Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de ingeniería química
versão impressa ISSN 1665-2738
Rev. Mex. Ing. Quím vol.10 no.1 Ciudad de México Abr. 2011
Ingeniería ambiental
Evaluation of the electro–coagulation process for the removal of turbidity of river water, wastewater and pond water
Evaluación del proceso de electrocoagulación para la remoción de turbidez de agua de río, agua residual y agua de estanque
S. Pérez–Sicairos1,2*, J.B. Morales–Cuevas1, R.M. Félix–Navarro2 and O.M. Hernández–Calderón1
1 Facultad de Ciencias Químico Biológicas de la Universidad Autónoma de Sinaloa. Blvd. Las Américas y Blvd. Universitarios, Ciudad Universitaria, C.P. 80010, Culiacán, Sinaloa.
2 Centro de Graduados del Instituto Tecnológico de Tijuana, Blvd. Industrial s/n, Mesa de Otay, C.P. 22500, Tijuana, B.C. *Corresponding author. E–mail: perezss@uas.uasnet.mx
Received 26 of September 2010.
Accepted 15 of March 2011.
Abstract
In this work, the removal of water turbidity was studied from three sources: Tamazula River, the Degremont Wastewater Treatment Plant and a pond of a botanical garden in the region of Culiacan, Sinaloa. For this purpose, parallel plate and continuous flow electrochemical reactors were constructed, both with perforated plate aluminum anodes and iron mesh cathodes, for the application of electro–coagulation. The process variables studied were the applied current and the concentration of supporting electrolyte, in the case of parallel plate reactor and also the volumetric flow rate, in the case of continuous flow reactor. According to the results, it is possible to eliminate the turbidity of the Tamazula river water with this process, achieving compliance with the official Mexican standards under the conditions studied. The general conclusion is that the electro–coagulation process has a great potential for application in turbidity removal for treatment of the three types of water sources used.
Keywords: electro–coagulation, turbidity, water treatment, wastewater, suspended solids.
Resumen
En este trabajo se estudió la remoción de turbidez de agua procedente de tres fuentes; río Tamazula, residual de la Planta Degremont y de un estanque del Jardín Botánico, en la región de Culiacán, Sinaloa, mediante la aplicación de electrocoagulación. Para esto, se construyó un reactor electroquímico de placas paralelas y uno de flujo continuo, ambos con ánodo de placa de aluminio perforada y cátodo de malla de hierro. Las variables de proceso estudiadas fueron la corriente aplicada y la concentración del electrolito de soporte para el caso del reactor de placas paralelas, y además el gasto volumétrico, para el caso del reactor de flujo continuo. De acuerdo con los resultados obtenidos, es posible eliminar la turbidez del agua del río Tamazula mediante este proceso, logrando cumplir, bajo las condiciones estudiadas, con las normas oficiales mexicanas en este rubro. La conclusión general es que el proceso de electro–coagulación presenta un potencial elevado de aplicación en el tratamiento de las tres fuentes de agua empleadas, para la eliminación de la turbidez.
Palabras clave: electro–coagulación, turbidez, tratamiento de agua, agua residual, sólidos suspendidos.
1 Introduction
One of the major issues that humanity faces in the twenty–first century is the need to provide water for the growing world population. Problems with water are expected to grow worse in the coming decades, with water scarcity occurring globally, even in regions currently considered water–rich. To address these problems, a tremendous amount of research must be conducted to identify robust new methods of purifying water at lower cost and with less energy, while at the same time minimizing the use of chemicals and the impact on the environment (Shannon et al., 2008).
Concern about water quality is related to two very important aspects: on one hand, contamination of drinking water or the poor quality of some sources and, on the other, the quality of the treated wastewater that is discharged into other water bodies, leading to a problem of dispersion of pollutants. Many regions of the world suffer from water shortages, while in others regions, the problem is not lack of resources, but poor management and distribution (Restrepo et al, 2006). Among the traditional technologies are biological processes and physicochemical processes, which include: filtration, ion exchange, chemical precipitation, chemical oxidation, adsorption, ultrafiltration, reverse osmosis and electrodialysis, among others.
Today, there exist emerging technologies that are based on electrochemistry and, currently, some alternatives offer competitive advantages over traditional technologies. This last group includes electro–coagulation, the electro–flotation and electro–decantation (Chen, 2004). Electro–coagulation is an electrochemical method of treating polluted water, whereby sacrificial anodes corrode to release active coagulant precursors (usually aluminum or iron cations) into solution. Accompanying electrolytic reactions involve gas evolution (usually as hydrogen bubbles) at the cathode.
