Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de ingeniería química
versión impresa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.10 no.2 Ciudad de México ago. 2011
Biotecnología
Comparative study for oxygenases produced by Aspergillus niger, ATCC 9642, in solid–state and submerged fermentation
Estudio comparativo de oxigenasas producidas por Aspergillus niger, ATCC 9642, en fermentación en estado sólido y sumergido
T.C. Flores–Flores1*, M. Gutiérrez–Rojas2, S. Revah3 and E. Favela–Torres2
1 Departamento de Ingeniería Química, Instituto Tecnológico de Celaya, Av. Tecnológico y García Cubas S/N, Col. Alfredo Bonfil, CP. 38010 Celaya, Gto., México. *Corresponding author. E–mail: tere@iqcelaya.itc.mx Tel: +461–611–7575 ext 136, Fax: +461–611–7744
2 Departamento de Biotecnología, Universidad Autónoma Metropolitana–Iztapalapa, Av. San Rafael Atlixco 186, Col. Vicentina, CP. 09340 México, DF, México.
3 Departamento de Procesos y Tecnología, Universidad Autónoma Metropolitana–Cuajimalpa, Avenida Constituyentes 1054, Col. Lomas Altas, Delegación Miguel Hidalgo, C. P 11950, México, D. F, México.
Received 25 of October 2010.
Accepted 18 of April 2011.
Abstract
In recent years, solid state fermentation (SSF) has shown much promise in the development of several bioprocesses and products. Some of these applications include bioremediation and biodegradation of hazardous compounds, biological detoxification of agroindustrial residues, production of micotoxins, antibio tics, gibberel lins, biope sticides, organic ac ids, flavour compounds, enzymes, etc. This study deals with the comparison of the oxygenases, produced by Aspergillus niger, ATCC 9642, under submerged fermentation (SmF) and SSF using hexadecane as inducer, in the presence and absence of glucose. In addition, the characterization of both systems is also presented here. The results show higher biomass yield (Yx/s), higher specific growth rates (μ) and higher rates of hexadecane consumption (qs) for SSF than for SmF, when only hexadecane is used as substrate. Mineralization is higher, for both systems, when a mixture of glucose and hexadecane is used as substrate. The oxygenase produced under SSF has no specificity difference for aliphatic hydrocarbons (linear and non linear). But, it increases for aromatic substrates as the complexity of the ring increases. Oxygenase produced under SmF has lower activities, at least 7–folds, compared to SSF.
Keywords: oxygenases, solid state culture, aromatic hydrocarbons, hexadecane, polyurethane matrix support, A. niger.
Resumen
En los últimos años, la fermentación en estado sólido (SSF) se ha considerado como un proceso con un gran potencial, en el desarrollo de bioprocesos y de productos de alto valor agregado. Algunas de sus aplicaciones incluyen: la bioremediación y la biodegradación de compuestos peligrosos, la detoxificación de residuos agroindustriales, la producción de micotoxinas, antibióticos, giberelinas, biopesticidas, ácidos orgánicos, saborizantes, enzimas, etc. En este estudio se hace la comparación de oxigenasas producidas por Aspergillus niger ATCC 9642 en fermentación sumergida (SmF) y en SSF, usando hexadecano como inductor, en presencia y ausencia de glucosa. Se presenta también la caracterización de ambos sistemas. Los resultados muestran un mayor rendimiento de biomasa (Yx/s) y velocidades específicas de crecimiento (μ) y de consumo de hexadecano (qs) más altas, para SSF que para SmF, cuando se usa hexadecano como único substrato. La oxigenasa producida en SSF no muestra diferencia de especificidad con hidrocarburos alifanticos (lineales o ramificados). Sin embargo, la especificidad para compuestos aromáticos se incrementa con la complejidad del anillo. La oxigenasa producida en SmF muestra actividades que son por lo menos 7 veces menores comparadas con las actividades de oxigenasa producida en SSF.
Palabras clave: oxigenasas, fermentación en estado sólido, hidrocarburos aromáticos, hexadecano, soporte de poliuretano, A. niger.
1 Introduction
Oxygenases are a group of intracellular enzymes with significant roles in metabolism and biosynthesis. They are important in the design of pharmaceuticals and the production of specialty chemicals (Burton, 2003; Kamerbeek et al., 2003; Hartman et al., 2006; Fraatz et al., 2009; Torres Pazmiño et al., 2010; Cresnar and Petric, 2011).
Besides, oxygenases are involved in biodegradation of hydrocarbons and related compounds that, due to their improper storage, use, and disposal have been released into the environment, where they are considered environmental pollutants. Some examples of these are chlorinated biphenyls (Dmochewitz and Ballschmiter, 1988); oil spills (Van Hamme et al., 2003; Radwan, 2008); nitrobenzene, 2,4– and 2,6–dinitrotoluene (Lessner et al., 2002; Parales et al., 2005); polycyclic aromatic hydrocarbons (Gibson and Subramanian, 1984; Romero et al., 2002; Feitkenhauer et al., 2003; Golubev et al., 2009); Short chain hydrocarbons (Hamamura et al., 2001); medium chain hydrocarbons (Volke–Sepúlveda et al., 2003; Volke–Sepúlveda et al., 2006; Bouchez–Näıtali and Vandecasteele, 2008) and volatiles aromatics collectively indicated as BTEX (Qi et al., 2002; Nikolova and Nenov, 2005, Maestre et al., 2007; García–Peña et al., 2008).
Although fungi have an important role in several ecosystems and they have predominance over bacteria in soil, oxygenases have been extensively studied in liquid cultures with bacteria, and analogous data for fungi are scarce. The use of fungi instead of bacteria offers some advantages with respect to stability and activity of the population, especially under reduced water activity and low pH conditions, which often prevail in soil bioremediation and in air biofilters.
On the other hand, several works dealing with remarkable differences between solid state fermentation (SSF) and submerged fermentation (SmF) have been described (Alazard and Raimbault, 1981; Barrios–González et al., 1988; Solís–Pereira et al., 1993; Viniegra et al., 2003; Volke–Sepúlveda et al., 2003; Hölker, et al., 2004; Fontana et al., 2005; Barrios–González et al., 2008). It is worth stressing that several biotechnological advantages have been reported for SSF compared to SmF, such as production of extracellular enzymes with higher activities, higher fermentation productivity, higher end–concentration of products, higher product stability, lower catabolic repression, higher hydrocarbon degradation, cultivation of microorganisms specialized for water–insoluble substrates or mixed cultivation of various fungi, and lower demand on sterility.
Among other factors, microbial synthesis of enzymes in a SSF system can be affected by: selection of a suitable substrate; pre–treatment, particle size and water content of the substrate; relative humidity; type and size of the inoculum; fermenting matter temperature, period of cultivation; maintenance of uniformity in the environment of SSF system, and the gaseous atmosphere (Pandey et al., 1999; Hölker, et al., 2004).
Most of the works dealing with enzyme production in SSF have been carried out for extracellular enzymes, although there are several reports of hydrocarbons degradation involving oxygenases, in SSF (Holden et al., 2002; Qi et al., 2002; Volke–Sepúlveda et al., 2003 and 2006; García–Peña et al., 2008). The aim of this work is to study the factors that affect Aspergillus niger oxygenase production in SSF and SmF in the presence and absence of glucose and using hexadecane as inducer. And to compare the characteristics (activity, specificity, stability) of the oxygenase(s) expressed under these conditions.
