Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Archivos de cardiología de México
versión On-line ISSN 1665-1731versión impresa ISSN 1405-9940
Arch. Cardiol. Méx. vol.78 no.4 Ciudad de México oct./dic. 2008
Comunicaciones breves
Left main coronary artery stenosis treatment with two paclitaxel–eluting stents in a patient with cardiac allograft vasculopathy
Tratamiento de una estenosis del tronco de la coronaria izquierda con dos stents liberadores de paclitaxel en un paciente con vasculopatía coronaria postrasplante
Marco A Martínez–Ríos,* Arturo Méndez–Ortíz,** Jorge Gaspar,* Rodolfo Barragán–García,*** Guillermo Fernández–de–la–Reguera,**** Carlos J González–Quesada*
* From the Interventional Cardiology Department.
** Clinical Cardiology Department.
*** Cardiothoracic Surgery Department.
**** Postoperative Intensive Care Unit.
Instituto Nacional de Cardiología Ignacio Chávez (Mexico City)
Correspondence:
Marco A Martínez–Ríos MD, FACC, FSCAI.
Instituto Nacional de Cardiología Ignacio Chávez.
(INCICH, Juan Badiano Núm. 1, Col. Sección XVI,
Tlalpan 14080. México D.F.).
Tel: 55–73–29–11 ext 1131
E–mail: mtzrios@cardiologia.org.mx.
Recibido: 4 de marzo de 2008
Aceptado: 13 de mayo de 2008
Abstract
Cardiac transplantation is a well defined therapy for end stage heart failure. After the first year of transplantation, allograft coronary artery disease (ACAD) is the second main cause of death. The ACAD is defined as a diffuse process affecting the entire length of epicardial vessels. Once ACAD has been established, treatments such as coronary angioplasty, coronary stenting, and coronary bypass are performed. We present a case of successful stenting of the left main coronary artery (LMCA) in a patient with ACAD. The patient's medical history was significant for heart transplantation due to ischemic heart failure. Four years aftertransplantation the patient was admitted again due to sudden worsening of New York Heart Association functional class and extreme fatigue. Coronary angiogram showed a severe stenosis in the proximal segment of the LMCA; we performed stenting with a paclitaxel–eluting stent (PES). Six months after the procedure, the patient had an elective angiogram, where we discovered a new severe occlusion distally to the former stent; a second PES was implanted. Fourteen months after the second stenting, a new elective angiogram was performed without evidence of in–stent restenosis. After a 8–year follow–up since transplantation, the patient is free from dyspnea, angina, and adverse cardiovascular events. Our report suggests the efficacy of PES as ACAD treatment of the unprotected LMCA.
Key words: Paclitaxel. Trunk. Vasculopathy. Transplantation.
Resumen
El trasplante cardíaco es uno de los principales tratamientos para la insuficiencia cardíaca avanzada. Una de las principales complicaciones de este procedimiento es la vasculopatía postrasplante (VCPT), cuyas opciones terapéuticas son la angioplastía y/o implantación de stent, y la cirugía de revascularización. En este reporte presentamos el caso de un paciente con VCPT en el tronco de la coronaria izquierda (TCI) tratado con implantación de stent. El motivo del transplante cardíaco fue insuficiencia cardíaca avanzada de etiología isquémica. Cuatro años posteriores al trasplante, el paciente fue reingresado debido a empeoramiento de su clase funcional y fatiga extrema. La angiografía coronaria mostró una estenosis severa en el segmento proximal del TCI, por lo que procedimos a implantar un stent liberador de paclitaxel (SLP). Seis meses después, se realizó una angiografía de control demostrándose una nueva oclusión severa inmediatamente distal al primer stent implantado, por lo que se decidió colocar un segundo SLP. Catorce meses después del segundo intervencionismo se realizó una nueva angiografía selectiva, la cual se reportó sin evidencia de reestenosis intrastent. Después de 7 años de seguimiento, el paciente se reporta sin disnea, angina o eventos cardiovasculares adversos. Nuestro reporte sugiere que la utilización del SLP puede ser efectiva en el tratamiento de la VCPT en el TCI no protegido.
