Introduction
The jackfruit (Artocarpus heterophyllus L.) is a tropical exotic fruit from India which has acquired importance in the state of Nayarit for being the main producer of Mexico (SIAP, 2019).
However, crop production is reduced by postharvest diseases. Jackfruit is mainly affected by postharvest soft rot, caused by Rhizopus stolonifer stage (Bautista-Baños, Bosquez-Molina & Barrera-Necha, 2014). The most used methods for its control are chemical fungicides, among which are Dicloran and Fludioxonil. (Singh & Sharma, 2018), which have caused alterations to the environment and residual problems, as well as the generation of resistant strains. Therefore, low toxicity alternatives of biological origin are sought, such as chitosan. This biopolymer that comes from the deacetylation of chitin has become a promising alternative due to its antifungal activity and inducer of defense mechanisms (Gutiérrez- Martínez et al., 2018). Likewise, the efficacy of generally recognized as safe (GRAS) in their application in food has also been evaluated (Palou, Ali, Fallik & Romanazzi, 2016). Among these compounds, immersion of citrus fruits in potassium sorbate solutions has been proven to be effective against P. digitatum and P. italicum (Montesinos-Herrero, Moscoso-Ramírez & Palou, 2016; Smilanick, Mansour, Gabler & Sorenson, 2008). These compounds are attributed to the activation of defense mechanisms in the fruit as a consequence of a specific recognition process between the fruit and the pathogen. Among the main oxidation-reduction enzymes involved in these signaling processes are peroxidase (POD) and polyphenoxidase (PPO) (Berúmen-Varela, Coronado-Partida, Ochoa-Jiménez, Chacón-Lopez & Gutiérrez-Martínez, 2015). Peroxidases are enzymes that contribute to induced resistance by generating reactive oxygen species (such as O2 and H2O2) that have antifungal activity against the attack of different phytopathogens (Peng & Kuc, 1992). POD has the function of oxidizing phenolic compounds and lignify the cell wall in plants (Yin et al., 2013). On the other hand, the PPO enzyme is in charge of catalyzing the oxidation of phenolic compounds to quinones, which are antimicrobial compounds toxic to the pathogen (Soliva, Elez, Sebastián & Martín, 2000). In addition, this enzyme is involved in the lignification of plant cells favoring defense against pathogens (Chen et al., 2014). Therefore, the objectives of this research were to i) evaluate the effects of chitosan (Chi), potassium sorbate (PS), and their combination on the in vitro growth and development of R. stolonifer, ii) to test the effectiveness of those treatments on soft rot severity, and iii) the POD and PPO activity in jackfruit treated fruits.
Materials and methods
Phytopathogen
R. stolonifer was isolated and identified from diseased stored jackfruits (Artocarpus heterophyllus L.) collected in San Blas, Nayarit, Mexico.
Treatments
Chitosan (75-85% deacetylation) (Sigma-Aldrich, St. Louis, MO, USA) was prepared in concentrations of 0.1, 0.5 and 1.0% (w/v) based on the methodology described by Ramos- Guerrero et al. (2018). Potassium sorbate solutions (Jalmek, Mexico) were prepared in concentrations of 1.0, 1.5, 2.0, 2.5 and 3.0% (w/v) in sterile distilled water. Once the antifungal effectiveness of the separate treatments was determined, treatment combinations of 1.0% Chi + 0.1% PS, 1.0% Chi + 0.5% PS and 1.0% Chi + 1.0% PS were selected, according the most effective concentrations in preliminary trials.
In vitro evaluations on R. stolonifera
The mycelial growth of the pathogen was evaluated by taking a 10 mm diameter disk from the edge of the colony. The disk was placed in the center of the Petri dish with PDA amended with each of the concentrations and/or combinations previously described. Petri dishes with only PDA were used as controls. Petri dishes were incubated at 25 ºC and the mycelial growth diameter was measured with a digital vernier (TruperTM) every 24 h until the pathogen completely colonize the Petri dishes in the control. The results were expressed in radial growth in mm of the circumference of the fungus in the Petri dish, as well as in percentage of inhibition of mycelial growth, comparing the treatments with the controls. The sporulation test was carried out after 48 h of incubation of the fungus using the same Petri dishes with the treatments and the control of the mycelial growth assay, using the methodology described by Cortes-Rivera, Blancas-Benitez, Romero-Islas, Gutiérrez-Martinez & González-Estrada (2019). In the spore germination assays, aliquots of 20 μL were taken from a spore suspension of 1 × 106 conidia mL− 1, which was placed on discs with the different treatments of Chi, PS, Chi-PS, and only PDA as a control. Finally, around 400 spores were observed in a microscope Motic BA 300 (Motic Instruments Inc., Canada) every hour for 6 h at 40X. The results were expressed as the percentage of germination compared to the control.