2 Materials and methods
2.1 Strain and conditions for inoculums preparation
Aspergillus niger (ATCC 9642) spores were stored at –20°C in protect–cryoblocks (bead storage system, Technical Service Consultants, Heywood, UK). Inocula were prepared by transferring a cryoblock in 250 ml Erlenmeyer flasks containing 50 ml of potato dextroxe agar (PDA), incubating for 5–7 days at 30 ºC. Spores were then scraped with a magnetic bar and 20 ml of sterile 0.1 % between 80 solution. Spores suspensions were used to inoculate culture media for SmF and SSF.
2.2 Cultivation systems and culture media
First of all, several fermentations were carried out, under both type of systems, SmF and SSF, where the glucose and hexadecane were used in different concentrations. The purpose of these fermentations was to assess the conditions, under which the enzyme was expressed, by relating the production of the oxygenase with the hexadecane degradation by A. niger.
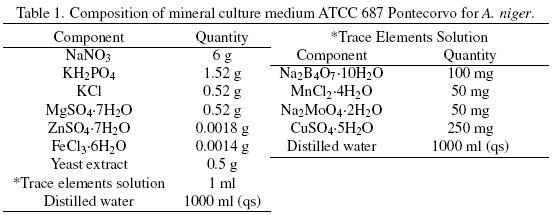
SmF and SSF were carried out with the mineral ATCC medium 687 with 0.05% yeast extract. The medium composition, for 12 g L–1 of total carbon and a C/N = 12.12, is described in Table 1. All the components were adjusted as required, depending on the amount of total carbon and on the C/N desired. The carbon source, in the culture medium, was supplied by different glucose:hexadecane ratios (Glu:Hxd) as shown in Table 2.
SmF were prepared in 250 ml Erlenmeyer flasks containing 50 ml of inoculated culture medium. Liquid cultures were started by inoculation of 1 × 106 spores ml–1 of A. niger. The initial pH was adjusted to 5.5 with 1N HCl. Erlenmeyer flasks were incubated at 30 ºC in an orbital shaker at 200 rev min–1 for at least 70 h. Every flask constituted a sample and each sample was prepared in duplicate.
SSF were carried out in 125 ml serological bottles containing approximately 1.13 g of grounded, sterilized polyurethane foam (PUF) and the inoculated culture medium described below. PUF was used as inert support to impregnate the culture medium (Zhu et al., 1994). Every bottle constituted a sample and each sample was prepared in duplicate.
Mineral composition of the SSF culture medium was the same used in SmF (Table 1). The carbon source, in the culture medium, was supplied by the Glu:Hxd ratios shown in Table 2. After sterilization of the serological bottles containing the PUF, culture broth was added to each bottle to have 70% humidity, and 1 × 107 spores g–1 of dry initial matter. The initial pH was 5.5. The bottles were sealed with rubber caps that were connected by means of syringe needles to GC equipment and to a water saturate air flow (2 ml min–1). Serological bottles were incubated in a water bath at 30 oC for at least 85 h.
2.3 Kinetic parameters
Growth curves were fitted by a Maquardt "Solver" computer program (Excel, Microsoft) using logistic equation as follows:
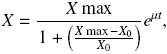
where X (g L–1) represents the biomass calculated, Xmax and X0 (g L–1) are the maximum and initial biomass values, respectively, μ (h–1) is the specific growth rate, and t (h) is the culture time. Substrate consumption curves were fitted using Pirt equation as follows:
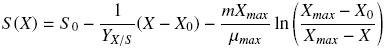
where S0 is the glucose or hexadecane concentration at t = 0.
The biomass yield, ΥX/S, was calculated using the next equations:
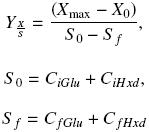
where Xmax and X0 (gL–1) are the maximum and initial biomass values, respectively. CiGlu and CiHxd (gL–1) are the initial carbon concentration values from glucose and hexadecane, respectively, and CfGlu and CfHxd (gL–1) are the final carbon concentration values, from glucose and hexadecane, respectively.
The specific substrate uptake rate, qs, is defined as follows:
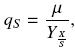
Where qs is given as grams of carbon, from substrates consumed, per gram of biomass per hour.
2.4 Oxygenase(s) production
Once the hexadecane degradation was studied in both types of systems, culture media c and f (Table 2) were selected to produce oxygenase(s) by SmF and SSF respectively. The fermentation conditions for oxygenase(s) production were the same as described before for hexadecane degradation. A. niger mycelium of 48, 72 and 120 h of growth was harvest from SSF and SmF and used to detect oxygenase activity. The oxygenase activity was analyzed by measuring the dissolved oxygen uptake rates, by resting cells, in the presence of substrate (above background respiration rate) and in the absence of it (background respiration rate or endogenous respiration). These rates were normalized with the total amount of biomass in the reaction vials and reported as nanomoles of oxygen removed per second per milligram of biomass. The oxygenase activity was then calculated by subtracting the oxygen uptake rate in the absence of substrate (RE) to the uptake rate in the presence of it (RS) and reported as units of enzyme (Sun and Wood, 1997). One unit of enzyme (U) was defined as the amount of enzyme that will catalyze the consumption of 1 nmol O2 per second.
2.5 Cell free crude enzyme extracts preparation
To study the characteristics of the induced oxygenases, cell free crude enzyme extracts were prepared from biomass growth in both types of cultures as explained above. Extract from SSF was prepared by washing the mycelium attached to the PUF (PUF–BioM), approx. 10 g, with 500 ml cold water (5 °C). Then it was suspended for 10 min in 200 ml cold (5 ºC) 50 mM tris buffer, pH 7.5 with 0.1–μmol dithiotreitol ml–1 and 1ml protease inhibitor cocktail (Sigma P–8215) per 20 g cells wet weight. The suspension was filtered through Whatman 41. The PUF–BioM was frozen with liquid nitrogen and disrupted in a coffee grinder (Brown, 2.5 oz). Afterwards, the PUF–BioM was wrapped in cheesecloth, unfrozen (around 4 ºC) and squeezed in a hydraulic press (ERCO model PH–51) at 2000 psi, around 130 kg (cm2)–1. The obtained suspension was centrifuged at 10,000 g for 15 min at 4 ºC. The supernatant was filtered with 0.45 μm membrane and the filtrate was kept in vials (1.5 ml) at –20 ºC until enzyme analyses.
Crude enzyme extract from SmF was prepared alike the extract from SSF. The only difference was that after the mycelium was washed and frozen, it was disrupted with a mortar, suspended in the buffer and finally treated as the SSF crude extract.
2.6 Analytical procedures
Schematic flow diagrams of the sample treatment and analyses for SmF and for SSF are given in Fig. 1 and Fig. 2 respectively. Glucose was measured using an enzymatic analyser (YSI model 2700). Hexadecane was determined by gas chromatography (Perkin Elmer autosystem XL) with a flame ionization detector under the following conditions: column, PE1 of 30 m length and 0.32 mm I.D; carrier gas, helium (15 ml min–1); the injector and detector temperature were constant at 250 and 300 °C respectively; the column temperature was kept initially at 100 °C for 2 min, increasing by 20 °C min–1 to 250 °C and then holding for 5 min. Samples of 1 μl were injected and decane was used as internal standard. Biomass concentration was determined as dry matter content.