Palabras clave: Paclitaxel. Tronco. Vasculopatía. Trasplante.
Introduction
Cardiac transplantation is a well defined therapy for end stage heart failure. After transplantation, median survival is from 9.6 to 12 years.1 The main causes of death in the first year after transplantations are infection, rejection, and graft failure. After the first year of transplantation, allograft coronary artery disease (ACAD) is the second main cause of death, just after malignancy.2 In a registry from the International Society of Heart and Lung Transplantation (ISHLT), ACAD is reported in only 2.4% of survivors at 1 year but in 8.9% at 5 years (A).1
The ACAD is defined as a diffuse process affecting the entire length of epicardial vessels, including intramyocardial branches. Histological studies reveal a lesion characterized by concentric intimal proliferation, with general sparing of the elastic lamina.3 This process is different from classical coronary artery disease (CAD), which tends to be focal and eccentric with marked involvement of the elastic lamina. In grafts with longer survival, there may be both focal and atheromatous disease as well as diffuse concentric intimal thickening.4
When ACAD has been established, treatments such as coronary angioplasty, coronary stenting, and coronary bypass offer only symptomatic relief and palliative care. The only effective treatment is to repeat transplantation, which is a main subject of ethical and moral discussion, especially with the shortage of donors.5
Percutaneous coronary intervention (PCI) of the unprotected left main coronary artery (LMCA) is currently not recommended as a routine procedure based on the history of inferior outcomes of left main (LM) percutaneous transluminal coronary angioplasty and bare metal stenting.6 Instead, surgical revascularization (coronary artery bypass grafting [CABG]) is considered to be the gold standard. There is renewed interest in LM–PCI because improved outcomes of PCI utilizing drug eluting stents (DES) in multiple randomized trials. Several single–center non–randomized registries have evaluated the role of DES for LMCA–PCI. Data suggest a low mortality and target vessel failure of ostial LM or mid–shaft lesions in contrast to bifurcation lesions.
Case report
A 49 year–old man was admitted because of sudden worsening of New York Heart Association (NYHA) functional class and extreme fatigue. The patient's medical history was significant for orthotopic heart transplantation four years earlier (September 2000) as treatment for ischemic heart failure due to massive myocardial infarction, DDD pacemaker implantation (August 2002) due to intermittent sinusal pauses and bradycardia, as well as hypertriglyceridemia and smoking. The patient did not have any history of severe graft rejection, angina or evidence of myocardial ischemia in the routine postransplant studies (99mTc–MIBI stress–rest myocardial perfusión scintigraphy, exercise stress test, radio–nuclide ventriculography, and echocardiography). Home medications were tacrolimus, mycophenolate mofetil, prednisone, pravastatin, amlodipine and isosorbide mononitrate. Complete blood count and a metabolic panel, including normal serial creatinine concentrations, were within normal limits. Low density lipoprotein ranged between 83.8 mg/dL, high density lipoprotein between 50 mg/dL, and triglyceride concentrations between 216 mg/dL over the preceding two years.
Once admitted, an echocardiogram showed a significant deteriorated systolic function (the left ventricular ejection fraction was 45%). Coronary angiogram showed only a significant (70%) focal coronary occlusion in the proximal segment of the left main stem. During the procedure, an endomyocardial biopsy was performed, which demonstrated a grade III graft rejection of the ISLHT (International Society for Heart & Lung Transplantation) classification. An intra–aortic balloon counterpulsation was implanted and treatment was initiated with basiliximab, and the dose of tacrolimus, mycophenolate mofetil and prednisone were adjusted. After three weeks of treatment another endomyocardial biopsy showed absence of rejection.