Electro–coagulation has a long history as a water treatment technology, having been employed to remove pollutants in a very broad range of nature and composition, including oily suspensions (Santos et al, 2006), wastes from the textile industry (Can et al, 2006, Kobya et al., 2003), electroplating wastes (Adhoum et al., 2004), heavy metals (Gao et al., 2005, Gomes et al., 2007, Parga et al., 2005, Ratna Kumar et al., 2004), surfactants (Önder et al., 2007)
or food processing wastes products (Barrera–Díaz et al., 2006, Roa–Morales et al., 2007) and this list far from exhaustive. However, electro–coagulation has never become accepted as a 'mainstream' water treatment technology. The lack of a systematic approach to electro–coagulation reactor design/operation and the issue of electrode reliability (particularly passivation of the electrodes over time) have limited its implementation. However recent technical improvements combined with a growing need for small–scale decentralized water treatment facilities have led to a re–evaluation of electro–coagulation (Holt et al., 2005).
Water quality is established considering three sets of characteristics: physical, chemical and biological. From the physical point of view, the total solids are water impurities that can be classified as filterable particles (suspended), not filterable (dissolved) and a third possibility is the intermediate case corresponding to colloids. Turbidity is defined as the optical property of a sample which scatters and absorbs light rather than transmitting in a straight line. The World Health Organization (WHO) and the NOM–127–SSA1–1994 indicate a reference value of 5 Nephelometric Turbidity Units (NTU) for drinking water. Turbidity is an indicator and does not provide results for a specific pollutant. However, it is a reflection of the full extent of contamination. Turbidity in water is caused by suspended matter such as clay, silt, and organic matter and by plankton and other microscopic organisms that interfere with the passage of light through the water.
2 Principles and fundamentals of electro–coagulation
Electro–coagulation is a technology that has been known for more than one hundred years. However, no systematic research has been developed that can be used to predict its chemical behavior, reactions and mechanisms, or can provide sufficient tools for the design and operation of the reactors (Moreno–Casillas et al., 2007).
The technique relies on the electrochemical dissolution of sacrificial Al or Fe electrodes. The generated cations contribute by diminishing the stability of the suspended entities, by decreasing their zeta potential. Also, upon formation of hydroxide ions at the cathode, metal ions complex with iron or aluminum hydroxides, which are known to be efficient coagulants. The hydrogen bubbles formed at the cathode adsorb the flocs formed by the process, and ensure their flotation, which simplifies their separation from the treated water (Zongo et al., 2009). The pollutants from many different effluents are removed by applying the principle of coagulation; however, in electro–coagulation, no use is made of a chemical coagulant. Electro–coagulation can be defined as a process in which the suspended pollutants are destabilized, emulsified or dissolved in an aqueous medium, by inducing electrical current in the water through parallel metal plates of different materials, iron and aluminum being the most commonly used (Holt et al., 2005, Chen, 2004).
Usually, the instability of pollutant molecules produces stable, less emulsified colloidal particles, yielding hydrophobic components that precipitate or float, facilitating their removal by some form of secondary separation. The metal ions released and dispersed in the liquid tend to form metallic oxides that bond contaminants that have been destabilized (Chen, 2004).
The presence of salts in sewage and industrial discharges aids the conduction of electricity, which in turn promotes the evolution of hydrogen and oxygen gas at the electrodes during electrolysis. These gases rise to the surface to produce three phenomena: separation of colloids from the electrode, decreasing the need for cleaning the surface; entrainment of destabilized colloids, which rise to the surface in a skin, enabling not only a classic sedimentation extraction but also, by flotation; and an increase in destabilization efficiency, due to the gas bubbles, that are produced updrafts and downdrafts of the solution, leading to a better contact surface.
3 Applications of the electro–coagulation (EC) in the treatment of contaminated water
The process has been used for removal of contaminants from different wastewaters. One of the best known and most popular applications of electro–coagulation has been the treatment of wastewater from the electroplating and metal plating industries, a process that strives to remove the bulk of the soluble metals in the discharge.
Adhoum et al. (2004) applied electro–coagulation, with aluminum sacrificial anode, in the treatment of metal ions (Cu2+, Zn2+ and Cr (VI)) contained in wastewaters. The method was found to be highly efficient and relatively fast compared to conventional techniques.
Gao et al. (2005) designed a combined process of electro–coagulation and electro–flotation first to reduce Cr6+ to Cr3+, followed by total removal of chromium from wastewater to a value below 0.5 mg/L. Acidic condition was employed in the reduction of Cr6+ and neutral conditions were found to be beneficial for the coagulation of the precipitates of Cr(OH)3 and Fe(OH)3.
A research team led by Can O. T. (2006) investigated the effect of initial addition of a chemical coagulant such as polyaluminum chloride (PAC) or alum on the chemical oxygen demand (COD) removal efficiency of EC treatment of textile wastewaters. They used each salt for the chemical coagulation, finding that both salts exhibited the same performance, but in the combined electro–coagulation (CEC), the PAC was found to significantly enhance the COD removal rate and efficiency.
Santos et al. (2006) successfully applied the electrochemical technology, employing a dimensionally stable anode DSA® (nominal composition Ti/Ru0.34 Ti0.66O2) for the treatment of oily emulsions.