Oxygen and carbon dioxide evolution on SSF were followed by gas chromatography (Gow Mac 580) with thermal conductivity detector. The column used was CTR8700 (Alltech), current detector 150mA, detector and injector temperature 80 °C, column temperature 50 °C. Acquisition and integration system Multivia Chroma V.30.
Oxygenase activity assays were performed, in cell free crude enzyme extracts. Two methods were used: oxygen uptake rate (oxymetry), based on a method described by Sun and Wood (1997) and a modified spectrophotometric method described by Zazueta–Sandoval et al. (2003).
Activity assays by oxygen consumption were done using an O2 micro–electrode (YSI 5300 Biological Oxygen Monitor; YSI Inc., Yellow Springs, OH, U.S.A.). The reaction mixture consisted of 1.3 ml 50mM Tris pH 7.5, 0.4 mM ml–1 NADH and 0.3 ml crude enzyme extract. After equilibration at the desired oxygen concentration, the reaction was started by adding 3 μl of substrate (hexadecane). O2–uptake was calculated by the difference in O2 concentration before and after substrate addition. The oxygenase activity was reported as specific activity (U mg–1 protein). One unit of enzyme (U) was defined as the amount of enzyme that will catalyze the consumption of 1 nmol O2 per second. Oxygenase activity measurements using the spectrophotometric method were carried out in the following way. In a 1.5 ml quartz cuvette, 1 ml of reaction mixture was added. This consisted of 300 μl of o–dianisidine dihydrochloride reagent (20 mg 3,3'–dimetoxibenzidine dissolved in 3 ml 0.025 M hydrochloride acid, added with agitation to 50 ml of 50 mM tris buffer pH 8.5 and brought up to 100 ml with the same buffer), 480 μl of 50 mM tris buffer pH 8.5 added with 0.4 μm of NADH, 20 μl of substrate and 200 μl crude enzyme extract. Measurements were performed at 30 °C by using a spectrophotometer (Shimadzu UV–160A) equipped with a thermojacketed cuvette holder and a water circulation system, absorbance at 460 nm was followed for one hour, against a blank, after the addition of substrate. The blank was prepared replacing 200 μl crude enzyme by 200 μl of buffer, besides a reference was made replacing the substrate by 20 μl of buffer.
Enzyme activity was calculated subtracting reference slope (absorbance at 460 nm vs time) from sample slope and interpolating this value on a peroxidase activity calibration curve made with different concentrations of hydrogen peroxide as substrate of the enzyme in the presence of o–dianisidine (Zazueta–Sandoval et al., 2003). This method is based in the characteristics of orto–dianisidine (orto–DNS) which is a chromogenic acceptor of oxygen and in the action of oxygenase on hydrocarbon molecule and peroxidase (POD) on hydrogen peroxide, accordingly with the next two net reactions, using hexadecane as example of substrate for a monooxygenase (MOX):
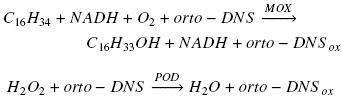
In the first reaction for each consumed oxygen molecule, one molecule of hexadecane and one molecule of orto–DNS are oxidized. Meanwhile, in the second reaction for each molecule of hydrogen peroxide reduced, one atom of oxygen is introduced in orto–DNS molecule. Therefore, through the interpolation of the absorbance value, generated from the first reaction, in the POD calibration curve, it is possible to know the amount of oxygen consumed in the first reaction. The oxygenase activity was reported as specific activity (U mg–1 protein). One unit of enzyme (U) was defined as the amount of enzyme that will catalyze the consumption of 1 nmol O2 per second.
The protein content was determined using the Bio–Rad Protein Assay, based on the method of Bradford, with bovine serum albumin as a standard.
2.7 Characterization of the oxygenase(s)
The concentration and storage effects on the enzyme activity and stability were examined by O2–uptake rate. Crude extracts from SSF and SmF were concentrated against sucrose with a 12,000 Da membrane (Millipore tubing membrane). They were stored at –20 °C from 0 to 84 days. Samples without concentrate were also kept at the same temperature. All these samples were used for O2–uptake analyses.
Oxygenase(s) substrate specificity was analyzed by the spectrophotometric method (Zazueta et al., 2003). Short (pentane, hexane, heptane), medium (decane, hexadecane) and large (eicosane) chain hydrocarbons as well as branched (2,6,10,14 tetramethyl–pentadecane) and aromatic (benzene, anthracene, phenanthrene, pyrene and toluene) compounds, were used as substrates.
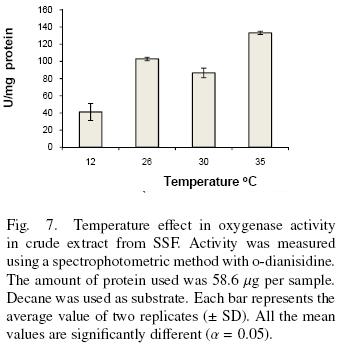
The effect of temperature in oxygenase activity was measured by the spectrophotometric method. The amount of protein used in these assays were 58.60 μg per sample contained in 200 μl of crude extract. The temperatures tested were 12, 26, 30 and 35 °C. Decane was used as substrate.
2.8 Polyacrylamide gel electrophoresis (PAGE)
The degree of purification and identification of proteins with oxygenase activity was monitored on native PAGE and zymograms. Native PAGE was performed according to Laemmli (1970), with 6% resolving and 4% stacking gels. Samples were electrophoresed at 10 mA and 4 °C through 1.5 mm gel in a vertical slab gel unit Mini Protean II Electrophoresis Cells (Bio–Rad Laboratories). The gels were loaded with 35μg of protein from cell free extracts obtained from SmF and SSF. The gels were stained with Coomasie Brilliant Blue R–250. A kaleidoscope prestained standard was also loaded as molecular weight standard.
Zymograms. Nondenaturing poliacrylamide gel electrophoresis was performed with both types of crude extracts as described before. Oxygenase activity was revealed by submerging the gel in a reaction mixture composed by 10 ml of 50 mM tris buffer pH 8.5 added with 0.4 μm ml–1 of NADH, 6.25 ml of o–dianisidine reagent (see section 2.5) and 1 ml of substrate (decane, hexadecane or bencene) and incubating the gel at room temperature with gentle agitation until the activity bands appeared. The protein loaded was 63.5 μg per lane.
3 Results and discussion
3.1 Growth of A. niger in SmF
Fig. 3 shows glucose and hexadecane degradation profiles in SmF. From this data it was evaluated that A. niger degraded 66.7±1.8 % of hexadecane in the presence of glucose (c), in only 48 h. However, the remaining 33.3 % did not undergo any change even after 140 h. Meanwhile, a small amount (17.9±14.6) of the added hexadecane was degraded when it was used as sole substrate (a), during the time of analyses (75 h) and none of it was degraded when the carbon source was 50 % from glucose and 50 % from hexadecane (b).
