Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Boletín médico del Hospital Infantil de México
versión impresa ISSN 1665-1146
Bol. Med. Hosp. Infant. Mex. vol.70 no.1 México ene./feb. 2013
ARTÍCULOS DE INVESTIGACIÓN
Identificación de Microsporidium spp en pacientes con diagnóstico de leucemia linfoblástica aguda
Identification of Microsporidium spp in patients with acute lymphoblastic leukemia
Leticia Eligio-García,1 Apolinar Cano-Estrada,1 Adrián Cortés-Campos,1 Aurora Medina-Sansón,2 Enedina Jiménez-Cardoso1
1 Laboratorio de Investigación en Parasitología
2 Departamento de Hemato-Oncología
Hospital Infantil de México ''Federico Gómez'', México, D.F., México
Corresponding author: Dra. Enedina Jiménez
Cardoso
E-mail: jimenezce@yahoo.com.mx
Fecha de recepción: 25-09-12
Fecha de aceptación: 24-01-13
Resumen
Introducción. El grupo Microsporidia incluye parásitos intracelulares obligados que afectan varios huéspedes. Los géneros que infectan humanos son Enterocytozoon y Encephalitozoon . Microsporidia es considerado un organismo oportunista en pacientes inmunocomprometidos e inmunocompetentes, que se ha convertido en un importante problema de salud pública. El objetivo del trabajo fue identificar Microsporidium spp en muestras fecales de pacientes con leucemia linfoblástica aguda, en diferente etapa de su tratamiento.
Métodos. Se analizaron 77 muestras de niños con diagnóstico de leucemia linfoblástica aguda menores de 12 años, por análisis coproparasitoscópico de Faust. Las muestras se tiñeron con Ziehl Nielsen, tricrómica de Masson y calcoflúor. Finalmente se realizó la amplificación por PCR del gen ribosomal.
Resultados. De los pacientes analizados, 16/77 (20.77%) resultaron positivos para Microsporidia, independientemente de que presentaran diarrea. El PCR fue más efectivo para la identificación que el análisis microscópico de las muestras teñidas.
Conclusiones. Este trabajo enfatiza la importancia de la microsporidiosis como infección emergente en pacientes con leucemia linfoblástica aguda bajo tratamiento quimioterapéutico, incrementando las complicaciones clínicas adicionales a la leucemia.
Palabras clave: Microsporidia, Enterocytozoon , Encephalitozoon , reacción en cadena de la polimerasa, tinción, leucemia linfoblástica aguda.
Abstract
Background. The phylum Microsporidia includes obligate intracellular parasites that affect several hosts. The most frequent genera to affect humans are Enterocytozoon and Encephalitozoon. Microsporidium is considered an opportunistic parasite in both immunocompromised and immunocompetent patients and have become an important health problem. The aim of this study was to identify Microsporidium spp in fecal samples of patients with acute lymphoblastic leukemia (ALL), with and without diarrhea, at different treatment stages.
Methods. Seventy seven samples from children <12 years old with diagnosis of ALL were analyzed by the Faust coproparasitoscopic method, Ziehl-Nielsen, trichrome and calcofluor staining methods and polymerase chain reaction.
Results. Results showed that 16/77 (20.77%) children presented Microsporidium and there was no relationship between microsporidial infection and diarrhea. Polymerase chain reaction was more effective than analysis by light microscopy of staining samples in the identification of the parasite.
Conclusions. This work emphasizes the importance of microsporidiosis as an emerging infection in patients with ALL undergoing chemotherapy, increasing the additional clinical complications of leukemia.
Key words: Microsporidia, Enterocytozoon , Encephalitozoon , polymerase chain reaction, staining, leukemia.