Finally, an angioplasty was attempted in the LMCA. Clopidogrel (300 mg) was administrated in the catheterization laboratory. The left main coronary artery was cannulated with a 8–French left guide catheter with side holes and the lesion was crossed with a 0.014–inch Hi–Torque Floppy coronary guidewire. The lesion was dilated twice with 3.0 x 20 mm Maverick balloon at 18 atm during four seconds. A 3.5 x 8 mm Taxus stent was deployed in the left main stem at 18 atm during thirteen seconds with good results. There was no residual stenosis of evidence of complications. The patient left the hospital the following day. One year of treatment with clopidogrel was recommended. On clinical follow–up after the angioplasty an echocardiogram showed improvement of the left ventricular ejection fraction and the patient reported a marked enhancement in his ability to perform sustained exercise without suffering from shortness of breath (NYHA functional class I) and angina (Fig. 1).
Six months after the interventional procedure, the patient underwent an elective follow–up angiogram. It showed a new 70% focal occlusion immediately distal to the former stent placed, in the distal segment of the LMCA. Similar technique as described in the first angioplasty was performed but in this case a 3.5 x 12 mm Taxus stent was placed covering the distal LMCA and proximal left anterior descendent coronary artery (Fig. 2).
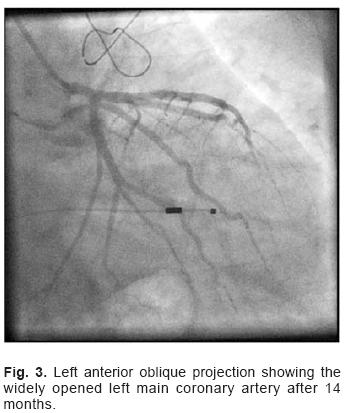
Fourteen months after the second stenting a new elective angiogram was performed. It showed patent stents in the LMCA, without evidence of other significant occlusions (Fig. 3). After a 7–year follow–up since transplantation, the patient is free from dyspnea, angina and major adverse cardiovascular events and presents a conserved left ventricular ejection fraction.
Discussion
ACAD represents a main obstacle to long–term survival after heart transplantation. There is a linear and steady risk of developing ACAD detected on angiograms after the first year from transplantation, so that by the fifth year, more than 50% of transplanted recipients will present some disease.7 Ahead of five years, the risk of presenting angiographically noticeable severe ACAD increases proportionally, illustrated by the fact that nine years after transplantation 29% of patients will have detectable disease.8 About 25% of all deaths between the first and tenth year after transplantation will be due to ACAD.7 Once three–vessel or severe ACAD is developed, the risk of death or retransplantation is extremely high within one year after diagnosis.9
The diagnosis of ACAD is challenging. Traditional symptoms of angina are often absent and patients have a tendency to present with heart failure, just as we show in the present report. Otherwise, ACAD is mainly diagnosed on angiography or intravascular ultrasound (IVUS), if routinely performed; as the former usually un–derdiagnose ACAD, a classification system was created by Stanford University that distinguishes ACAD going from typical atherosclesis to concentric arterial occlusion.10 Angiograms should be interpreted consecutively as new concentric lesions may be missed on one–time angiograms analysis. The IVUS, however, has the benefit of picturing the entire arterial wall and is more sensitive and specific. The Stanford group also proposed a classification system.11 A study by Kapadia et al established that all three–vessels imaging with IVUS revealed up to two times the amount of ACA at 1 year (58% versus 27%) and this trend persisted for three years.12
Before transplantation the medical team should focus on preventing endothelial injury and brain death, reducing cold ischemic time, and improving myocardial preservation during storage and transportation.13 Following transplantation, the focus should be on prevention of ACAD by attenuation of adverse nonimmunological and immunological reaction. Modification of traditional risk factors such as diabetes, dyslipidemia, hypertension, and smoking is a key factor in the prevention of endothelial dysfunction and thus development of ACAD.14
Certain therapies for ACAD have been shown to slow the progression of the disease, and therefore they tend to decrease mortality. Drugs such as 3–hydroxy–3–methylglutaryl coenzyme A (HMG–CoA) reductase inhibitors and calcium–channel blockers have some protective effect, but they do not eliminate entirely the problem.3 Newer immunosuppressants such as sirolimus and everolimus can be useful.15 The CABG has been associated with a high peri–operative mortality of up to 30%.16 The ineffective medical treatment and the limited suitability of CABG and retransplantation have motivated interest in PCI for the subset of heart transplant recipients presenting with allograft vasculopathy in proximal segments.