Another remarkable result in medium c (Fig. 3c) was the way that hexadecane was consumed. The first 20 h, A. niger consumed glucose only, but around 25 h, it consumed hexadecane and glucose simultaneously. Around 50 h the glucose has been totally consumed and the hexadecane uptake stopped. The biomass production of A. niger ATCC 9642, in SmF, reached the highest value (14.5 ± 0.27 g L–1) in the culture medium d (glucose alone), whereas medium a (hexadecane alone) gained the lowest value (0.63±0.17 g L–1). Table 3 presents the values of the kinetic parameters associated with the fungal growth: the specific growth rate (μ) and the maximum growth (Xmax).
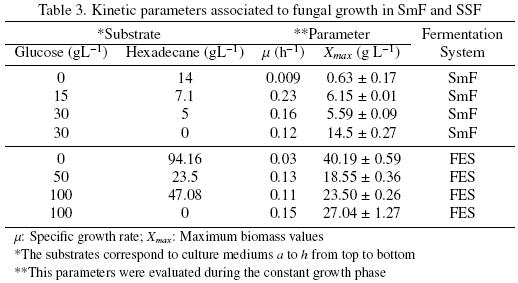
The specific growth rate increased when glucose plus hexadecane were used as substrates (mediums b and c). The highest value for μ (0.23 h–1) corresponded to medium b. But it had zero hexadecane consumption. Noordman et al. (2002) reported a μ value of 0.02 h–1 for P. aeruginosa grown in a liquid culture with hexadecane concentrations ranging from 0.1 to 2.0 g L–1, this value is 2.2 timeshigher than the μ value obtain in the present work for A. niger, under SmF, when only hexadecane was used as substrate. However, the μ values for bacteria are known to be higher than that of fungi (Volke–Sepúlveda et al., 2006).
Table 4 shows the kinetic parameters associated to substrate consumption. It can be observed that substrate uptake rate (qs) increased when mixtures of hexadecane plus glucose were used as substrates, compared with the qs of culture medium with glucose or hexadecane alone. The biomass/substrate yields (YSX) had higher values when A. niger growth at the expense of glucose only and smaller values when hexadecane was consumed, either alone or in the presence of glucose. This implies a higher mineralization when hexadecane was consumed. Volke–Sepúlveda et al. (2003) reported biomass/oxygen and oxygen/hexadecane yields, for an initial hexadecane concentration of 20 (gL–1) under SmF, of 0.29 (mg X mg–1 O2) and 3.34 (mmol O2/mmol Hxd) respectively. With these data a biomass/substrate yield (YSX) of 0.14 (mg X mg–1 Hxd) can be calculated. García–Peña et al. (2008) reported a YSX = 0.13 (mg X mg–1 benzene). In our study for medium a (14 g L–1 hexadecane) YS X = 0.2 (mg X/mg Hxd). In all these cases when the hydrocarbon was the only carbon source the biomass/substrate yields were very low. Prenafeta–Boldú et al. (2002) attributed a low degradation pattern of benzene by Cladophialophora sp to the accumulation of dead–end oxidation sub products, however in the case of hexadecane the reason of low hexadecane degradation in FEL is attributed to the low hexadecane solubility in water.
From the data shown so far it can be observed that even though, the amount of carbon consumed for medium c was the highest under SmF and it had the highest substrate uptake rate (0.35 g C–substrate g–1 X h–1), the Xmax was only 5.59±0.09 g L–1 and the substrate yield was one of the lowest under SmF, 0.40 (gX g–1 C–substrate). These results suggest high mineralization, which was confirmed by a carbon balance; data is presented in Table 5.
The degree of mineralization was higher when hexadecane was consumed, either alone or in the presence of glucose, than when only glucose was uptaken. This confirms what was suggested through the analysis of YSX. The respiratory quotients (RQ) are also presented in Table 5. RQ is related to the respiratory metabolism of the microorganism and to the substrate oxidation level (Volke–Sepúlveda et al., 2003). The RQ for SmF varied in the opposite direction than the degree of mineralization, i.e. higher for glucose uptake and smaller for hexadecane consumption. RQ values smaller than 1 are an indication of predominant mineralization of aliphatic organic compound, having low oxygen content. Ratios greater than1are obtained when organic compounds with high content of oxygen are extensively decomposed (Dilly, 2001).
In a similar study Wang et al. (1996) found for Pseudomonas putida (ATCC 17514) that when glucose was the sole carbon and energy source, the culture utilized glucose following Monod kinetics. When phenol was the sole carbon and energy source, the culture biodegraded it following Andrews (inhibitory) kinetics. When both glucose and phenol were present in the medium, the culture used them simultaneously but with lower specific rates. They stated that reduction of the specific substrate utilization rates indicates that the two substances are involved in a cross–inhibitory pattern which can be classified as uncompetitive. García–Peña et al. (2008) working with Paecilomyces variotti, found that the degradation of binary mixtures of BTEX rates obtained with single substrates (BTEX) and binary combinations of them were sensibly reduced in the mixtures. The results shown here are similar to Wang et al. (1996) results, except that in this work higher specific substrate utilization rates were obtained with mixtures of substrates. An increment in the specific substrate utilization rate suggests a synergistic effect of glucose in hexadecane consumption in culture medium c. However, in culture medium b, glucose inhibits hexadecane consumption, that could be by glucose repression. However, once glucose was depleted the enzyme was not derepress, or at least its action was not observed.
3.2 Growth of A. niger in SSF
Fig. 4 shows glucose and hexadecane degradation profiles in SSF. It can be observed that A. niger degraded hexadecane in the presence and absence of glucose, regardless of concentration and type of carbon source. The highest hexadecane degradation was attained in culture medium f, 95.5±1.9 % of the initial hexadecane in less than 60 h, followed by medium e with 89.8±2.1 % and 62.7±7.2% for medium g. A. niger in the presence of glucose and hexadecane (Fig. 4 f and g) started consuming glucose first and once this was completely exhausted, A. niger consumed hexadecane. Apparently there was catabolite repression which was more severe for culture medium g which contained the double of glucose than medium f. In Fig. 4 (f and g) it can be seen that hexadecane consumption started around 20 h for medium f, immediately after all the glucose was assimilated, while it started after 50 h for g, although glucose was depleted in 30 h.
In SSF higher Xmax were attained for culture mediums with glucose or hexadecane as sole carbon sources (see Table 3). In cultures mediums with glucose and hexadecane Xmax decreased with an increase in glucose and hexadecane concentration. The specific growth rates in SSF (Table 3) were similar for all the culture mediums studied, except for the culture medium e (hexadecane as sole carbon source) that was 3.6 times slower. For A. niger with an initial hexadecane concentration of 360 mg g–1 PUF, Volke–Sepúlveda, et al. (2006) reported a μ value of 0.70±0.02 that is similar to the one reported here in the presence of 344 mg g–1 PUF of hexadecane (0.72).
Table 4 shows the kinetic parameters associated to substrate consumption in SSF. It can be seen that substrate uptake rates (qs) had the same trend as in SmF. The biomass/substrate yields (YSX) were higher for glucose alone and had the minimum value when hexadecane is the sole source of carbone. From data reported by Volke–Sepúlveda et al. (2003) a YSX = 0.67(mg X mg–1 Hxd) was calculated for a sample with 90 g L–1 of inital hexadecane concentration. In the present study for medium e (94.16 g L–1) YSX = 0.56 (mg X mg–1 Hxd).
