Case presentation
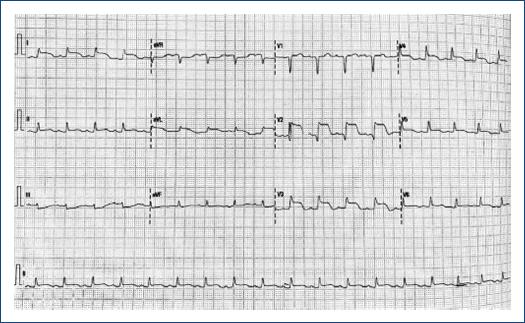
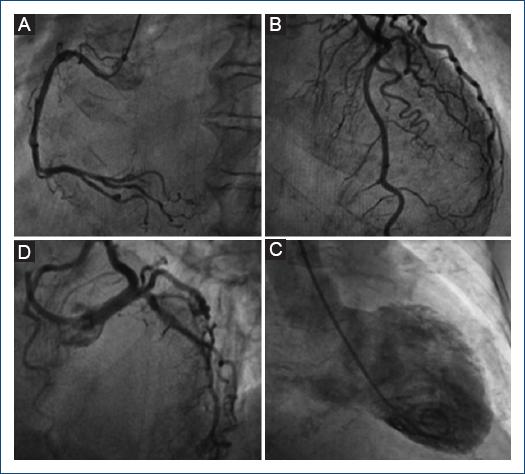
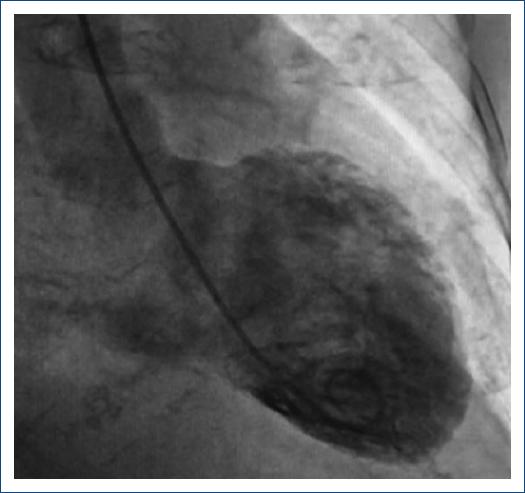
A 75-year-old female with a lymphoma record, diabetes mellitus 2, and hypertension, was hospitalized in another institution with a diagnosis of urosepsis; she required vasopressor treatment and a 3rd generation cephalosporin 2 weeks ago with a partial clinical improvement; but two weeks later she returned to the same hospital with dyspnea and peripheral edema. At the physical examination highlights general rales and peripheral edema, an electrocardiogram (ECG) showed sinus rhythm with ST-segment elevation in V2-V6, DI, and aVL. The troponin I level was 620 ng per liter on a high-sensitivity assay, and she was referred to our institute. Upon her arrival to the emergency department at our insistution, she had a Glasgow score of 10 points, respiratory distress and medium blood pressure <65 mmHg. The ECG persists with a ST-segment elevation in V2-V6, DI, and aVL (Fig. 1). The patient was intubated and required invasive mechanical ventilation, we started anti-ischemic treatment and vasopressor. In the context of ST-segment elevation myocardial infarction and cardiogenic shock, she was transferred to the cardiac catheterization laboratory, where it was reported the absence of injuries in the right coronary, circumflex, and anterior descending arteries (Fig. 2). With anterior and inferior akinesia, and apical dyskinesia, suggestive of Takotsubo cardiomyopathy (Fig. 3). After that, she was transferred to the coronary care unit where the diagnosis of cardiogenic shock was integrated. We iniated treatment with an inodilator (levosimendan) and inserted an intra-aortic balloon pump (IABP) as a ventricular-assistant device. A transthoracic echocardiogram was performed, reporting basal hypokinesia, apical akinesia. LVEF 12%, without a dynamic obstruction of the left ventricular outflow tract and a lung ultrasound with a B-profile. Urine and blood culture were negative; she presented a clinical improvement, so the vasopressor and inodilator were retired and started deflating the IABP. We performed another echocardiogram obtaining a LVEF 27%. As she presented clinical improvement as the days went by, we removed the ventricular assistant device. Unfortunately, in a sudden way, the patient started with ventricular tachycardia requiring pharmacological, electrical defibrillation, and resuscitating maneuvers without a response.
Discussion
The patient had three of four criteria according to the Mayo Clinic diagnostic criteria for takotsubo syndrome and 38 points of InterTAK diagnostic score. Cardiogenic shock as a presentation of takotsubo syndrome is not common, in this case, female gender and a decreased LVEF are well-known risk factors associated with takotsubo syndrome and it's complications. In addition, diabetes mellitus presents only in 12% of patients and is associated as a protective factor due to slow release of catecholamines1. Some prospective studies enhance that 50% of patients developed complications but only 2-3% died during the acute phase; being our case report part of this statistic2. In this context, patients that receive cardiac mechanical support (IABP, Impella, or extracorporeal membrane oxygenation [ECMO]) have a lower in-hospital mortality rate (12.8%) than those without cardiac mechanical support (28.3%), making this intervention a key factor in the evolution of the patients3. It is important to mention that IABP is the predominantly used mechanical support device all over the world. However, it is also established and supported by the Heart Failure Association of the European Society of Cardiology that in case of having the source and according to the clinical evolution of the patient progression of mechanical support to Impella or V-A ECMO is indicated to avoid refractory cardiogenic shock4,5. Early implantation of mechanical devices should be considered as a bridge to recovery therapy to reduce the high mortality rate during the acute phase6. Identifying more predictor data of shock valuable for an appropriate algorithm of treatment strategies are imperative6,7. According to the results of the international registry of takotsubo syndrome, identifying variables such as apical takotsubo syndrome, physical stress, lower LVEF, and atrial fibrillation should be components to include in a primary risk stratification model3.











 nueva página del texto (beta)
nueva página del texto (beta)