In vivo evaluation in jackfruit
The jackfruit was harvested at physiological maturity in San Blas, Nayarit, Mexico, and then transported to the food biotechnology laboratory of the TecNM / Technological Institute of Tepic. Fruits were washed with a 2% (v / v) sodium hypochlorite solution (NaClO) and left to dry at room temperature. Next, 30 µLof a 1 x 106 spore/mLspore suspension were inoculated by wound using a sterile needle (2.5 mm deep and 3 mm wide). Fruits were allowed to dry for 30 min and then the jackfruits were subjected to spray treatment with a Chi-PS solution, allowed to dry at room temperature for 60 min and then were stored at 24 ºC in humid chambers (80% humidity) for 96 h. Finally, the disease severity was evaluated according to Velázquez-del Valle, Bautista-Baños, Hernández- Lauzardo, Guerra-Sánchez & Amora-Lazcano (2008). Control fruits were sprayed with water.
Enzyme activity
The enzyme activity was evaluated at 0, 24, 48, and 72 h after the application of the treatment Chi (1.0%) + PS (1.0%) in jackfruit fruits at physiological maturity. The enzymatic extract was obtained from the cuticle of the jackfruit using the methodology described by Chen Bélanger, Benhamou & Paulitz (2000). POD expression was evaluated using the technique proposed by Chance & Maehly (1955) with some modifications. Briefly, 0.5 mL of crude extract (supernatant) were mixed with 2 mL of a guaiac buffer solution, and then incubated for 5 min at 30 ºC. Subsequently, 1 mL of H2O2 was added to the mixture and the absorbance was measured at 460 nm every 5 s for 90 s in a UV / visible spectrophotometer (JENWAY 67 series). On the other hand, PPO activity was determined according to Yue-Ming (1999). Briefly, 0.5 mL of crude extract were mixed with 3 mL of catechol (as substrate) and then the absorbance was measured at 420 nm every 10 s for 180 s. The activity of both enzymes was expressed as U mg protein-1. The protein content was determined by the Bradford method (1976). Data were analyzed by analysis of variance (ANOVA) with a 5% level of significance using a completely randomized block design. A comparison of means was performed by Tukey’s test when the ANOVAshowed significant differences. The statistical package IBM SPSS statistics 25 was used.
Results and discussion
The Mycelial growth inhibition, sporulation, and germination are shown in Table I, observing a significant difference in all the treatments compared to the control. In 48 h, the control has a growth of 80.0 mm. The lowest mycelial growth was observed at a concentration of 1.0% of Chi (41.6 mm), obtaining a 48% inhibition of mycelial growth. The inhibition of the sporulation of R. stolonifer was observed from the 0.1% treatment, obtaining a lower concentration of spores with the 1.0% treatment (1.28 x 105 spores mL-1). Regarding the germination of spores, the germination in the PDA discs was inhibited with the treatments up to 60% in the 1.0% concentration of the biopolymer compared to the one that presented 100% at 4 h. The parameters evaluated of the PS at different concentrations are shown in Table I. One % PS inhibited mycelial growth by 70%, while complete inhibition was observed at 1.5. In the sporulation test, the solution with a concentration of 3.0% of PS presented the lowest number of spores (2.1 x 105 spores mL-1). Table I shows the results of the PS concentrations used in this experiment, observing a statistically significant control (0.0%) in the germination of spores with a concentration of 1.0%, which was monitored for up to 6 h. In Table I, it can be seen that the combination of Chi and PS treatments completely inhibited mycelial growth from the lowest concentration of (0.1% Chi-1.0% PS). The mycelial growth inhibition and germination of R. stolonifer isolated from the jackfruit are due to the synergistic effect of chitosan and sodium sorbate. Taking this into account, chitosan is attributed to its polycationic nature, since its molecule is positively charged by the presence of amino groups, which interact with the negative charges of the cell wall of the microorganism, achieving a break in its structure, carrying out the loss of protein compounds and intracellular constituents (Ayala Valencia, 2015). Regarding the effect of potassium sorbate, Smilanick et al. (2008) describe that this compound generates alterations in the structure of the cell as well as alterations in the cell membrane and the inhibition of enzymes that are involved in metabolism in the transport functions. In previous studies, the affectivity of chitosan added with potassium sorbate to inhibit the mycelial growth of P. citrinum isolated from garlic has been reported with an effectiveness of 99.5%, attributing it to the decrease in intracellular pH and ionization by of K+ in the chemical structure of PS, affecting the development of the fungus (González-Estrada et al., 2020).