Introduction
The phylum Microsporidia encompasses a group of obligate intracellular parasitic eukaryotes that parasitize several hosts including mammalians, birds, reptiles and fish.1 The phylum contains >1200 species and represents ~150 genera.2,3 At least 15 species have been implicated in human infections. Among the most frequent species that parasitize humans are Enterocytozoon bieneusi , E. intestinalis , E. hellem , and E. cuniculi .4 In recent years a high number of cases have been reported as opportunistic infections in immunocompromised patients, for example, those who have received an organ transplant, HIV-infected patients and individuals undergoing cancer chemotherapy. In some cases the infection has also been found in immunocompetent persons associated with persons who live or work together with animals.5 Some clinical manifestations that have been observed are diarrhea, sinusitis, keratitis, myositis, osteomyelitis, and encephalitis, among others, but the disease encompasses a wide spectrum and includes most organ systems. In some cases it may even be confused with other infectious pathologies.6,7
The usual treatment against Microsporidia includes the use of albendazole and fumagillin and some derivative drugs, but there is no totally effective and safe treatment for microsporidian species that infect humans.8 Microsporidia produce an environmentally resistant spore that represents the infectious form. It has a sophisticated mechanism to inoculate its genetic contents into a host cell by the extrusion of its internal polar filament. The spore has been of interest to researchers because it is an alternative for an effective anti-microsporidian therapy by inhibition of the spore germination process.9
According to the international literature, there are only a few studies about the prevalence of Microsporidium in immunocompromised patients, specifically those who are undergoing anti-cancer chemotherapy.10,11 Data are variable because of the difficulties in the identification of the organism and because the parasitic search is focused on more common parasites. The successful diagnosis of Microsporidium is related to the identification of spores in biological samples by the coproparasitoscopic method (CPS) method and staining, but the size of the organism, inability of the analysis and the number of biological samples analyzed (< 3) limit correct identification. In Mexico, according to our knowledge, there are no reports about Microsporidium related to leukemia. Routine diagnosis is performed with microscopy of fecal samples stained with fluorescent or chromotrope-based stains.12 However, because correct identification of species has clinical importance in order to provide efficient treatment and considering that the species cannot be differentiated from each other by light microscopy, several molecular techniques have been reported.
The aim of this work was to establish the frequency of Microsporidium spp. in fecal samples of patients with acute lymphoblastic leukemia (ALL), with and without gastrointestinal symptomatology at different times of treatment using Faust coproparasitoscopic method, different staining techniques and polymerase chain reaction (PCR).
Methods
Biological samples
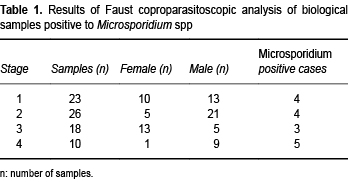
From March 2010 through December 2011, we collected 77 samples from children < 12 years old from the Hospital Infantil de Mexico Federico Gomez (HIMFG) with a diagnosis of ALL (23 samples were taken at the time of diagnosis, 26 samples during the second stage, 18 in the third stage and 10 in the fourth stage). Ethical considerations were followed in accordance with the institutional committee of the HIMFG that reviewed and approved the project. Written consent was obtained from a parent or legal guardian. Clinical data were gathered about patient's age, gender, time of chemotherapy treatment and clinical symptoms, especially diarrhea.
Faust coproparasitoscopic method and staining
Fecal samples were collected and examined by microscopy after staining with Lugol's iodine12 in order to detect spores of Microsporidium spp. Sample slide preparations were done from each sample and fixed with methanol for 5 min. Slides were stained with Ziehl-Neelsen, trichrome and calcofluor method as follows.13,14
Ziehl-Neelsen method
The fixed preparation was rinsed with concentrated carbol-fuchsin for 20 minutes and then washed with 7% sulfuric acid and counterstained with Malachite green.
Trichrome method
The fixed slide was covered with phenol-alcoholic fuchsin solution for 10 min, discolored with 0.5% HCl in ethanol and trichrome solution (Cromotrope 2R, blue aniline and phosphotungstic acid) was added to the preparation and rinsed first with acetic acid and then with 90% ethanol.
Calcofluor method
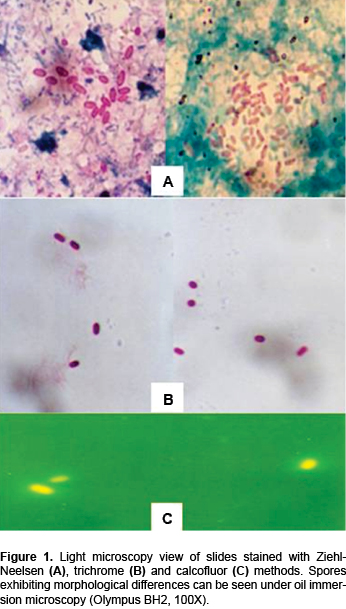
The preparation was covered with 0.1% calcofluor and rinsed with water. Slides were dried and observed by light microscopy (Olympus BH2). The sample was viewed under oil immersion lens. Calcofluor preparation was viewed under UV light at a wavelength of 380-400 nm.