Roa–Morales et al. (2007) evaluated the removal of organic pollutants from pasta and cookie processing industrial wastewater by aluminum electro–coagulation and combined electro–coagulation/H2O2 processes. They found that treatment reduced the COD by 90%, biochemical oxygen demand (BOD5) by 96%, total solids by 95% and fecal coliforms by 99.9%.
Kobya et al. (2003) reported the treatment of textile wastewaters using iron and of aluminum electrode materials. They concluded that iron is superior to aluminum, as the sacrificial electrode material, for COD removal efficiency and energy consumption.
3.1 Mechanism of reaction
The most important reactions that the contaminant particles experience during the electro–coagulation are: hydrolysis, electrolysis, ionization and free radical formation.
The electro–coagulation process is affected by different factors. Among the most important are the nature and concentration of pollutants, wastewater pH and conductivity. These factors determine and control the reactions occurring in the system, as well as the formation of the coagulant.
When the aluminum acts as the anode, the reactions are as follows (Holt et al., 2005):
At the anode:
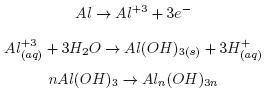
At the cathode:
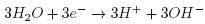
Al+3 ions combine with OH– react to form monomeric species, such as Al(OH) 2+, Al2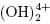 , Al
, Al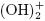 , and polymers, such as Al6
, and polymers, such as Al6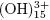 , Al7
, Al7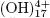 , Al8
, Al8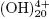 , Al13O4
, Al13O4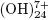 y Al13
y Al13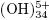 , that cause the precipitation of Al(OH)3 as shown in the anode reaction. Al(OH)3(s) is an amorphous substance, which exposes a large surface area and which is conducive to the adsorption and attraction of particulate pollutants. (Restrepo et al., 2006).
, that cause the precipitation of Al(OH)3 as shown in the anode reaction. Al(OH)3(s) is an amorphous substance, which exposes a large surface area and which is conducive to the adsorption and attraction of particulate pollutants. (Restrepo et al., 2006).
In this work, results are reported for the removal of turbidity from local river waters, such as the Tamazula, Wastewater Treatment Plant Degremont and a pond of the botanical garden in the region of Culiacan. Table 1 shows a typical analysis of the quality of wastewater from the sewage system in the city, which is treated in the wastewater plant Degremont. The wastewater generated in the city of Culiacan, consists mainly of household waste and commercial establishments selling food, so the main component is organic matter, fat and grease since the presence of industries is relatively negligible in the city. These water bodies, sampled on different days under distinct environmental conditions, were treated in a prototype of batch and continuous flow electrochemical reactors, in order to eliminate turbidity to meet levels required by Mexican regulations. The operating variables studied were the applied current, supporting electrolyte concentration (NaCl) and the feed flow rate. In any electrochemical process the electrolyte plays a very important role, its presence in the solution promotes the current passage across the solution, reducing the electrical resistance of the system and hence the energy consumption. Sodium chloride is a natural compound in water and an excellent electrolyte for many aqueous systems also is a substance of relatively low cost. In the surface of aluminum electrodes develops an insulating film of alumina (Al2O3) due to natural oxidation with oxygen gas. This surface oxide film has been reported to be constituted of an adherent compact layer covered with a more porous layer. At the opposite, common electrolytes such as NaCl, are often used for conductivity reasons but without anticipating the powerful corrosive properties of chloride ions that promote (Trompette and Vergnes (2009), even at low concentrations, film breakdown through pitting corrosion, ensuring a sufficient release of aluminum cations, so allowing further satisfactory separation conditions even when operating at low electrical potential.
Batch conditions were tested initially and, later, in the one pass continuous flow reactor. With the results obtained from different experiments, reactor operating parameters were determined to establish the functionality and feasibility of the process.
4 Experimental materials and methods
4.1 Materials
The electrodes employed were made with commercial materials. The anode was a perforated aluminum rectangular plate with a thickness of 1.72 mm, a height of 113 mm and length of 142 mm; these were perforated in order to increase the contact surface area, which increases electron transfer. The cathode was constructed with iron mesh, which provides a large area for the reaction. The turbidity measurements of samples collected from the electro–reactors were performed twice for each sample (Solak et al., 2009), the first on the sample taken directly from the reactor and the second, after filtering the sample with Whatman #4 (particle retention > 25 μm). This was done to simulate the sedimentation stage that traditionally accompanies the stage of coagulation in treatment plants. The filter retains only relatively large flocs present in the samples formed after contact of the constituents with species formed during electrocoagulation but cannot remove the colloidal particles with sizes smaller than 20–25 μm, which cross the filter. Also, this procedure let us saving time in the experiments running.
4.2 Design and construction of the electro–coagulation reactors
4.2.1 Batch reactor
For the preliminary study of the process variables (applied current and concentration of supporting electrolyte), a parallel–plate reactor (Fig. 1) was constructed. The reactor was an acrylic tank, with a capacity of approximately 4.2 liters. The dimensions were 20 cm in length, 14 cm in height and a 15 cm width. The electrodes used in this cell were separated 6 cm.