The mineralization and the RQ for SSF are shown in Table 5. As for SmF the higher mineralization was attained when glucose and hexadecane were present in the culture medium. The RQ also had the same trend as in SmF, the lowest RQ values were found for cultures mediums where only hexadecane was consumed. Volke–Sepúlveda et al. (2006) reported, for degradation of hexadecane in SSF with A. niger, that RQ values were nearly independent of the hexadecane concentration and they were higher in C/N variable (0.73 ± 0.03) than in C/N constant (0.66 ± 0.02). For hexadecane degradation under SmF Volke–Sepúlveda et al. (2003) reported higher and variable RQ values. In the present work, degradation of hexadecane alone under SSF or SmF, produced RQ values smaller (0.57 and 0.64 respectively) than the values reported by them. These results must be related to the higher mineralization that was attained in our case.
3.3 Solid state fermentation compared to submerged fermentation
Examination of figs. 3 and 4 shows very different profiles of substrates consumption and biomass production for liquid vs. solid cultures. Among the principal differences the following can be mentioned: 1) In SSF independently of the initial hexadecane concentration, always there was hexadecane degradation. 2) Hexadecane degradation tended to 100% in all the culture media studied in SSF. 3) When the carbon source was 50 % from glucose and 50% from hexadecane apparently there was catabolic repression under SmF as well as in SSF. However, in SSF the enzyme was derepress and hexadecane was consumed, even when high glucose concentrations were used. While, under SmF glucose was depleted, hexadecane degradation never started and biomass concentration started decreasing. Some authors working with similar systems have reported that high hydrocarbon concentrations can be associated with heavy undispersed oils slicks in water, inhibiting biodegradation due to a nutrient and/or oxygen limitation (Leahy and Colwell, 1990). del Castillo and Ramos (2007) reported that under SmF in a minimal medium with 16 mM of glucose and 6 mM of toluene as carbon sources there was simultaneous catabolite repression, however both substrates were consumed, although with lower rates than the separated substrates. Diaz–Godinez et al. (2001) have shown for A. niger C28B25 using SmF and SSF with a mixed substrate (5 g/L pectin and 50 g/L sucrose), better pectinase productivity because of higher biomass production and lower protein breakdown in SSF. Several authors have proposed that SSF system minimizes the catabolite repression (Ramesh and Lonsane, 1991; Aguilar, et al., 2001; Fontana and Moura da Silveira, 2005). Generally they report lower catabolite repression with glucose concentrations ≥100 g L–1, compared to SmF. In light of the findings it appears that breakdown of biomass occurred, before the enzyme is derepressed in culture medium b (15 g L–1 glucose and 7.1 g L–1 hexadecane) and this could be due to oxygen limitation. 4) The values for μ were similar for both systems and are higher when more glucose is consumed. Glucose had a stimulatory effect because provides easily accessible carbon source, which gives rise to an increased biomass (Ostberg et al., 2007). 5) When the culture medium had glucose and hexadecane the qs values were 1.36–2.27 times faster for SmF than for SSF. For culture medium with hexadecane as sole source of carbon, qs was 2.2 times faster for SSF than for SmF, this could be due to a better hydrophilic substrate assimilation in SmF and a better hydrophobic substrate uptake in SSF.
As it was said before one of the objectives of studying the degradation of hexadecane under SmF and SSF was to assess the conditions, under which the enzyme was expressed, by relating the production of the oxygenase with the hexadecane degradation by A. niger. Wu et al. (2011) reported that an increase in P450 cytochrome monooxygenase activity was linearly correlated with hexadecane degradation. Other purpose of this type of study was to find the conditions under which a considerable amount of biomass with good characteristics, i.e. with the enzyme induced and non sporulating, was formed.
Evaluation of the parameters mentioned above resulted in the selection of medium c and f (see Table 2) to grow A. niger. Mycelium was harvested at 48, 72 and 120 h to evaluate the production of oxygenase(s) and to observe if the enzyme activity corresponded with the time of hexadecane consumption.
3.4 Production of oxygenase(s)
Culture media c and f were selected to produce oxygenase(s) by SmF and SSF respectively. The oxygenase activity was measured using resting cells as described in section 2.4. The results are shown in Table 6.
It can be observed in Table 6 that background respiration (RE) rate was relatively high. To avoid the interference of endogenous respiration with the oxygenase activity, two respiration inhibitors were used (NaN3 and NaCN). The two inhibitors drastically reduced the background respiration rate. However, they also reduced the oxygenase activity. This essay did not give reproducible results for SSF (data not shown). Resting cells from SmF showed higher activity for the 48 h mycelium. It was observed that 48 h coincided with the period of steady growth of A. niger and with the time that hexadecane was consumed, in culture medium c, under SmF (Fig. 3).
Since oxygen uptake rate by A. niger resting cells was not an appropriate method of measuring oxygenase activity, it was decided to use a cell free crude enzyme extract to achieve this objective. The time selected to harvest biomass for crude extracts preparation was 35 h for SSF and 45 h for SmF.
3.5 Characterization of the oxygenase(s)
3.5.1 Enzyme stability
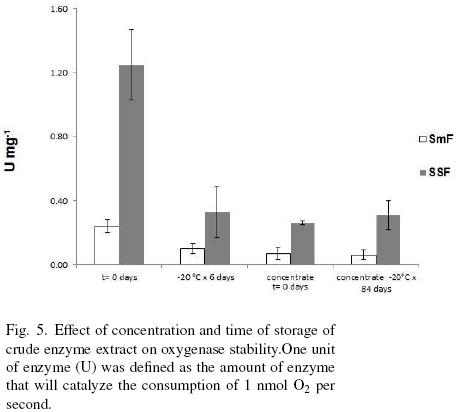
Figure 5 shows the concentration by dehydration and storage effects on the oxygenase(s) stability. First able it can be observed that oxygenase activity is at least 3.7 times higher for SSF sample than for SmF. Crude extracts from both types of fermentations had a drastic loss of activity, around 75%, when they were concentrated or stored at –20 °C for more than 7 days. Samples dehydrated and stored for 84 days at –20 °C had no further decrease of activity. The loss of activity with time of storage can be due to the disappearance of the cofactor. It is known that most of oxygenases are cofactor dependent. Among the cofactors we can cite NADH, FAD, NADPH. These cofactors are continuously forming while the microorganism is intact, however, in the extract there is a small amount of it that is lost very easily since this type of molecules are very labile. Another reason for the lost of activity it could be an adduct formation (Gray, 1989).
3.5.2 Oxygenase(s) substrate specificity
Crude extracts from SSF oxidized aliphatic and cyclic substrates (Fig. 6A). There was no significant difference (α = 0.05) in substrate specificity for low (pentane, hexane, heptane), medium (decane, hexadecane) and high (eicosane) molecular weight aliphatic hydrocarbons, as well as for linear compared to non linear (2,6,10,14 tetramethyl–pentadecane). However, for aromatic substrates, activity was significantly higher (α = 0.05) as the number of the rings increased (benzene<anthracene<phenanthrene<pyrene). The activity with toluene was relatively high, similar to that presented by pyrene. Substrate specificity for crude extracts obtained from SmF showed no specific pattern and the activities were lower for them, at least 7–folds compared to SSF (Fig. 6B).
