were stored at 4 °C, and the viability of L. rhamnosus was determined during 25 days, at intervals of 5 days, as indicated above. The experimental data (log10 cfu ml–1) were converted into survival percentage by dividing the final viable population between the initial viable population, and multiplying by 100.
2.9 Tolerance ofL. rhamnosus in simulated gastrointestinal conditions
LCBR (8.34 ± 0.21 log10 cfu ml–1) and GCBR (8.54 ± 0.11 log10 cfu ml–1), and non–encapsulated L. rhamnosus cells (8.47 ± 0.03 log10 cfu ml–1) were placed separately into 9 ml of of 0.05 M sodium phosphate buffer solution adjusted to pH 2.3 with 0.1 N HCl (Pimentel–González et al., 2009). Samples were incubated at 37 ºC for 2 h with agitation at 100 rpm. Afterwards, the beads exposed to acid solution were recovered by filtration, washed with sterile physiological solution and put into 9 ml of fresh MRS broth with 0.3 g of bile salts (B8631, Sigma–Aldrich Chemical Co., Steinheim, Switzerland) per 100 ml. On the other hand, 1 ml of the non–encapsulated L. rhamnosus cells acid suspension was placed into the same bile solution as mentioned above. Samples were incubated at 37 ºC for 24 h with continuous stirring at 100 rpm. At the end of acid and bile exposure periods, L. rhamnosus viable cells were enumerated.
2.10 Statistical analysis
All the assays were independently carried out by triplicate. The experimental data were subjected to Simple Classification Variance Analysis and whenever it was adequate to Tukey's test. The significance was established at P < 0.05. All data were analyzed using the NCSS 2000, version 5, software (Wireframe Graphics, Kaysville, UT, USA).
3 Results and discussion
3.1 Characterization of LCBR and GCBR
3.1.1. Beads diameter and volume
Both the LCBR and GCBR displayed spherical morphologies, with average external diameters (De) of 1.37 ± 0.25 mm, which were non–significantly different. The average thickness of alginate gelled layer (GLt) was 0.27 ± 0.01 mm and the liquid alginate core volume (Vlc) fell in–between 45–56 % for LCBR. All the beads obtained exhibited an adequate mechanical stability against rupture under manipulation.
3.1.2 Textural properties of capsules
The extent of the crosslinking reaction between calcium–alginate polymeric networks influenced the mechanical responses to deformation of the beads. LCBR showed significant lower hardness and chewiness, higher cohesiveness, and comparable springiness values than GCBR (Table 1). Hardness, considered as the force required for compressing the sample, and chewiness, which grades the resistance and energy when chewing the sample (Segnini and Dejmek, 1999), was directly proportional to the thickness of the biopolymer gelled fraction in the bead, which on turn was related to extent of the reaction time, so that these parameters were higher for GCBR. Cohesiveness, considered as the amount of sample that deforms rather than splits apart (Segnini and Dejmek, 1999), may be considered as directly proportional to the liquid core fraction in the bead, and this parameter was higher for LCBR. Springiness, is the rate at which the sample returns to its original shape (Moiny, 2002), and both beads possessed an equivalent overall ability to reform broken linkages when stress application was released. (Figura 1)
3.2 Survivability ofLCBR and GCBR
3.2.1 Survivability ofL. rhamnosus during storage
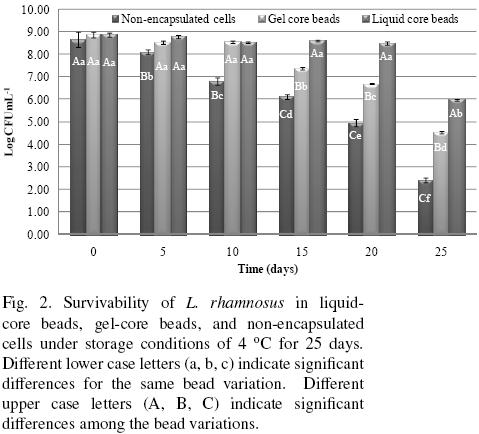