There is some evidence that supports PCI as a therapy in ACAD. In 1995 Halle et al,16 reported a 55% restenosis incidence at 8 ± 5 months after angioplasty and angiographic distal arteriopathy adversely affected allograft survival. In 1998 Musci et al17 studied 46 patients who underwent 76 angioplasties at a mean of 50 ± 30 months; they reported 96% primary success rate and angiographic restenosis occurred in 42% with no procedure–related death. In 2000 Schnetzler et al18 reported their experience in 29 transplanted patients with 53 coronary sites dilated; they described a primary success rate of 94.3% with a 32.5% stenosis rate at 1.27 ±1.2 years, and they did not found any factor predictive for restenosis in this population; although they informed about 2 patients with a LMCA stenosis who were treated with PCI, one of them had restenosis and underwent to CABG.
ACAD is one of the most insidious complications limiting long–term survival of transplanted patients. The option of using stents eluting different drugs might help in the treatment of this feared condition. Most reports suggest a low major adverse cardiac events rate for PCI in ostial or mid–shaft LMCA disease, while data are more heterogeneous for PCI in distal LM disease involving the bifurcation.19,20 Stent implantation is technically viable and is related to an optimal early outcome. The real problem is the unknown risk of restenosis in these patients with often ominous clinical presentation. Routine follow up angiography comes forward to be necessary for the opportune diagnosis of restenosis. To the best of our knowledge, this is the first reported case of successful double paclitaxel stenting for LMCA stenosis in a patient with ACAD. The effect of drug eluting stents on LMCA occlusions needs further investigation, as this treatment modality may be an effective alternative for this very high risk group of patients.
Acknowledgements
The authors thanks to all the staff of the Ignacio Chavez National Institute of Cardiology transplant Committee for their contribution and assistance with the report (Valentín Herrera–Alarcón MD, Humberto Martínez–Hernández MD, Sergio Olvera–Cruz MD, Javier Molina–Méndez MD, Pedro Reyes–Lopez MD, Maria del Carmen Ávila–Casado MD, Carlos Martínez–Sánchez MD, Carlos Zamora–González MD Enrique López–Mora MD, Gustavo Sánchez–Miranda MD, Oscar Fiscal–Lopez MD, Hermes Ilarraza MD, Julio Sandoval MD, AnaLuis Flores –Moya RN, María Vázquez–Suárez RN, Rocío Ramírez (social worker)
References
1. TRULOCK EP, EDWARDS LB, TAYLOR DO, BOUCEK MM, KECK BM, HERTZ MI: International Society for Heart and Lung Transplantation. Registry of the International Society for Heart and Lung Transplantation: twenty–third official adult lung and heart–lung transplantation report – 2006. J Heart Lung Transplant 2006 25: 880–892. [ Links ]
2. International Society of Heart and Lung Transplantation (ISHLT) registry, http://www.ishlt.org/registries. [Accessed May 9th, 2007] [ Links ]
3. AVERY RK: Cardiac–allograft vasculopathy. N Engl J Med 2003; 349: 829–830. [ Links ]
4. BILLINGHAM ME: Histopathology of graft coronary artery disease. J Heart Lung Transplant 1992; 11(3 Pt 2): S38–S44. [ Links ]
5. MINIATI DN, ROBBINS RC: Heart transplantation: a thirty year perspective. Annu Rev Med 2002; 53: 189–205. [ Links ]