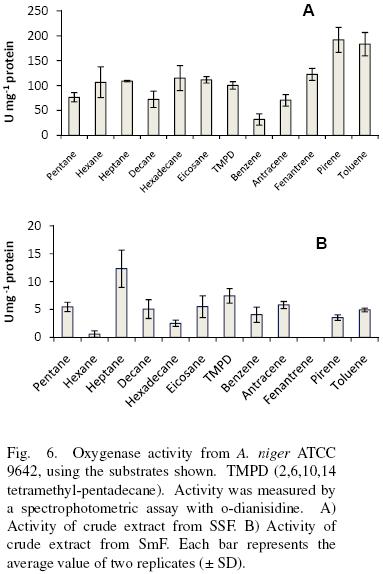
van Beilen et al. (1994) studied the hydroxylation of a wide range of linear, branched, and cyclic alkanes and alkylbenzenes by the alkane hydroxylase system of Pseudomonas oleovorans GPo1 in vivo and in vitro. In vitro hydroxylation was determined with a reconstituted hydroxylase system consisting of AlkB (the membrane–bound catalytic monooxygenase component), AlkG (rubredoxin), and spinach ferredoxin reductase. van Beilen et al. (1994) found that this system was able to oxidize all types of substrates, except when a tertiary or quaternary carbon was present.
Zazueta–Sandoval et al. (2003) partially purified an oxygenase from an unknown fungus strain, grown in liquid culture. They reported for it a higher activity with cyclic substrates than with aliphatic ones and they found a decrease in activity as the complexity of the substrate molecule increased. They conclude the existence of one enzyme with activity as mono and dioxygenase.
Vatsyayan et al. (2008) found a cytochrome P450 monooxygenase (CYP) of Aspergillus terreus grown in hexadecane (cytosolic fraction) and in glucose (membrane bound). The CYP studied by them have similar specificities than the ones found in this work under SmF, albeit the activities are different. For example they report 1.28±0.011 μmol O2 min–1 mg–1 protein for hexadecane equivalent to 21.33 nmol O2 s–1 mg–1 protein. This is one of the highest activities reported for the enzyme extract obtained from A. terreus growth with hexadecane. The enzyme was active also with octane, tetracosane, benzene and toluene among other substrates. All the activities shown for the CYP were smaller than the ones calculated in this work under SSF, but higher than in SmF.
3.5.3 The effect of temperature on oxygenase(s) activity
There are two optimum temperatures for the oxidation of decane as shown in Fig. 7. One is at 26 °C and the other is equal or greater than 35 °C. This result together with the ones showed above suggests the presence of more than one enzyme. In addition the results show the dependence of enzyme activity with temperature. Zazueta–Sandoval et al. (2003) did not observe effect of incubation temperature at 28 or 37 °C, for the unknown strain they used. Faber et al. (2001) purified and characterized a benzoate–parahydroxylase of A. niger. This enzyme is a cytochrome P450 (CYP53A1). They did not report an optimum temperature. Vatsyayan et al. (2008) observed that the optimum temperature for CYP activity was 37°C. They report that activity is rapidly lost at 40 °C and it decreases slower when the temperature is below 37 °C. They did not study the activity below 30°C.
3.6 Polyacrylamide gel electrophoresis (PAGE)
Figure 8 exhibits the results of PAGE. Lanes 1–3 were revealed for oxygenase activity (zymogram). They were loaded with cell free extract from SSF. Crude extract from SmF presented no activity with any of the substrates used even though, the amount of protein was the same for both kind of extracts or even 4 times greater for SmF(results not shown). The rest of the lanes were revealed for protein. Lane 4, was loaded with SSF sample and lane 5 with SmF extract, 35 μg of protein were used for each lane. The last lane corresponds to a molecular weight standard. It can be observed that the SSF samples show two well defined protein bands (a, b). As for SmF sample only band a is clear. Protein bands a and b matches with decane, hexadecane and bencene activities. Because, activity bands are too broad, it is not possible to allocate such activity to one of the protein bands a or b. Besides, both proteins have similar molecular weights, around 219 kDa. Vatsyayan et al. (2008) reported a CYP with a molecular weight that is half of the calculated in this work. Although, they run an SDS–PAGE so it may be a similar protein than the reported here.
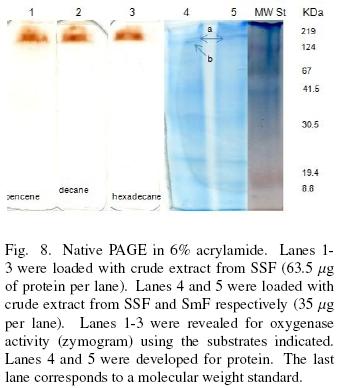
García–Peña et al. (2008) reported a zymogram for an oxygenase produced by P. variotti grown under SmF with toluene and with a mixture of toluene and glucose. The zymogram was revealed with benzene. They found that bands corresponding to the enzyme produced with toluene and glucose shown less activity, despite the fact that they did not observe catabolite repression during the toluene degradation. These results totally agree with our observations. Although in our case no bands of activity were visible for SmF.
Qi et al. (2002) commented that Woertz and Kinney (2000) reported that E. Lecanii–corni was not able to degrade benzene in liquid cultures. While they found, for the same strain, it was able to degraded benzene under solid culture.
The results shown for characterization of oxygenase(s) can be explained by the presence of a cytochrome P450 system. Faber et al. (2001) reported a CYP53A1and a NADH:cytochrome P450 reductase (CPR) from A. niger. The CYP51A showed only one band of apparent mass of 58 kDa and the CPR had also only one band of 78 kDa. However, this mass was calculated in SDS–PAGE like Vatsyayan et al. (2008) did.
Conclusions
The results discussed above show the importance of the type of fermentation since not only extracellular enzymes, but also intracellular enzymes, can be obtained with better characteristics by SSF. The enzymes induced by SSF had not only higher activity but also a broader specificity. Enzyme stability, of the intracellular oxygenases studied here, does not depend of the type of fermentation system. A. niger, ATCC 9642, produces labile oxygenases under SSF and SmF as well.
One possible reason for the higher oxygenase activity found by SSF compared with the results by SmF is the higher biomass production. Another reason could be the direct contact of the hifas of A. niger with the hydrocarbon when the fungus is grown by SSF, that promotes a better enzyme induction. Meanwhile in liquid culture A. niger grows as pellets and only a small surface is in contact with the substrate.
A. niger is a filamentous fungus that can be used to degrade hydrocarbons such as hexadecane in SSF, in the presence or absence of glucose as a cosubstrate. However, the Glu:Hxd should be carefully selected to reach the extent of degradation desired at a reasonably period of time. Or to select a Glu:Hxd depending if the objective is to have a small amount of biomass with highly active enzyme (as for the case of biofilters) or to produce a high amount of biomass with oxygenase activity (as for the case of bioremediation).
Highly active oxigenases that can oxidize a broad spectrum of substrates (linear, non linear and aromatic hydrocarbons) can be induced in A. niger, under SSF using hexadecane as inducer. It is important to highlight the high activity presented by oxygenases of A. niger with recalcitrant compounds such as pyrene, toluene and phenanthrene.