PCR amplification
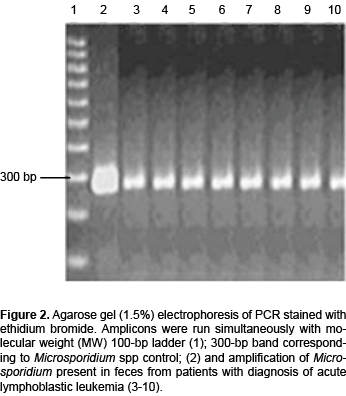
DNA was extracted from all feces using the QIAamp DNA stool mini kit (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions. DNA concentrations were determined using a spectrophotometer (EPOCH, Biotek). PCR was then performed as follows: V1 (5´-CACCAGGTTGATTCTGCCTGAC-3´) and PMP2 (5´-CCTCTCCGGAACCAAACCCTG-3´) primers described by Chabchoub15 were used to obtain a 300-bp DNA fragment. PCR was performed in a 25-μL reaction mixture containing 1X PCR amplification buffer, 1.2 μM each dATP, dGTP, dCTP and dTTP, 0.5 ng each primer and 1.5 U of Taq DNA polymerase (Roche, Mannheim, Germany). The amplification procedure included 5 min of initial denaturation at 95 °C followed by 35 cycles at 94 °C for 30 sec, an annealing step at 55 °C for 30 sec, an extension step at 72 °C for 1 min and a final extension step at 72 °C for 10 min, following the last cycle. The amplified products were electrophoretically resolved on a 2% agarose gel (V3121, Promega, Madison, WI, USA) and stained with ethidium bromide to visualize the amplified PCR products under UV illumination.
Results
Biological samples
From 77 samples analyzed, 29/77(37.66%) corresponded to females and 48/77(62.33%) to males. Only five patients (6.4%) had diarrhea (Table 1).
Coproparasitoscopic method and staining
Of the total 77 children, 16 (20.77%) presented Microsporidium and only one presented symptoms of diarrhea (Table 1). In adition to these results, 2/77 (2.59%) presented Giardia intestinalis , 1/77 (1.29%) had Isospora belli , 1/77 (1.29%) presented Uncinaria and 8/77 (10.38%) were infected with Cryptosporidium spp. This parasite was widely associated with Microsporidium spp. There was no relationship between microsporidial infection and diarrhea. Staining with different methods is observed in Figures 1 A-C. We can observe the spore and noted morphological differences by light microscopy.
PCR amplification
PCR products are presented in Figure 2 where we can observe a 300-bp amplicon corresponding to Microsporidium spp.
Discussion
The presence of Microsporidium species in biological samples of immunosuppressed patients and specifically in children undergoing chemotherapy treatment for ALL has not been investigated in Mexico and its magnitude is undervalued. Worldwide, reported data are limited and incomplete.16
The efficiency of Microsporidium diagnosis by stool analysis depends on the methodology and experience of the analyst. The main difficulty for identification is the small size of the spore (~1-3 μm), which requires the use of oil immersion and staining methods to highlight the morphology. Diagnostic efficiency is increased if three serial stool samples are analyzed, incorporating staining techniques to facilitate identification of the spores and to avoid false negatives of these parasites. In this study we performed the FAUST coproparasitoscopic analysis to identify the parasites. To improve the results, the Ziehl-Neelsen, trichrome and calcuofluor methods were used to stain the slides. This in fact increased the number of positive cases and allowed us to appreciate morphological differences (Figure 1). Finally, molecular analysis allowed us to establish the specie, which cannot be determined by light microscopy (Figure 2).
The frequency of Microsporidium spp was 20.77% in children with ALL and the highest number of cases was observed in the fourth stage, with five positive cases compared with three and four in early stages. This suggests that, at the beginning of the disease, the immunology of the patient is more effective than during later stages due to the fact that chemotherapy received weakens their immune system. In most cases the infection goes unnoticed because children do not present symptomatology.
