Introduction
Venous thromboembolism (VTE) includes both, deep vein thrombosis (DVT) and pulmonary embolism (PE); the latter represents a common cause of inpatient and outpatient morbidity and mortality1. The annual incidence of VTE has been estimated from 0.1 to 0.27%, affecting up to 5% of individuals of the general population at least 1 time in their life2. This entity is the result of a combination of hereditary factors as thrombophilias and acquired risk factors as hypercoagulable states that can lead to the most feared complication PE3. In this respect, it has been reported that PE is the third most common cause of hospital-related death and one of the most common preventable causes1,3; approximately 20% of the affected patients die at the time of diagnosis and 11% within 3-month period2.
Although anticoagulation remains the primary management for VTE, inferior vena cava filters (IVCFs) constitute an important alternative of mechanical prophylaxis4. The use of IVCF has increased markedly in recent years5 and, since the first device was approved in 1972, by 2012, approximately a total of 250,000 devices had been implanted6. The insertion of this mechanical prophylactic modality has demonstrated to be safe; however, there are complications that may occur during the implantation and retrieval or when the filter is retained for a long time7-10. Although there are reasons to believe that IVCFs provide benefit for patients who cannot be initiated on anticoagulation therapy and can be lifesaving in patients at high risk of PE recurrence11,12, there are few clinical studies that have demonstrated a significant advantage of filter placement in the setting of VTE in addition to the continuation of anticoagulation13.
At the National Institute of Medical Sciences and Nutrition Salvador Zubiran in Mexico City, we evaluated retrospectively patients with a history of DVT and/or PE that underwent IVCF placement. To determine the value of anticoagulation, we examined the outcomes for patients with IVCF who subsequently were initiated on therapeutic levels of anticoagulation after filter placement and compared them with those patients with IVCFs that were not placed on anticoagulation.
Methods
This was a retrospective review of patients that underwent insertion of IVCF. For the purpose of our analysis, the patients were divided and studied in two groups, those that were initiated on anticoagulation as soon as safety allowed this therapy and were compliant with the management (A) and those without anticoagulation (NA). Patients on anticoagulation were closely followed in our clinic and maintained with an international normalized ratio within a therapeutic range of 2-3. Variables such as indications for filter placement, demographics, comorbidities, recurrence of thrombotic events, optimal anticoagulation therapy, development of device-related complications, post-thrombotic syndrome (PTS), and reinterventions were examined.
Study setting
Academic and Research Medical Center which is a tertiary referral facility serving a catchment area of 23 million people. Institutional review board approved this study.
Statistical analysis
Descriptive statistics were conducted; categorical data were analyzed with Cox regression test to find association of variables, Fisher exact test or likelihood ratio χ2 was used with dichotomic outcomes. All tests were performed using the statistical program STATA version 14.1.
Results
From April 2007 to March 2014, a total of 54 patients with IVCF met our inclusion criteria; 33 were female (61%), with a mean age of 54 years (range 20-85, standard deviation 19). Table 1 summarizes the patients demographics, comorbidities, and the statistical analysis of the comparison groups. Indications for filter placement were as follows: patients with confirmed diagnosis of DVT and contraindication for anticoagulation in 24 patients (44%), PE in 18 (33%), 7 individuals (13%) underwent IVCF in anticipation for a surgical procedure that placed them at high risk for thrombotic events, and prophylactic anticoagulation was contraindicated. 5 (9%) patients secondary to repeat thrombotic episodes despite optimal anticoagulation (Table 2). From these, 28 (52%) were initiated on anticoagulation therapy when was safe, 13 (24%) patients were placed on lifelong therapy, 9 (17%) patients were placed from 1 to 6 months period, 5 (9%) from 6 to 12 months, and 1 patient for 1 year or longer. 26 (48%) were not placed on anticoagulation (NA group) (Table 3). During a mean follow-up period of 28 months (standard error ±5), five patients experienced recurrent thrombotic events, 3 (60%) of them were on the A group, and 2 (40%) in the NA cohort (p=0.5); a comparative analysis in patients that developed PTS showed that seven patients were in the A group and seven in the NA, without statistical significance for this variable: three patients developed venous ulcers, two were in the A, and one in the NA group (p=0.5). Only three of them underwent filter removal, the period of time for IVCF removal ranged from 1 to 214 months, with a median of 24 months; in this series, the reasons for not retrieving the device were permanent filters insertion in 15 patients (28%) and 1 (2%) could not be removed due the technical failure. The rest of patients (35) did not return to clinic or refused removal of the device.