Much work needs to be done in order to elucidate the type of enzymes that are induced. It will be desirable to do zymograms with enzyme extracts from SmF and SSF using hexadecane as sole carbon source in order to observe if the activity for SmF sample increases. It is also recommended to change the conditions for the PAGE to have a better resolution of the proteins. To further purify the enzyme(s) it will be useful to use cloning and expression techniques.
It will be interesting to look for molecules that act as signal molecules in catabolite repression. Besides to study glucose repression from the physiological and molecular points of view.
Acknowledgements
The results presented here are part of the research conducted for F–F,T.C. in order to obtain the degree of Doctor in Biotechnology from UAM. This author thanks Conacyt and ITC for the financial support received. F–F,T.C. also thanks Dr R. Zazueta–Sandoval for his help in conducting PAGE and zymograms.
References
Aguilar, C. N., Augur, C., Favela–Torres, E., Viniegra–González, G. (2001). Induction and repression patterns of fungal tannase in solid–state and submerged cultures. Process Biochemistry 36, 565–570. [ Links ]
Alazard, D. and Raimbault, M. (1981). Comparative study of amylolytic enzymes production by Aspergillus niger in liquid and solid state cultivation. European Journal of Applied Microbiology and Biotechnology 12, 113–117. [ Links ]
Barrios–González, J., Baños, J. G., Covarrubias, A. A. and Garay–Arroyo, A. (2008). Lovastatin biosynthetic genes of Aspergillus terreus are expressed differentially in solid–state and in liquid submerged fermentation. Applied Biochemistry and Biotechnology 79(2), 179–186. [ Links ]
Barrios–González, J., Tomasini, A., Viniegra–Gonzalez, G. and López, L. (1988). Penicillin production by solid state fermentation. Biotechnology Letters 10(11), 793–798. [ Links ]
Bouchez–Naïtali, M. and Vandecasteele, J.P, (2008). Biosurfactans, a help in the biodegradation of hexadecane? The case of Rhodococcus and Pseudomonas strains. World Journal of Microbiology and Biotechnology 24, 1901–1907. [ Links ]
Burton, S. (2003). Oxidizing enzymes as biocatalysts. Trends in Biotechnology 21(12), 543–549. [ Links ]
Cresnar, B. and Petric, S. (2011). Cytochrome P450 enzymes in the fungal kingdom. Biochimica et Biophysica Acta 1814, 29–35. [ Links ]
del Castillo, T. and Ramos, J. L. (2007). Simultaneous catabolite repression between glucose and toluene metabolism in Pseudomonas putida is channeled through different signaling pathways. Journal of Bacteriology 189(18), 6602–6610. [ Links ]
Díaz–Godínez, G., Soriano–Santos, J., Augur, C. and Viniegra–González, G. (2001). Exopectinases produced by Aspergillus niger in solid–state and submerged fermentation: a comparative study. Journal of Industrial Microbiology and Biotechnology 26(5), 271–275. [ Links ]
Dilly, O. (2001). Microbial respiratory quotient during basal metabolism and after glucose amendment in soils and litter. Soil Biology and Biochemistry 33, 117–127. [ Links ]
Dmochewitz, S. and Ballschmiter, K. (1988). Microbial transformation of technical mixtures of polychlorinated biphenyls (PCB) by the fungus Aspergillus niger. Chemosphere 17(1), 111–121. [ Links ]
Faber, B. W., van Gorcom, R. F. M. and Duine, J. A. (2001). Purification and characterization of benzoate–para–hydroxylase, a cytochrome P450 (CYP53A1), from Aspergillus niger. Archives of Biochemistry and Biophysics 394(2), 245–254. [ Links ]
Favela–Torres, E., Córdova–López, J., García–Rivero, M. and Gutiérrez–Rojas, M. (1998). Kinetics of growth of Aspergillus niger during submerged, agar surface and solid state fermentations. Process Biochemistry 33(2), 103–107. [ Links ]
Feitkenhauer, H., Müller, R, and Märk, H. (2003). Degradation of polycyclic aromatic hydrocarbons and long chain alkanes at 60–70 oC by Thermus and Bacillus spp. Biodegradation 14, 367–372. [ Links ]
Fontana, R.C. and Moura da Silveira, S.S.M. (2005). Influence of pectin and glucose on growth and polygalacturonase production by Aspergillus niger in solid–state cultivation. Journal of Industrial Microbiology and Biotechnology 32, 371–377. [ Links ]
Fraatz, M. A., Berger, R. G. and Zorn, H. (2009). Nootkatone– a biotechnological challenge. Applied Microbiology and Biotechnology 83, 35–41. [ Links ]
García–Peña, I., Ortiz, I., Hernández, S., Revah, S. (2008). Biofiltration of BTEX by the fungus Paecilomyces variotii. International Biodeterioration & Biodegradation 62, 442–447. [ Links ]
Gibson, D.T. and Subramanian, V., (1984). Microbial degradation of aromatic hydrocarbons. In Microbial Degradation of Organic Compounds (Gibson, D.T. ed.) Pp. 181–252. Marcel Dekker, New York. [ Links ]
Golubev, S.N., Scheludko, A.V., Muratova. A.Y., Makarov, O.E. and Turkovskaya, O.V. (2009). Assessing the potential of Rhizobacteria to survive under phenanthrene pollution. Water, Air, & Soil Pollution 198, 5–16. [ Links ]
Gray, M. (1989). Substrate inactivation of enzymes in vitro and in vivo. Biotechnology Advances 7, 527–575. [ Links ]
Hamamura, N., Yeager, C. M. and Arp, D. J. (2001). Two distinct monooxygenases for alkane oxidation in Nocardioides sp. strain CF8. Applied and Environmental Microbiology 67(11), 4992–4998. [ Links ]
Hartman, R. Hany, R., Plestcher, E., Ritter, A., Witholt, B. and Zinn, M. (2006). Tailor made olefinic medium chain length poly ([R]–3–hydroxyalkanoates) by Pseudomonas putida GPo1: batch versus chemostat production. Biotechnology and Bioengineering 93, 737. [ Links ]
Holden, P.A., La Montagne, M.G., Bruce, A.K., Miller, W.G. and Lindow, S.E. (2002). Assessing the role of Pseudomonas aeruginosa surface–active gene expression in hexadecane biodegradation in sand. Applied and Environmental Microbiology 68(5), 2509–2518. [ Links ]
Hölker, U., Höfer, M. and Lenz, J. (2004). Biotechnological advantages of laboratory–scale solid–state fermentation with fungi. Applied Microbiology and Biotechnology 64(2), 175–186. [ Links ]
Kamerbeek, N.M., Janssen, D.B., van Berkel, W. J. H., Fraaije, M.W. (2003). Baeyer–Villiger monooxygenases, an emerging family of flavin–dependent biocatalysts. Advanced Synthesis & Catalysis 345(6), 667–678. [ Links ]
Leahy, J. G., Colwell, R.R(1990). Microbial degradation of hydrocarbons in the environment. Microbiological Reviews 54, 305–315. [ Links ]