Table I Effect of Chi, PS, and Chi-PS at different concentrations on mycelial growth, percentage of mycelial growth inhibition, sporulation, and percentage of germination of R. stolonifer.
| Treatment | Mycelial growth (mm) (48 h) | Mycelial growth inhibition (%) | Sporulation (spores/ mL) | Germination (6 h) (%) |
|---|---|---|---|---|
| Control | 80.0 ± 0.20 a | 0 ± 0.0 a | 143.2x106 ± 0.15 a | 100 ± 0.0 a |
| Chi 0.1 % | 60.0 ± 0.38 b | 25 ± 0.28 b | 4.24x105 ± 0.09 b | 80 ± 0.3 b |
| Chi 0.5 % | 44.8 ± 0.24 c | 44 ± 0.35 c | 3.06x105 ± 0.03 c | 75 ± 0.4 c |
| Chi 1.0 % | 41.6 ± 0.32 d | 48 ± 0.23 d | 1.28x105 ± 0.04 d | 60 ± 0.5 d |
| Control | 80 ± 0.2 a | 0 ± 0.0 a | 143.2x106 ± 0.15 a | 100 ± 0.0 a |
| PS 1.0 % | 24 ± 0.22 b | 70 ± 0.3 b | 12x106 ± 0.82 b | 0.0 ± 0.0 b |
| PS 1.5 % | 0.0 ± 0.0 c | 100 ± 0.0 c | 8x105 ± 0.35 c | 0.0 ± 0.0 b |
| PS 2.0 % | 0.0 ± 0.0 c | 100 ± 0.0 c | 8x105 ± 0.52 c | 0.0 ± 0.0 b |
| PS 2.5 % | 0.0 ± 0.0 c | 100 ± 0.0 c | 5.6x105 ± 0.48 d | 0.0 ± 0.0 b |
| PS 3.0 % | 0.0 ± 0.0 c | 100 ± 0.0 c | 2.1x105 ±0.32 e | 0.0 ± 0.0 b |
| Control | 80.0 ± 0.2 a | 0 ± 0.0 a | 143.2x106 ± 0.15 a | 100 ± 0.0 a |
| 0.1% Chi-1.0% PS | 0.0 ± 0.0 b | 100 ± 0.0 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| 0.5% Chi-1.0% PS | 0.0 ± 0.0 b | 100 ± 0.0 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| 1.0% Chi-1.0% PS | 0.0 ± 0.0 b | 100 ± 0.0 b | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
Values are the means of three repetitions. Different letters per column indicate a significant difference between treatments (p ≤ 0.05).
In vivo evaluation
Once the best treatments were established, combination treatment with Chi (1.0%) - PS (1.0%) was applied in the jackfruit fruits. The jackfruit inoculated with R. stolonifer and treated only with water showed an accelerated infection, detecting signs of rot at 48 h after inoculation. In addition, at 96 h the control presented 100% infection (Figure 1D). An accelerated softening of the fruit was also observed, evidence of the advance of the ripening process. Further, in the control treatment (fruits with natural infection) (Figure 1B), the development of soft rot symptoms was visualized in different areas of the fruit. At 96 h, the fruit was completely deteriorated by the fungus. The fruits treated with Chi-PS (with and without inoculation of the pathogen) had a 0.0% severity of the infection (Table II). The effectiveness of the Chi-PS treatment on the jackfruit can be due to the influence of the pH of the compound since it has been reported that its application on the cuticle of certain citrus fruits, reporting a value of 5.5 and when a wound occurs, it can drop to 5.1. The application of the treatment regulates this value, maximizing the activity to inhibit the invasion of the pathogen (Smilanick et al., 2008). On the other hand, it has been described that chitosan generates a modified atmosphere in the fruit, regulating its maturation and senescence processes, preventing the development of pathogens that could infect the fruit after harvest, as well as the potential to induce enzymes against the attack of pathogens (Bautista-Baños, Ventura-Aguilar, Correa- Pacheco & Corona-Rangel, 2017), and phenolic compounds in plants (Benhamou, 1996).