Table 1 Demographics and comorbidities of patients with and without anticoagulation therapy
| Variable | A group (%) | NA group (%) | Total | p |
|---|---|---|---|---|
| Gender | ||||
| Female | 9 (32) | 12 (46) | 21 (36) | NS |
| Male | 19 (68) | 14 (54) | 33 (64) | NS |
| Age (years) | 52 | 56 | ||
| Comorbidities | ||||
| Autoimmune disease | ||||
| SLE | 9 (32) | 4 (15) | 13 (24) | 0.2 |
| APS | 8 (29) | 1 (4) | 9 (17) | 0.02* |
| Wegener | 2 (6) | 0 (0) | 2 (4) | 0.4 |
| Scleroderma | 0 (0) | 2 (8) | 2 (4) | 0.2 |
| Protein C deficiency | 1 (4) | 0 (0) | 1 (2) | NS |
| Malignancies | 8 (29) | 17 (65) | 25 (46) | 0.01* |
| Total of patients | 28 | 26 | 54 (100) | |
*Statistically significant. Statistical analysis performed with Fishers exact test. A: anticoagulation; NA: without anticoagulation; APS: antiphospholipid syndrome; SLE: systemic lupus erythematosus; NS: non-significant.
Table 2 Indications for inferior vena cava filter placement in the 54 patients
| Indication | n (%) |
|---|---|
| Contraindication for anticoagulation | 24 (44) |
| Pulmonary embolism | 18 (33) |
| Anticipation for surgical procedure | 7 (13) |
| Recurrent thrombotic event | 5 (9) |
| Total (patients) | 54 (100) |
Table 3 Distribution of the duration of anticoagulation therapy following inferior vena cava filters placement
| Duration of anticoagulation | n (%) |
|---|---|
| 0-6 months | 9 (17) |
| 6-12 months | 5 (9) |
| >1 years | 1 (2) |
| Lifelong anticoagulation | 13 (24) |
| No anticoagulation | 26 (48) |
| Total (patients) | 54 (100) |
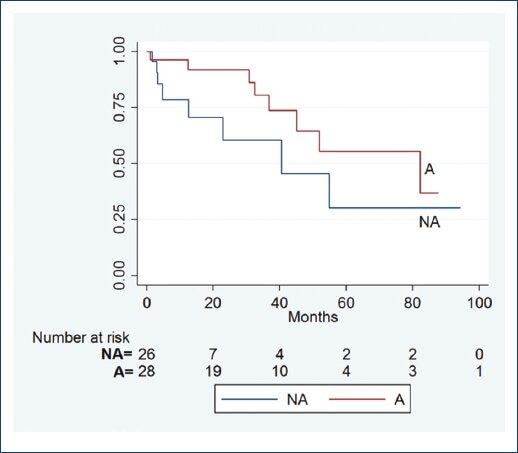
The filter that was used with more frequency was the OptEase (Cordis, Piscataway, NJ, USA) in 26 (48%) cases, followed by Greenfield (Medi-tech/Boston Scientific, MA, USA) in 19 (35%) and the TrapEase (Cordis, Miami Lakes, FL) in 3 patients (5%), and finally, 2 individuals (4%) for each of the following devices: VenaTech (B. Brain, Bethlehem, PA), Simon-Nitinol (C.R. Bard Inc., Covington, GA), and Gianturco-Roehm Birds Nest (Cook Medical, Bloomington, Indiana), respectively. With respect to patients comorbidities associated with hypercoagulable states, 13 (24%) patients had a history of systemic lupus erythematosus, 9 (17%) antiphospholipid syndrome, 2 (4%) with scleroderma and Wegener, respectively, 1 (2%) protein C deficiency, and 25 (46%) suffered from some type of malignancy. 11 (20%) patients died during the follow-up period and 4 (36%) of them were in the A group. With respect device-related complications and reinterventions, 2 (4%) patients that experienced IVC rupture/perforation requiring surgical treatment were in the A group (p=0.5) and the only case of IVCF migration occurred in the A group (p=1) (Table 4). From the 28 patients maintained on anticoagulation, 23 (82%) were on Vitamin K antagonists, 4 (14%) placed on new oral anticoagulants, and 1 (3%) patient anticoagulated with low-molecular-weight heparin (LMWH). Our analysis of KaplanMeier showed no difference in the number of complications and survival in patients with or NA (Fig. 1).