Laemmli , V. K. (1970) Cleavage of the structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [ Links ]
Lessner, D.J., Johnson, G.R., Parales, R.E., Spain, J.C. and Gibson, D.T., (2002). Molecular characterization and substrate specificity of nitrobenzene dioxygenase from Comamonas sp. strain JS765. Applied and Environmental Microbiology 68(2), 634–641. [ Links ]
Maestre, J. P., Gamisans, C., Gabriel, D. and Lafuente, J. (2007). Fungal biofilters for toluene biofiltration: evaluation on the performance with four packing materials under different operating conditions. Chemosphere 67, 684–692. [ Links ]
Nikolova, N. and Nenov, V. (2005). BTEX degradation by fungi. Water Science & Technology 51(11), 87–93. [ Links ]
Noordman, W. H., Wachter, J. H., de Boer, G. J., Janssen, D. B. (2002). The enhancement by surfactans of hexadecane degradation by Pseudomonas aeruginosa varies with substrateavailability. Journal of Biotechnology 94, 195–212. [ Links ]
Östberg, T. L., Jonsson, A. P., Bylund, D. and Lundström, U. S. (2007). The effects of carbon sources and micronutrients in fermented whey on the biodegradation of n–hexadecane in diesel fuel contaminated soil. International Biodeterioration and Biodegradation 60, 334–341. [ Links ]
Pandey, A., Selvakumar, P., Soccol, C. R. and Nigam, P. (1999). Solid state fermentation for the production of industrial enzymes. Current Sciences 77(1), 149–162. [ Links ]
Parales, R.E., Huang, R., Yu, C.L., Parales, J.V., Lee, F.K.N., Lessner, D.J. , Ivkovic–Jensen, M. M., Liu, W., Friemann, R., Ramaswamy, S. and Gibson, D.T. (2005). Purification, characterization, and crystallization of the components of the nitrobenzene and 2–nitrotoluene dioxygenase enzyme systems. Applied and Environmental Microbiology 71(7), 3806–3814. [ Links ]
Prenafeta–Boldu, F. S., Vervoort, J., Grotenhuis, J. T. C., van Groenestijn, J. W. (2002). Substrate interactions during the biodegradation of benzene, toluene, ethylbenzene and xylene (BTEX) hydrocarbons by the fungus Cladophialophora sp. strain T1. Applied and Environmental Microbiology 68, 2660–2665. [ Links ]
Qi, B., Moe, W. M. and Kinney, K. A. (2002). Biodegradation of volatile organic compounds by five fungal species. Applied Microbiology and Biotechnology 58, 684–689. [ Links ]
Radwan, S. (2008). Microbiology of oil–contaminated desert soils and coastal areas in the Arabian Gulf region. In: Microbiology of Extreme Soils. Soil Biology 13, (P. Dion and C. S. Nautiyal Eds.) Pp 275–298. Springer–Verlag Berlin Heidelberg. [ Links ]
Ramesh, M. V. and Lonsane, B. K. (1991). Ability of solid state fermentation technique to significantly minimize catabolic repression of a–amylase production by Bacillus licheniformis M27. Applied Microbiology and Biotechnology 35, 591–593. [ Links ]
Romero, M.C., Salvioli, M.L., Cazau, M.C. and Arambarri, A.M. (2002). Pyrene degradation by yeasts and filamentous fungi. Environmental Pollution 117(1), 159–163 [ Links ]
Solís–Pereira, S.E., Favela–Torres, E., Viniegra–Gonzalez, G., Gutierrez–Rojas, M. (1993). Effect of different carbon sources on the synthesis of pectinases by Aspergillus niger CH4 in submerged and absorbed substrate fermentation. Applied Microbiology and Biotechnology 39(1), 36–41. [ Links ]
Sun, A. K. and Wood, T. K. (1997). Trichloroethylene mineralization in a fixed–film bioreactor using a pure culture expressing constitutively toluene ortho–monoxygenase. Biotechnology and Bioengineering 55(4), 674–685. [ Links ]
Torres Pazmiño, D. E., Winkler, M., Glieder, A., and Fraaije, M. W. (2010). Monooxygenases as biocatalysts: Classification, mechanistic aspects and biotechnological applications. Journal of Biotechnology 146, 9–24. [ Links ]
van Beilen, J. B., Kingma, J. and Witholt, B. (1994). Substrate specificity of the alkane hydroxylase system of Pseudomonas oleovorans GPo1. Enzyme and Microbial Technology 16(10), 904–911. [ Links ]
Van Gorcom, R. F. M., Boschloo, J. G., Kuijvenhoven, A., Lange J., Van Vark, A. J., Bos, C. J., Van Balken, J. A. M., Pouwels, P. H. and Van den Hondel, A. A. M. J. (1990). Isolation and molecular characteristics of the benzoate–para–hydroxilase gene (bpha) of Aspergillus niger. A member of a new gene family of the cytochrome P450 superfamily. Molecular and General Genetics 223, 192–197. [ Links ]
Van Hamme, J. D., Singh, A. and Ward, O. P. (2003). Recent advances in petroleum microbiology. Microbiology and Molecular Biology Reviews 67(4), 503–549. [ Links ]
Vatsyayan, P., Kiran Kumar, A., Goswami, P. and Goswami, P. (2008). Broad substrate cytochrome P450 monooxygenase activity in the cells of Aspergillus terreus MTCC 6324. Bioresource Technology 99, 68–75. [ Links ]
Viniegra–González, G., Favela–Torres, E., Aguilar, C. N., Romero–Gómez, S. J., Díaz–Godínez, G. (2003). Advantages of fungal enzyme production in solid state over liquid fermentation Systems. Biochemistry and Bioengineering Journal 13, 157–167. [ Links ]
Volke–Sepúlveda, T., Gutiérrez–Rojas, M., Favela–Torres, E. (2003). Biodegradation of hexadecane in liquid and solid–state fermentation by Aspergillus niger. Bioresource Technology 87, 81–86. [ Links ]
Volke–Sepúlveda, T., Gutiérrez–Rojas, M., Favela–Torres, E. (2006). Biodegradation of high concentrations of hexadecane by Aspergillus niger in a solid–state system: kinetic analysis. Bioresource Technology 97, 1583–1591. [ Links ]
Wang, K., W., Baltzis, B. C. and Lewandowki, G. A. (1996). Kinetics of phenol biodegradation in the presence of glucose. Biotechnology and Bioengineering 51(1), 87–94 [ Links ]
Wu, R. R., Dang, Z., Yi, X. Y., Yang, C., Lu, G. N., Guo, C. L. and Liu, C. Q. (2011). The effects of nutrient amendment on biodegradation and cytochrome P450 activity of an n–alkane degrading strain of Burkholderia sp. GS3C. Journal of Hazardous Materials 186, 978–983. [ Links ]
Zazueta–Sandoval, R., Zazueta–Novoa, V., Silva–Jimenez, H. and Cabrera–Ortiz, R. (2003). A different Method of Measuring and detecting mono– and dioxygenase activities. Applied Biochemistry and Biotechnology 105(108), 725–736. [ Links ]
Zhu, Y., Smits, J. P., Knol, W. and Bol, J. (1994). A novel solid–state fermentation system using polyurethane foam as inert carrier. Biotechnology Letters 16(6), 643–648. [ Links ]