Microsporidium spp was frequently associated with Cryptosporidium and results show that diarrhea is not a symptom involved in this parasite infection. Diarrhea is a common gastrointestinal symptom in infected subjects and is cause for a wide range of etiologies related to other parasites, bacteria, viruses, toxins and drug administration.
Although Microsporidium was long considered a nonpathogenic organism, currently it has obtained great importance as being causative of emergent infection. It has been associated with HIV-infected and immunosuppressed patients but there are also reports about the presence of this parasite in immunocompetent patients. Because the infection is mainly asymptomatic, it facilitates constant human-to-human transmission and even transmission from animals to humans.
In conclusion, we have shown the importance of determining Microsporidium spp in patients undergoing chemotherapy to treat ALL because this parasite can cause serious complications and interfere with disease control, which decreases the quality of life of children. It is also important to highlight the importance of investigating efficient methodologies for safe and reliable diagnosis.
REFERENCIAS
1. Ghosh K, Cappiello CD, McBrided SM, Occi JL, Cali A, Takvorian PM, et al. Functional characterization of a putative aquaporin from Encephalitozoon cuniculi , a microsporidia pathogenic to humans. Int J Parasitol 2006;36:57-62. [ Links ]
2. Weiss LM. Microsporidia: emerging pathogenic protists. Acta Trop 2001;78:89-102. [ Links ]
3. Didier ES, Didier PJ, Snowden KF, Shadduck JA. Microsporidiosis in mammals. Microbes Infect 2000;2:709-720. [ Links ]
4. Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clin Microbiol Rev 1994;7:426-461. [ Links ]
5. Levkutová M, Hípiková V, Faitelzon S, Benath G, Paulík S, Levkut M. Prevalence of antibodies to Encephalitozoon cuniculi in horses in Israel. Ann Agric Environ Med 2004;11:265-267. [ Links ]
6. Cali A. General microsporidian features and recent findings on AIDS isolates. J Protozool 1991;38:625-630. [ Links ]
7. Franzen C, Müller A, Hartmann P, Salzberger B. Quantitation of microsporidia in cultured cells by flow cytometry. Cytometry A 2004;60:107-114. [ Links ]
8. Franzen C, Salzberger B. Analysis of the β-tubulin gene from Vittaforma corneae suggests benzimidazole resistance. Antimicrob Agents Chemother 2008;52:790-793. [ Links ]
9. Wolk DM, Johnson CH, Rice EW, Marshall MM, Grahn KF, Plummer CB, Sterling CR. A spore counting method and cell culture model for chlorine disinfection studies of Encephalitozoon syn. Septata intestinalis . Appl Environ Microbiol 2000;66:1266-1273. [ Links ]
10. Rezk H, el-Shazly AM, Soliman M, el-Nemr HI, Nagaty IM, Fouad MA. Coccidiosis among immuno-competent and -compromised adults. J Egypt Soc Parasitol 2001;31:823-834. [ Links ]
11. Karaman U, Atambay M, Daldal N, Colak C. The prevalence of Microsporidium among patients given a diagnosis of cancer. Turkiye Parazitol Derg 2008;32:109-112. [ Links ]
12. Faust EC, D'Antoni JS, Odom V, Miller MJ, Peres C, Sawitz W, et al. A critical study of clinical laboratory techniques for the diagnosis of protozoan cysts and helminth eggs in feces. Am J Trop Med Hyg 1938;18:169-183. [ Links ]
13. Botero D, Restrepo M. Parasitosis humanas. Medellín, Colombia: Corporación para Investigaciones Biológicas; 2007. p. 473. [ Links ]
14. Becerril MA. Parasitología Médica. México, D.F., McGraw Hill; 2011. p 320-326. [ Links ]
15. Chabchoub N, Abdelmalek R, Mellouli F, Kanoun F, Thellier M, Bouratbine A, et al. Genetic identification of intestinal microsporidia species in immunocompromised patients in Tunisia. Am J Trop Med Hyg 2009;80:24-27. [ Links ]
16. Botero JH, Castaño A, Montoya MN, Ocampo NE, Hurtado MI, Lopera MM. A preliminary study of the prevalence of intestinal parasites in immunocompromised patients with and without gastrointestinal manifestations. Rev Inst Med Trop Sao Paulo 2003;45:197-200. [ Links ]