Table 4 Number of device-related complications and number of patients affected by post-thrombotic syndrome
| Complications | A = 28 patients (%) | NA = 26 patients (%) | p |
|---|---|---|---|
| Device related | |||
| IVC rupture or perforation | 2 (7) | 1 (4) | 0.5 |
| Filter migration | 1 (4) | 0 | NS |
| Other | |||
| Recurrent thrombosis | 3 (11) | 2 (8) | 0.5 |
| Post-thrombotic syndrome | 7 (25) | 7 (27) | NS |
Statistical analysis performed by Fishers exact test. IVC: inferior vena cava; A: anticoagulation; NA: without anticoagulation.
Discussion
There are well-recognized groups of patients who are considered to be candidates for IVCF placement: the failed group with individuals who have experienced recurrent VTE despite optimal coagulation therapy, another group is composed of those who have a history VTE, but who also have contraindication for anticoagulation such high risk for hemorrhagic complications or recent hemorrhage4,14. A third group is represented of those patients who have not sustained VTE, but in whom a filter is inserted prophylactically in relation with a surgical procedure or event that is associated with high incidence of thrombosis such as trauma from different causes15. Literature has reported important evidence that indicates that IVCFs effectively reduce the incidence of PE; however, as many as 76% of the study patients received concurrent anticoagulation therapy16. Conversely, the use of IVCF has been associated to adverse events and the frequency of which may increase overtime as they do not have prophylactic effect on the occurrence of lower extremity DVT and in situ IVCs thrombosis, and they may favor the development of PTS17. In addition, the rates of IVCF thrombosis and recurrent DVT are highly variable, and the long-term efficacy of many IVCFs remains unknown16. In the PREPIC study, initially published in 1998 included 400 patients with DVT, the insertion of IVCF in combination with standard anticoagulation was associated with reduction in the occurrence of PE alone, but filters showed no impact in mortality18. In 8-year follow-up, after the insertion of filter in patients with proximal DVT with or without PE19, the latter entity was reduced significantly, although not eliminated compared with patients without filters. However, these benefits were offset by an increase occurrence of DVT the in lower limbs; interestingly, in this study, IVCFs did not increase the risk of PTS. In our study, we observed that 5 (9%) of our patients had recurrent thrombotic events in contrast with the 38% reported in literature, where the main risk factor was history of malignancy20.
In 2008, Ray and Prochazka published a meta-analysis; in this study, the authors observed a trend toward decreased VTE rates in patients with post-filter anticoagulation (12.3% vs. 15.8%), but the analysis failed to reach statistical significance21. In 2015, update of the PREPIC study group was confirmed that the use of retrievable IVCF in patients who can receive anticoagulation was not superior to anticoagulation alone22. In 2017, Yunes and Aizman completed a meta-analysis that included three systematic reviews and four clinical trials, the authors concluded that might not exist a difference in the occurrence of DVT adding an IVFC in patients on anticoagulation, and they could not find difference in regard to the occurrence of PE and mortality due to the low level of evidence available23, as we found in our study.