4.2.2 Continuous flow reactor
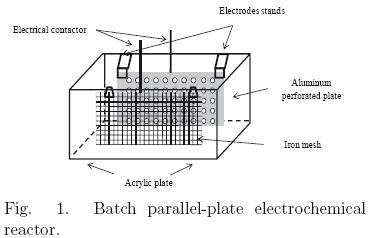
To study the electrochemical process in continuous flow, an acrylic electrochemical reactor was designed with a volume of 15 liters, which consists of a set of four cells, each with both electrodes. It also features three screens, whose function was to increase the retention time of water inside the reactor and break the laminar flow regime. The reactor also has a blowdown system, a skimmer to remove floated matter and a hose to feed the wastewater. The electrodes were mounted on special bases designed to keep the electrodes separated from internals walls allowing the solution to circumvent the surface of electrodes. The distance between the electrodes in each section of the reactor, is approximately 10 cm. Fig. 2 shows a diagram of the electro–coagulation reactor with continuous feed stream. Gravity is the driving force for the system feed.
4.2.3 Determination of the effect of current intensity and of the supporting electrolyte concentration on the reaction of electro–coagulation in a parallel–plate cell
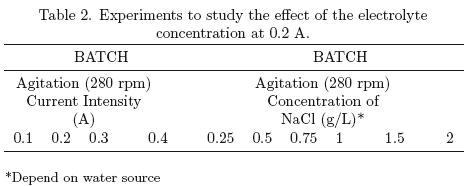
In these experiments, 2.5 L of the sample (wastewater, river or pond) were transferred to the electrochemical cell with parallel plates. Electrolyte (sodium chloride) was added in different concentrations from 0.25 to 2.0 g/L, 0.25 to 0.75 g/L and 0.25 to 1.0 g/L, for river, pond and wastewaster, respectively. Once the water to be treated was transferred, the magnetic stirrer was initiated (approximately 280 rpm), the terminals of the electrodes were connected to the power supply and the circuit current (ranged 0.1 a 0.4 A) was established. The initial turbidity was determined and the system was then allowed to react, measuring turbidity at different time intervals. For experiments with different salt concentrations, the current was kept fixed. Once the experiment was completed, the salt concentration was altered, repeating the procedure described above. Table 2 details the conditions under which these experiments were developed. For experiments with different currents, the electrolyte concentration was held constant. When the experiment was concluded, the applied current was changed, repeating the procedure described above.
4.2.4 Determination of current intensity and the concentration of the supporting electrolyte on the electro–coagulation reaction in the continuous flow reactor
During these experiments, common salt was added in the feed tank in different concentrations (0.25, 0.5, 0.75 g/L), the current range was adjusted from 0.4 to 1.6 A, and the volume flow rate was adjusted to 0.5 L/minute. The experiments had a duration of 30 minutes. Table 3 shows the conditions under which these experiments were developed. Samples were taken and turbidity measured at different reaction times (at 5 minute intervals) at the exit of the reactor.
The electrical resistance of the solution to current flow is highly dependent on the concentration of supporting electrolyte.
4.2.5 Effect of volumetric flow through the electrochemical reactor on the reaction rate
In these experiments, the volume flow rate through the reactor was varied. The volumetric flow of water to be treated affects two important phenomena: on the one hand, the rate of mass transfer from the surface of the electrodes into the bulk solution and, on the other, renewal of the nearest layer to the electrode surface, which affects the rate of charge transfer at the solid–liquid interface.
The volumetric flow was varied in the range of 0.25 to 1 L/min, with retention times of 60 to 15 minutes, respectively. The applied current and the electrolyte concentration were held constant. The procedures are similar to those described above.
4.2.6 Calculation of the efficiency of the process by measuring the turbidity of water treated with electro–coagulation process and energy consumption of the process
To determine the efficiency of the process, the turbidity of treated water was quantified, which provides information on the process from the point of view of water treatment. However, to determine the efficiency of electrochemical process, the energy consumption was estimated, although not the specific consumption, that means, per unit of mass electrolyzed, as it was not possible to quantify the aluminum in solution, so the calculations of the required energy include all side effects.
5 Results and discussion
Two electrochemical reactors were designed for the development of the experimental protocol; one with parallel plates to operate in batch and other with continuous flow.
5.1 River water treatment
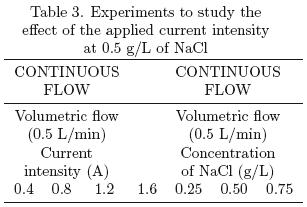
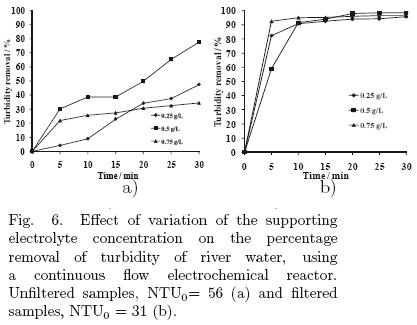
The turbidity in water of river Tamazula is mainly caused by dissolved humic acids, whose concentration depends on the season of the year (rainy or dry season). The average turbidity for the samples used in the experiments was 56 NTU. The results for the experiments of varying current density and electrolyte concentration in the parallel plate reactor are shown in figs. 5 and 6, respectively.