6. LENZEN MJ, BOERSMA E. BERTRAND ME, MAIER W, MORIS C, PISCIONE F, ET AL: European Society of Cardiology. Management and outcome of patients with established coronary artery disease: The Euro Heart Survey on coronary revascularization. Eur Heart J 2005; 26: 1169–79. [ Links ]
7. COSTANZO MR, NAFTEL DC, PRITZKER MR, HEILMAN JK 3RD, BOEHMER JP, BROZENA SC, ET AL: Heart transplant coronary artery disease detected by coronary angiography: A multi–institutional study of preoperative donor and recipient risk factors. J Heart Lung Transplant 1992; 17: 744–753. [ Links ]
8. BOEHMER JP, BROWN CV, LEIER CV: Advanced allograft coronary artery disease: Interaction between pre– and post–transplant risk factors from a ten–year multi–institutional study (abstract). J Heart Lung Transplant 2002; 21: 19. [ Links ]
9. KEOGH A, VALANTINE H, HUNG SA, SCHROEDER JS, MCINTOSH N, OVER PE, ET AL: Impact of proximal or mid–vessel discreet coronary artery stenosis on survival after heart transplantation. J Heart Lung Transplant 1992; 11: 892–901. [ Links ]
10. BOFFA G, FAGGIAN G, BUJA G: Coronary artery spasm in heart transplant recipients. J Heart Lung Transplant 1989; 8: 154–158. [ Links ]
11. ST GOAR FG, PINTO FJ, ALDERMAN EL, VALANTINE HA, SCHROEDER JS, GAO SZ, ET AL: Intracoronary ultrasound in cardiac transplant recipients: in vivo evidence of 'angiographically silent' intimal thickening. Circulation 1992; 85: 979–987. [ Links ]
12. KAPADIA SR, ZIADA KM, L'ALLIER PL, CROWE TD, RINCON G, HOBBS RE, ET AL: Intravascular ultrasound imaging after cardiac transplantation: advantage of multivessel imaging. J Heart Lung Transplant 2000; 19: 167–172. [ Links ]
13. RAMZY D, RAO V, BRAHM J, MIRIUKA S, DELGADO D, ROSS HJ, ET AL: Cardiac allograft vasculopathy: a review. Can J Surg 2005; 48: 319–327. [ Links ]
14. ARANDA JM, HILL J: Cardiac transplant vasculopathy. Chest 2000; 118: 1792–1800. [ Links ]
15. EISEN HJ, TUZCU EM, DOREN R, KOBASHIGAWA J, MANCINI D: Everolimus for the prevention of allograft rejection and vasculopathy in cardiac–transplant recipients. N Engl J Med 2003; 349: 847–58. [ Links ]
16. HALLE AA III, DISCIASCIO G, MASSIN EK, WILSON RF, JOHNSON MR, SULLIVAN HJ, ET AL: Coronary angioplasty, atherectomy and bypass surgery in cardiac transplant recipients. JAMA 1995; 26: 120–8. [ Links ]
17. MUSCI M, LOEBE M, WELLNHOFER E, MEYER R, PASIC M, HUMMEL M, ET AL: Coronary angioplasty, bypass surgery, and retransplantation in cardiac transplant patients with graft coronary disease. Thorac Cardiovasc Surg 1998; 46: 268–74. [ Links ]
18. SCHNETZLER B, DROBINSKI G, DORENT R, CAMPROUX AC, GHOSSOUB J, THOMAS D, ET AL: The role of percutaneous coronary angioplasty in heart transplant recipients. J Heart Lung Transplant 2000; 19:557–65. [ Links ]
19. MORICE MC, SERRUYS PW, SOUSA JE, FAJADET J, BAN HAYASHI E, PERIN M, ET AL; RAVEL Study Group Randomized study with the sirolimus–coated bx velocity balloon–expandable stent in the treatment of patients with de novo native coronary artery lesions: A randomized comparison of a sirolimus–eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346: 1773–80. [ Links ]
20. STONE GW, ELLIS SG, COX DA, HERMILLER J, O'SHAUGHNESSY C, MANN JT, ET AL: TAXUS–IV Investigators: A polymer–based paclitaxel–eluting stent in patients with coronary artery disease. N Engl J Med 1002; 350: 221–31. [ Links ]