With respect the type of anticoagulation, Decousus et al. classified the patients in two groups: one with the unfractionated heparin and the second group with LMWH; the authors observed similar efficacy in both, without significant difference regarding the number of recurrent thrombotic events, hemorrhagic complications, or mortality18. Iwamoto et al. followed IVCF patients ranging from 1 to 9 years, during this study period, the author demonstrated that underlying diseases and the presence of cardiac thrombus were significant factors for the prognosis of patients with DVT who underwent IVCF with anticoagulation therapy24. In 2010, Hadjuk et al. published a prospective study that included 121 patients; the authors concluded that patients who have received IVCF after thromboembolic episodes and receive anticoagulation when it is not contraindicated and undergoes appropriate management have an acceptable prognosis if it not otherwise limited by cancer or VTE-unrelated terminal cardiopulmonary disease12. In our series, anticoagulation therapy did not have an impact in survival and mortality was associated to malignancies. In 2014, Akl et al.25 published a comprehensive search for studies of anticoagulation in patients with cancer; this study included an electronic search of the Cochrane Central Register of Controlled Trials. The authors concluded that for long-term treatment of VTE in cancer patients, LMWH compared with Vitamin K antagonist reduces venous thromboembolic events but not mortality. In addition, the authors emphasized that the decision for a patient with cancer and history VTE to start long-term LMWH versus oral anticoagulation should balance the benefits and harms and integrate the patients values and preferences for the important outcomes and alternative management. In 2016, Kang et al. published a retrospective study, including 180 patients with cancer-associated PE, with 143 of them receiving and a total of 37 not receiving post-IVCF anticoagulation treatment, this study showed no difference in mortality in both groups26. In our study, 8 of 25 patients with malignancies were initiated on anticoagulation, during the follow-up period, there were two recurrent thrombotic events, these two patients were on Vitamin K antagonist and new oral anticoagulants, respectively. Due to the retrospective nature of our study, this variable was not in our control, but this supports the need of appropriate drug selection in this group of patients. Other important issues are the complications related directly with the device itself; there are numerous reports of IVCF dislodgement, vessel perforation or rupture9, and even device migration to the right ventricle as the report by Peters et al.27
Although removal can be performed by endovascular means, in cases of perforation or migration, open surgery is sometimes necessary with a significant morbidity in this frail population28. In a retrospective study that included 265 IVCF patients that underwent computed tomography, 39% of them had IVCF penetration in the vessel wall and 13.2% to surrounding organs as the duodenum, aorta, vertebral bodies, muscles, pancreas, liver, diaphragm, and suprarenal glands29. Complications that occurred in our study included IVC rupture in 2 (4%) patients, 1 (2%) case of device migration, 5 (9%) experienced recurrent thrombotic events, and 26% (14 patients) of the population developed PTS. In the MAUDE database, the adverse effects of IVCF were reported; the BARD filters had complications in 27% of the cases, wall penetration occurred in 30% with Celect IVCF. Failure during device deployment and placement occurred with OptEase in 30% and 45% of the cases with the Günter Tulip, the most common complication in the registry was device malfunction in 47% of patients30. These adverse effects could be related to the design of the devices and operator skills and experience.
We recognized limitations in our study, including the retrospective nature, non-randomized studied subjects, the relatively small number, and the differences of the comorbidities of studied groups.
Conclusions
Our initial observations suggest that in patients with IVCF in whom an optimal and structured anticoagulation therapy regime had equivalent rates of thrombotic events, device-related complications as vessel rupture, perforation, and filter migration than in those patients NA. In addition, anticoagulation therapy showed no impact in long-term survival. Further research in this area is warranted.











 nueva página del texto (beta)
nueva página del texto (beta)