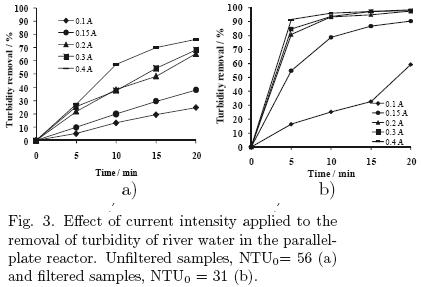
5.1.1 Effect of the applied current
The applied current determines the speed of reaction. Since the amount of Al3+ generated is proportional to the current, thus a higher concentration increases the removal of turbidity. However, this represents an increase in operation costs and of secondary reactions, decreasing the efficiency. The undesirable reaction is the electrolysis of water. To estimate the cost of energy consumption (kW·h), the amperage was multiplied by the applied voltage and the operating time. Energy consumptions ranged from 0.0000332 to 0.000748 kW·h/Liter of treated water. For unfiltered samples, the highest percentage of turbidity removal was 68% (Fig. 3) applying a current of 0.4 A with an energy consumption of 0.000748 kW·h/L of water treated. However, for filtered samples (Whatman #4 filter paper), the results are quite different, observing no significant difference between 0.4 A, 0.3 A and 0.2 A, with values of 98, 97 and 96% for turbidity removal, respectively. For this reason, the best condition of the applied current in the parallel plate reactor was found to be 0.2 A, due to its high levels of turbidity removal and lower energy consumption (0.00016 kW·h/L), compared with other values used.
5.1.2 Effect of the concentration of electrolyte (sodium chloride)
These experiments were performed at concentrations ranged from 0.25 g/L to 2.0 g/L with a current of 0.2 A. The results for unfiltered samples showed the best results using the solution with 1.5 g/L of electrolyte. For filtered samples, the best performance was with a concentration of 0.5 g/L. The remaining values of turbidity removal are very similar, with a 1 % different in concentrations of 0.25 g/L and 0.75 g/L with removal of turbidity values of 97 and 98%, respectively. It was decided that the most convenient electrolyte concentration was 0.5 g/L, because in both cases (filtered and unfiltered samples) acceptable results were obtained. The energy consumption ranged from 0.000268 to 0.00008 kW·h/L.
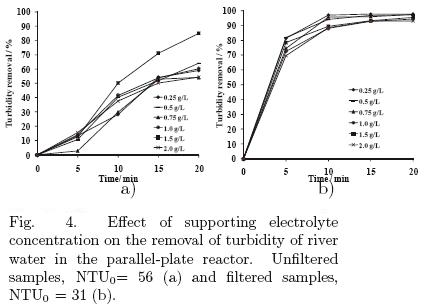
5.1.3 Effect of the current intensity and the concentration of the supporting electrolyte on the electro–coagulation reaction in the continuous flow rea ctor
The results for these experiments are shown in figs. 5 and 6, respectively.
5.1.4 Effect of the applied current
Experiments were performed applying four current; 0.1 A, 0.2 A, 0.3 A and 0.4 A (using 0.5 g/L of NaCl and 0.5 L/min); the energy consumptions were 0.0000093, 0.00004, 0.000085 and 0.00016 kW·h/L, respectively. The results of these experiments appear in Figure 5, where similar values for applied currents of 0.2, 0.3 and 0.4 A are observed for the filtered samples. For that reason, an applied current of 0.2 A was chosen for the continuous flow electrochemical reactor.
5.1.5 Effect of the electrolyte concentration
For this system, the use of low concentrations of electrolyte yielded the best results (Fig. 6), so experiments were conducted at concentrations of 0.25 g/L, 0.5 g/L and 0.75 g/L. For the unfiltered water, the use of an electrolyte concentration of 0.75 g/L produced the worst results. In the case of filtered samples the turbidity removal were 96%, 97% and 98% and energy consumptions 0.000048, 0.00004 and 0.000031 kW·h/L for 0.25 g/L, 0.5 g/L and 0.75 g/L, respectively.
5.1.6 Effect of the volumetric flow through the electrochemical reactor on the reaction rate
The results for experiments, varying the volumetric flow rate, are shown in Fig. 7. The effect arising from the variation of volumetric flow rate is a consequence of the wastewater residence time in the system. The study of this variable is important to maximize the volumetric capacity of water to be treated and to prevent the secondary reactions that do not benefit water clarification. The results of experiments demonstrated that a rate of 0.5 L/min is the best for the operation of the reactor, because in both the unfiltered and the filtered samples, this condition produces the highest percentage of turbidity removal. This variable had no significant effect on energy consumption, fluctuating near 0.00004 kW·h/L.