Bacteria survivability data during storage are presented in Fig. 2. The GCBR, LCBR and non–encapsulated free cells initial counts (t = 0) were non–significantly different. However, the non–encapsulated free cells showed a significant decay in cell numbers as storage time increased, experiencing a loss of about 6.24 log cycles after 25 days storage. In contrast, GCBR showed non–significant differences in cell numbers during the first 10 days of storage, but at longer storage showed a progressively significant decrease in cell numbers, accounting for about 4.34 log cycles at the end of storage time. Likewise, LCBR showed a remarkably high survivability that was non–significantly different at day 20 from the initial count, but a significant diminution in cell count of about 2.9 log cycles was shown at the end of storage time. These results clearly indicate that the survivability of LCBR was significantly higher than for GCBR, which on turn was significantly higher than for the non–encapsulated free cells. One of the main expected properties of probiotic microorganisms is that they possess the capability to survive refrigerated storage (4 °C) as formulated products (Brusch–Brinques and Zachia–Ayub, 2011). The results of this work suggest that immobilization of probiotics in liquid center beads constitutes a promising technology for improving the survivability conditions of the microorganisms than those achievable in gel–core beads, and of course, those obtainable with non–encapsulated cells. While for GCBR, active cell growth took place with colonies occupying gel cavities close to the gel surface (Arnaud et al., 1992), in LCBR it occurred at the bead liquid core, where cell mobility was considerably less restricted, and allowed a greater survivability.
3.2.2 Survivability under simulated gastrointestinal conditions
Data of survivability of non–encapsulated free cells, and GCBR and LCBR exposed to sequential simulated gastrointestinal conditions (pH 2.3 and bile salts) are given in Fig. 3. Exposure to pH 2.3 conditions did not affect significantly the viability of LCBR, but did affect significantly that of GCBR and the non–encapsulated cells, producing cell count reductions of about 0.76 log and 1.87 log cycles, respectively. Further exposure to bile salts, resulted in a significant reduction of the cells viability in the three treatments, but LCBR showed a significantly lower cell count reduction (~ 0.77 log cycles) than GCBR (~ 0.88 log cycles), and the latter lower than the non–encapsulated cells (~ 1.00 log cycle). The survivability percentage was of 89.9 % for LCBR, 80.8 % for GCBR, and 66.1% for non–encapsulated cells. From these results, it may be inferred, that the protective biopolymer coating formed around LCBR and GCBR, effectively isolated L. rhamnosus from the simulated gastrointestinal conditions, hindering the interaction between cells with this harsh environment. Moreover, the protection provided by the biopolymer shell in LCBR was superior to that provided by the completely gelled bead (GCBR).
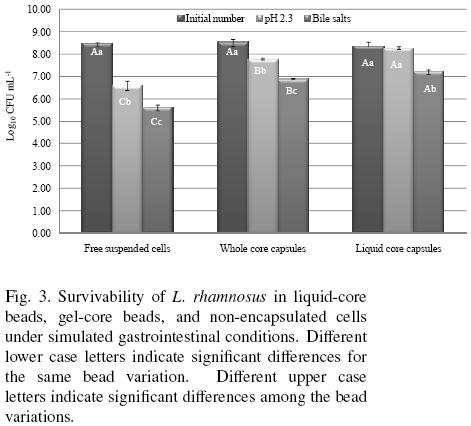
The highest survival of cell culture encapsulated on liquid core beads observed in these work, can be supported in studies that have demonstrated that cells entrapped in gel core beads, tend to grow at the periphery of the calcium alginate beads (Seki et al., 1990). The better performance of the liquid core beads versus gel core beads might partially be attributed to the prevention of cell release from the liquid core capsules. As the results of extensive surface growth, cells are released from the gel core beads into the fermentation medium, thus decreasing cell population recorded in the beads (Morin et al., 1992). In gel core beads, active cell growth takes place with microcolonies occupying gel cavitities close to the gel surface (Arnaud et al., 1992). Therefore, cells exposed in gel surface are in direct contact with adverse conditions and survival can decrease in comparison with liquid core bead.