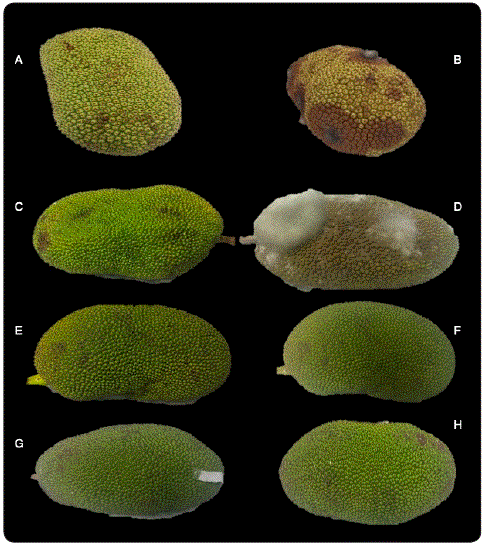
Figure 1 Jackfruit fruits with the different treatments: (A) No inoculated at 0 h, (B) No inoculated at 96 h, (C) Inoculated at 0 h, (D) Inoculated at 96 h, (E) No inoculated treated with Chi-PS at 0 h, (F) No inoculated treated with Chi-PS at 96 h, (G) Inoculated treated with Chi-PS at 0 h and (H) Inoculated treated with Chi-PS at 96 h. Source: self-made.
Table II Disease severity in jackfruit fruits treated with chitosan and potassium sorbate with and without inoculation of Rhizopus stolonifer.
| Treatment | Disease severity (%) |
|---|---|
| Control | 100 ± 0.0 a |
| Control (inoculated) | 100 ± 0.0 a |
| Chi + PS | 0 ± 0.0 b |
| Chi + PS (inoculated) | 0 ± 0.0 b |
Values are the means of three repetitions. Different letters per column indicate a significant difference between treatments (p ≤ 0.05).
Enzyme activity
The effect of Chi and PS (with and without inoculation of the pathogen) maintained a high enzymatic activity compared to control in which it was relatively low. In the fruits inoculated and sprayed with the combined treatment (Chi-PS) at 24 h the highest level of activity was presented by this enzyme subsequently decreasing in relation to the control (p < 0.001) (Figure 2 A), as well as the non-inoculated fruits showed higher activity at 72 h after treatment application. In Figure 2 B, the activity of the PPO is presented, recording an increase in its activity as time passes, observing a difference (p < 0.001) between the controls (with inoculation and without inoculation) and the fruits treated with the solutions of chitosan and potassium sorbate, showing greater activity at 48 h after their application. It is observed that in the treated fruits, the activity of the enzyme (Figure 2B) was induced from 12 h and according to time it increased, observing that at 24 h it reached its maximum induction. The observed increases in POD activity seem to be related to the pathogen to the fruit, stimulating a series of mechanisms in the synthesis of reactive oxygen species such as superoxide radicals and hydrogen peroxide, acting as a signal, and regulating gene expression and strengthening the cell wall via protein cross- linking (Blechert et al., 1995). These results agree with those obtained by Liu, Tian, Meng & Xu (2007) in tomato fruits, observing that enzymatic activity of POD was increased at chitosan 1.0% concentration, stored at 25 ° and 2 °C. POD and PPO enzymes are part of a defense system in plants against stress situations generated by the invasion of pathogens (García-Garrido & Ocampo, 2002; Kazan, Murray, Goulter, Llewellyn & Manners, 1998). Berumen-Varela et al. (2015), observed that the 1.0% chitosan induced the enzymatic activity of POD in mango fruits, observing the highest activity at 24 h (without inoculation) and 72 h (with inoculation) after applying the treatment. Polyphenoloxidases have been shown to catalyze the oxidation of phenolic compounds to quinones using molecular oxygen as an electron acceptor (Sommer, Petersen & Bautz, 1994) which are toxic to pathogens and pests (Weir, Park & Vivanco, 2004). Polyphenoloxidases have been suggested to be directly involved in auxin biosynthesis because the o-quinones produced can react with tryptophan to form indole-3-acetide (Jukanti, 2017). The use of GRAS compounds can protect against infections of R. stolonifer by activating mechanisms of fruit defense.
Conclusions
The combined treatment of chitosan and potassium sorbate inhibited the germination of spores of R. stolonifer and significantly reduced soft rot in jackfruit without damaging the quality of the fruit. Chi-PS treatmentled toasignificant increase in the enzymatic activity of PPO and POD. These results show that the application of these GRAS compounds is a promising method for the control of fungal diseases in the post-harvest stage and represents an efficient, reliable and safe method to replace fungicides.











 nueva página del texto (beta)
nueva página del texto (beta)