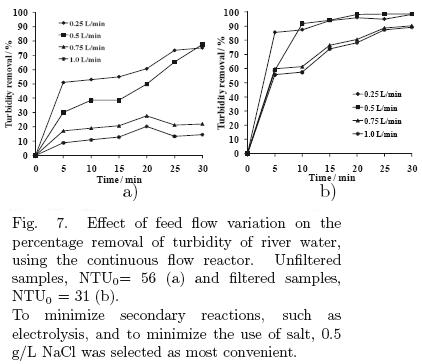
In conclusion, the best operating conditions were found to be an electrolyte concentration of 0.5 g/L and a current of 0.2 A, using a volumetric flow rate of 0.5 L/min through the electrochemical reactor.
5.2 Pond water treatment (Botanical Garden)
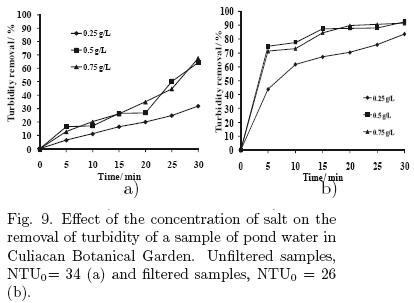
In these experiments, using the parallel plate cell, the effect of the applied current and the electrolyte concentration was determined. The samples were collected from a pond of the Botanical Garden in Culiacan, where there is a significant presence of suspended and dissolved material, due to plant residues and wastes of some aquatic species. The results are shown in figs. 8 and 9, respectively. The initial condition of turbidity for the samples was about 34 NTU.
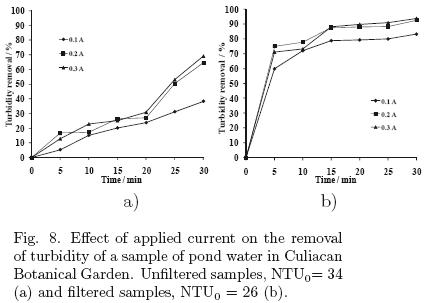
5.2.1 Effect of the applied current
The highest percentage was about 70% for samples analyzed without previous filtration and about 94% for filtered samples, in both cases the applied current was 0.3 A. In both cases, an applied current of 0.1 A shows the lowest percentage of turbidity removal, due to reduced formation of aluminum ion at the anode.
Energy consumption for these experiments was in the range of 0.000088 to 0.00125 kW·h/L, applying currents of 0.1, 0.2 and 0.3 A.
5.2.2 Effect of the supporting electrolyte concentration
Of the three concentrations of NaCl used (0.25, 0.5 and 0.75 g/L) in these experiments, 0.25 g/L had the lowest removal of turbidity. The highest percentage was 68% without previous filtration and approximately 92% for the filtered samples with 0.75 g/L (Fig. 9).
The energy consumption for these experiments was 0.00256, 0.00192 and 0.00144 kW·h/L for concentrations of 0.25, 0.5 and 0.75 g/L, respectively, because increasing the salt concentration, decreased the cell voltage for a constant applied current of 0.3 A.
5.3 Wastewater treatment (Degremont North Plant, Culiacan)
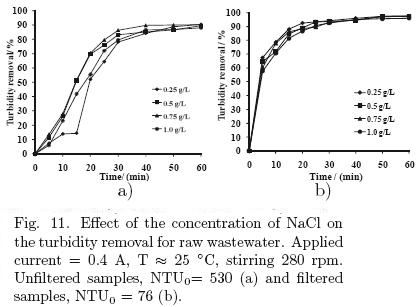
To evaluate the performance of the process in wastewater treatment, different experiments were performed with the parallel–plate cell. Only the applied current and the concentration of supporting electrolyte were studied. The initial condition of turbidity for the samples was about 530 NTU and all samples were collected at different day but at same hour, 2:00 P.M. The results for this set of experiments are presented in figs. 10 and 11.
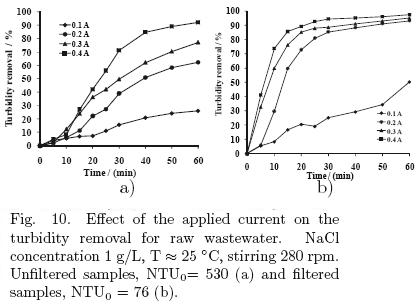
5.3.1 Effect of the applied current intensity
Fig. 10a shows the results of the effect of the applied current (0.1, 0.2, 0.3 and 0.4 A) in the electrochemical cell on the reduction of turbidity for the treated wastewater, when the samples were not filtered. Fig. 10b relates to the results for the same variable, when the samples were filtered. According to both figures, it can be observed that higher applied currents remove a greater percentage of turbidity, due to the larger amount of aluminum released in the solution (which favors the formation of clots) and of gas produced on the surfaces of the electrodes (favoring the flotation of colloidal matter) due to water electrolysis.
However, energy consumption also increases with the applied current, being 0.000088, 0.0004, 0.001 and 0.00124 kW·h/L for 0.1, 0.2, 0.3 and 0.4 A, respectively.