Conclusions
Microencapsulation in liquid–core calcium alginate beads by the extrusion technique provided better protection to L. rhamnosus cells during storage and from simulated gastrointestinal conditions than when microencapsulation was done in extruded gel–core calcium alginate beads or when cells were non–encapsulated. Thus, it is implied that liquid–core calcium alginate beads enhance the protection of the sensitive cells from degradation by reducing the reactivity from outside detrimental environmental factors.
Acknowledgements
The authors extend their thanks to CONACyT for the partial financing of this Project through grants U–45992–Z and the Biol. Yolanda Hornelas–Uribe of the Instituto de Ciencias del Mar y Limnología of the Universidad Nacional Autónoma de México for the expertise provided for obtaining the SEM micrographs.
References
Anal, A. K. and Singh, H. (2007). Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends in Food Science and Technology 18, 240–251. [ Links ]
Arnaud, J. P, Lacroix, C. and Choplin, L. (1992). Effect of agitation rate on cell release rate and metabolism during continuous fermentation with entrapped growing Lactobacillus casei subsp. casei. Biotechnology and Technology 6, 265–269. [ Links ]
Artignan, J. M., Corrieu, G. and Lacroix, C. (1997). Rheology of pure and mixed kappa–carrageenan gels in lactic acid fermentation conditions. Journal of Texture Studies 28,47–70. [ Links ]
Bennett, A. N. (1989). Alginate gels. – Patent EP0345886. [ Links ]
Bourne, M. C. (2002). Food texture and viscosity: Concept and measurement (2nd ed.). Academic Press, New York. [ Links ]
Brusch–Brinques, G. and Zachia–Ayub, M.A. (2011). Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. Journal of Food Engineering 103, 123–128. [ Links ]
Chandramouli, V., Kailasapathy, K., Peiris, P. and Jones, M. (2004). An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. Journal of Microbiological Methods 56, 27–35. [ Links ]
Dembczynski, R. and Jankowski, T. (2002). Growth characteristics and acidifying activity of Lactobacillus rhamnosus in alginate/starch liquid–core capsules. Enzyme and Microbial Technology 31, 111–115. [ Links ]
Figueroa–González, I., Hernández–Sánchez, H., Rodríguez–Serrano, G., Gómez–Ruíz, L., García–Garibay, M. and Cruz–Guerrero, A. (2010). Antimicrobial effect of Lactobacillus casei strain Shirota co–cultivated with Escherichia coli UAM0403. Revista Mexicana de Ingeniería Química 9, 11–16. [ Links ]
Fiszman, G.L., Karara, A.L., Finocchiaro, L.M.E. and Glikin, G.C. (2002). A laboratory scale device for microencapsulation of genetically engineered cells into alginate beads. Electronic Journal of Biotechnology 5, 23–24. [ Links ]
Koyama, K. and Seki, M. (2004). Cultivation of yeast and plant cells entrapped in the low–viscous liquid–core of an alginate membrane capsule prepared using polyethylene glycol. Journal Bioscience and Bioengineering 97, 111–118. [ Links ]
Lobato–Calleros, C., Ramos–Solís, L., Santos–Moreno, A. and Rodríguez–Huezo, M. E. (2006). Microstructure and texture of panela type cheese–like products: use of low methoxyl pectin and canola oil as milk–fat substitutes. Revista Mexicana de Ingeniería Química 5, 7179. [ Links ]
Lobato–Calleros, C., Reyes–Hernández, J., Beristain, C.I., Hornelas–Uribe, Y., Sánchez–García, J.E. and Vernon–Carter, E.J. (2007). Microestructure and texture of white fresh cheese made with canola oil and whey protein concentrate in partial or total replacement of milk fat. Food Research International 40, 529–537. [ Links ]