5.3.2 Effect of the supporting electrolyte concentration
The results for varying the salt concentration on turbidity removal in treated wastewater by electro–coagulation, are shown in Fig. 11, in the range from 0.25 to 1.0 g/L. In the case of the unfiltered samples, it appears that there is a marked difference in the results, except for the concentration of 0.25 g/L, where the first 30 min of reaction has the lowest percentage of turbidity removal. However, towards the end of the experiment, the removal percentages are very similar for the four concentrations of salt used. It is worthy to note that the applied current in all these experiments was 0.4 A, conditions under which the turbidity had the largest decrease.
In the case of the filtered samples, a similar trend for all salt concentrations was observed. The energy consumption was 0.00256, 0.00192, 0.00144 and 0.00124 kW·h/L for 0.25, 0.5, 0.75 and 1.0 g/L NaCl, respectively.
Fig. 12 presents the results of the turbidity removal of treated wastewater, where the process was allowed to stand for two hours after application of electro–coagulation, and samples were taken at different intervals and no filtration was made.
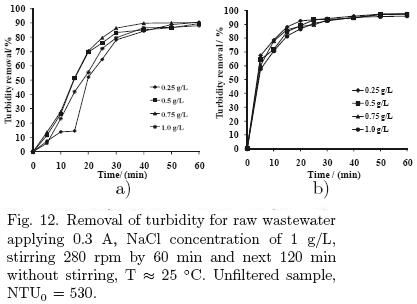
In this case, this time for sedimentation indicate that after 30 min of finished the electro–coagulation, is enough to achieve 98% of turbidity removal because of the gravity sedimentation. This condition is more approximate to that taking place in the real stage of sedimentation in a typical treatment plant.
Conclusion
Contaminated water from different sources, river, wastewater treatment plant and pond, were treated. Three variables were studied: applied current, supporting electrolyte concentration and volumetric flow rate, although the latter was only varied in the continuous flow reactor.
Tamazula River Water Treatment
Parallel Plate Reactor
The best operating conditions found for the treatment of river water in the batch reactor were the following: 0.5 g/L for the NaCl supporting electrolyte and an applied current of 0.2 A. Apparently, there is a very marked impact in this water of the electrolyte concentration on the turbidity removal. In experiments conducted under these conditions, the values of turbidity removal were never greater than those permitted by the standard NOM–127–SSA1–1994, which indicates a maximum allowable value for turbidity of 5 NTU.
Continuous Flow Reactor
Results for the system with continuous flow indicate that the best conditions for turbidity removal were at 0.2 A with an electrolyte concentration of 0.5 g/L and a feed volumetric flow of 0.5 L / min. For this type of reactor the volumetric flow rate determines the residence time of wastewater in the reactor. All results obtained with these parameters comply with the standard.
Botanical Garden Pond Water Treatment
In the treatment of pond water, when either 0.2 or 0.3 A is applied after approximately 15 minutes of reaction, the turbidity decreases by 88% and up to 94% with 30 minutes of reaction. In this case it is important to mention that the composition of water differs from that of river water, due to the incorporated substances are breakdown products of some aquatic species; additionally, dissolved oxygen is lower than in river water.
Of the three salt concentrations studied in the electro–coagulation of pond water, the least efficient was 0.25 g/L. It is likely that due to the constituents of this water, the different organic compounds (humic substances, metabolites, etc.) interfere with the charge transfer processes. According to the results, there is no significant difference between using 0.5 and 0.75 g/L of salt on the turbidity removal efficiency.
Wastewater Treatment Plant Degremont Culiacan
The process was found adequate to remove the turbidity of wastewater, although it was unable to remove odors. It is clear that two main processes are involved in the elimination of residual water turbidity, aggregate formation and foaming. This implies that the formation of small bubbles on the surface of the electrodes and their rise is another important mechanism for water treatment, helping to remove floatable material.
In this process, an applied current of 0.4 achieved the best results, removing about 98% of the initial turbidity, but with the largest energy consumption (0.0031 kW.h). In electrochemical processes, the applied current to the systems is straightly related to the electrolysis, according the Faraday's Law, so at higher values of current intensity higher aluminum mass is electrolyzed.
As in the treatment of other types of water, the concentration of the supporting electrolyte has no significant effect on the electrochemical process, but its presence is necessary to increase the efficiency of the process due to two main factors: aluminum oxidation, by dissolved oxygen in water, promotes a passive layer on its surface that imply a very high electrical potential to be applied to breakdown the passive film, in this case the supporting electrolyte (NaCl) provides the corrosive chloride ions that breakdown the passive film at too much lower potential. By the other hand, sodium chloride increases conductivity of the solution for current passage, reducing the energy consumption, this allowed to reduce the applied cell potential from 16 V (at 0.2 A, without NaCl addition) to 5.5 V (at 0.2 A and 1 g/L NaCl added) in wastewater, for example.