Moiny, V. (2002). Uniaxial compression of cheddar cheese at various loading rates and its correlation to sensory texture profiles. Journal of Texture Studies 33, 237–254. [ Links ]
Monshipouri, M. and Price, R. R. (1995). Emulsification preparation of calcium alginate beads in the presence of sequesterant. Journal of Microencapsulation 3(12), 255–262. [ Links ]
Morin, N., Bernier–Cardon, M., Champagne, C.P. (1992). Production of concentrated Lactococcus lactis subsp. cremoris suspension in calcium alginate beads. Applied and Environmental Microbiology 58, 545–550. [ Links ]
Muthukumarasamy, P., Allan, P. W. and Holley, A. R. (2006). Stability of Lactobacillus reuteri in different types of microcapsules. Journal of Food Science 71 (1), 20–24. [ Links ]
Nedović, V.A., Obradović, B., Leskošek–Cukalović, I., Trifunović, O., Pešić, R. and Bugarski, B. (2001). Electrostatic generation of alginate microbeads loaded with brewing yeast. Process Biochemistry 37, 17–22. [ Links ]
Nussinovitch, A., Gershon, Z. and Nussinovitch, M. (1996a). Liquid–core hydrocollioid capsules. Food Hydrocollids 10, 21–26. [ Links ]
Nussinovitch, A., Nussinovitch, M., Shapira, R. and Gershon, Z. (1996b). Influence of immobilization of bacteria, yeasts and fungal spores on the mechanical properties of agar and alginate gels. Food Hydrocolloids 8, 361–372. [ Links ]
Picot, A. and Lacroix, C. (2004). Encapsulation of bifidobacteria in whey protein–based microcapsules and survival in simulated gastrointestinal conditions and in yoghurt. International Dairy Journal 14(6), 505–515. [ Links ]
Pimentel–González, D. J. Campos–Montiel, R. G., Lobato–Calleros, C., Pedroza–Islas, R. and Vernon–Carter, E. J. (2009). Encapsulation of Lactobacillus rhamnosus in double emulsions formulated with sweet whey as emulsifier and survival in simulated gastrointestinal conditions. Food Research International 42, 292–297. [ Links ]
Rao, A. V., Shiwnarain, N. and Maharaj, I. (1989). Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Journal ofCanadian Institute on Food Science and Technology 22, 345–349. [ Links ]
Sandoval–Castilla, O., Lobato–Calleros, C., García–Galindo, H.S., Alvarez–Ramírez, J. and Vernon–Carter, E.J. (2010). Textural properties of alginate–pectin beads and survivability of entrapped Lb. casei in simulated gastrointestinal conditions and in yoghurt. Food Research International 43, 111–117. [ Links ]
Seki, M., Shigematsu, K. and Furusaki, S. (1990). Cell–growth and reaction characteristics of immobilized Zymomonas mobilis. Annual N.Y. Academic Science 613, 290–302. [ Links ]
Segnini, S. and Dejmek, P. (1999). Relationship between instrumental and sensory analysis of texture and color of potato chips. Journal of Texture Studies 30, 677–690. [ Links ]
Sultana, K., Godward, G., Reynolds, N., Arumugaswamy, R., Peiris, P. and Kailasapathy, K. (2000). Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. International Journal of Food Microbiology 62, 47–55. [ Links ]
Tanaka, H., Matsumara, M. and Veliky, I. A. (1984). Diffusion characteristics of substrates in Ca–alginate gel beads. Biotechnology and Bioengineering 26, 53–58. [ Links ]
Wang, H., Seki, M. and Furusaki, S. (1995). Characteristics of immobilized Lactobacillus delbruekii in a liquid–solid fluidized bed bioreactor for lactic acid production. 1. Chemical Engineering Japan 28, 198–203. [ Links ]