References
Adhoum,N., Monser, L., Bellakhal, N., Belgaied, J. E. (2004). Treatment of electroplating wastewater containing Cu2+, Zn2+ and Cr(VI) by electro–coagulation. Journal of Hazardous Materials 112, 207–213. [ Links ]
Barrera–Díaz, C., Roa–Morales, G., Avila–Córdoba, L., Pavón–Silva, T., Bilyeu, B. Electrochemical Treatment Applied to Food–Processing Industrial Wastewater. Industrial and Engineering Chemistry Research 45, 34–38. [ Links ]
Can, O.T., Kobya, M., Demirbas, E., Bayramoglu, M. (2006). Treatment of the textile wastewater by combined electrocoagulation. Chemosphere 62, 181–187. [ Links ]
Chen, X., Chen, G., Yue, P. L. (2002). Novel Electrode System for Electroflotation of Wastewater. Environmental Science and Technology 36, 778–783. [ Links ]
Chen, G. (2004). Electrochemical technologies in wastewater treatment. Separation and Purification Technology 38, 11–41. [ Links ]
Gao, P., Chen, X., Shen, F., Chen, G. (2005). Removal of chromium(VI) from wastewater by combined electrocoagulation–electroflotation without a filter. Separation and Purification Technology 43, 117–123. [ Links ]
Gomes, J.A.G., Daida, P., Kesmez, M., Weir, M., Moreno, H., Parga, J.R., Irwin, G., McWhinney, H., Grady, T., Peterson, E., Cocke, D.L. (2007). Arsenic removal by electro–coagulation using combined Al–Fe electrode system and characterization of products. Journal of Hazardous Materials 139, 220–231. [ Links ]
Holt, P. K., Barton, G. W., Mitchell, C. A. (2005). The future for electrocoagulation as a localized water treatment technology. Chemosphere 59, 355–367. [ Links ]
Kobya, M., Can, O.T., Bayramoglu, M. Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. Journal of Hazardous Materials 100, 163–178. [ Links ]
Kumar, P.R., Chaudhari, S., Khilar, K.C., Mahajan, S.P. (2004). Removal of arsenic from water by electrocoagulation. Chemosphere 55, 1245–1252. [ Links ]
Moreno–Casillas, H.A., Cocke, D.L., Gomes, J.A.G., Morkovsky, P., Parga, J.R., Peterson, E. (2007). Electrocoagulation mechanism for COD removal. Separation and Purification Technology 56, 204–211. [ Links ]
Mouedhen, G., Feki, M., De Petris Wery, M., Ayedi, H.F. (2008). Behavior of aluminum electrodes in electrocoagulation process. Journal of Hazardous Materials 150, 124–135. [ Links ]
Önder, E., Koparal, A.S., Ö?ütveren, U.B. (2007). An alternative method for the removal of surfactants from water: Electrochemical coagulation. Separation and Purification Technology 52, 527–532. [ Links ]
Parga, J.R., Cocke, D.L., Valenzuela, J.L., Gomes, J.A., Kesmez, M., Irwin, G., Moreno, H., Weir, M. (2005). Arsenic removal via electrocoagulation from heavy metal contaminated groundwater in La Comarca Lagunera México. Journal of Hazardous Materials 124, 247–254. [ Links ]
Restrepo Mejía, A. P., Arango Ruiz, A., Garcés Giraldo, L. F. (2006). La electrocoagulación: retos y oportunidades en el tratamiento de aguas. Producción + Limpia 1, 58–77. [ Links ]
Roa–Morales, G., Campos–Medina, E., Aguilera–Cotero, J., Bilyeu, B., Barrera–Díaz, C. (2007). Aluminum electrocoagulation with peroxide applied to wastewater from pasta and cookie processing. Separation and Purification Technology 54, 124–129. [ Links ]
Santos, M.R.G., Goulart, M.O.F., Tonholo, J., Zanta, C.L.P.S. (2006). The application of electrochemical technology to the remediation of oily wastewater. Chemosphere 64, 393–399. [ Links ]
Shannon, M.A., Bohn, P.W., Elimelech, M., Georgiadis, J.G., Mariñas, B.J., Mayes, A.M. (2007). Science and technology for water purification in the coming decades. Nature 452, 301–310. [ Links ]
Solak, M., Kili,c, M., Yazici, H., S,encan, A. (2009). Removal of suspended solids and turbidity from marble processing wastewaters by electrocoagulation: Comparison of electrode materials and electrode connection systems. Journal of Hazardous Materials 172, 345–352. [ Links ]
Trompette, J. L., Vergnes, H. (2009). On the crucial influence of some supporting electrolytes during electrocoagulation in the presence of aluminum electrodes. Journal of Hazardous Materials 163, 1282–1288. [ Links ]
Zongo, I., Maiga, A. H., Wéthé, J., Valentin, G., Leclerc, J.P., Paternotte, G., Lapicque, F. (2009). Electrocoagulation for the treatment of textile wastewaters with Al or Fe electrodes: Compared variations of COD levels, turbidity and absorbance. Journal of Hazardous Materials 169, 70–76. [ Links ]














