Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Agrociencia
versión On-line ISSN 2521-9766versión impresa ISSN 1405-3195
Agrociencia vol.49 no.8 Texcoco nov./dic. 2015
Fitociencia
Assessment of cadmium on wheat (Triticum aestivum L.) in hydroponics medium
Evaluación de cadmio en trigo (Triticum aestivum L.) en un medio hidropónico
Saiba Idrees, Sumera Shabir, Noshin Ilyas*, Nazima Batool, Sidra Kanwal
Department of Botany, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, Pakistan. *Author for correspondence. (noshinilyas@yahoo.com) (noshinilyas@uaar.edu.pk).
Received: June, 2015.
Approved: September, 2015.
Abstract
Cadmium is a non-essential heavy metal that adversely affects plant growth. Its growth retarding effect has been revealed in different crops. The present research was devised to evaluate the Cd effect on the wheat (Triticum aestivum L.) cultivars NARC-11 and Galaxy in the hydroponic system. The effect of three concentrations of CdCl2•5H2O (150 μM, 200 μM and 250 μM) were evaluated on seed germination and seedling growth. The study lasted 36 d and Cd concentrations were applied after the interval of 7 d. The experimental design was completely randomized. The results showed that Cd significantly reduced the seed germination (20 % and 30 %), seedling fresh (32 % and 28 %) and dry weight (31 % and 30 %), shoot length (13 % and 14 %), root length (12.5 % and 9.5 %), total chlorophyll content (10 % in both), relative water content (24 % and 36 %), and membrane stability (18.5 % and 27 %) in NARC-11 and Galaxy. A remarkable difference was observed in both wheat cultivars.
Key words: Wheat, CdCl2•5H2O, hydroponics, chlorophyll content.
Resumen
El cadmio es un metal pesado no esencial que afecta adversamente el crecimiento de las plantas. Se ha encontrado un efecto de demora en el crecimiento de distintos cultivos. Este estudio se diseñó para evaluar el efecto de Cd en los cultivares de trigo (Triticum aestivum L.) NARC-11 y Galaxy en el sistema hidropónico. El efecto de tres concentraciones de CdCl2•5H2O (150 μM, 200 μM y 250 μM) en la germinación de semillas y el crecimiento de plántulas se evaluó. El estudio duró 36 d y las concentraciones de Cd se aplicaron después de un intervalo de 7 d. El diseño experimental fue aleatorio. Los resultados mostraron que el Cd redujo significativamente germinación de semillas (20 % y 30 %), peso fresco (32 % y 28 %) y seco (31 % y 30 %) de plántulas, longitud del tallo (13 % y 14 %) y de la raíz (12.5 % y 9.5 %), contenido total de clorofila (10 % en ambas), contenido relativo de agua (24 % y 36 %), y estabilidad de las membranas (18.5 % y 27 %) en NARC-11 y Galaxy. Una diferencia notable se observó en las dos variedades de trigo.
Palabras clave: Trigo, CdCl2•5H2O, hidroponia, contenido de clorofila.
INTRODUCTION
Various biotic and abiotic stresses adversely affect the food productivity in the world, and reduction of these losses under changing climate is a major concern to optimize food security. Environmental abiotic stresses, such as extreme temperature, cold or high salinity, heavy metals and drought affect plant growth and productivity (Anjum et al., 2011). Growth and yield of annual crops is limited up to 50 % due to abiotic stresses. Under stress conditions morphological and physiological charateristics are affected (Rodriguez et al., 2005).
With increase in industrialization, the heavy metal contents are also increasing adversely in soil. The industrial waste has the mixture of various organic, inorganic, radioactive and heavy metals (Ahmad et al., 2012). These heavy metals act as essential micronutrients and play an important role in plants, e.g. as cofactors of metabolic enzymes. When their amount increases beyond the limits in the soil, they become heavy metal stress in plants (Stobrawa and Lorence-Plucinska, 2007; Montenegro et al., 2009; Bhatti et al., 2013).
Cadmiun being a mobile heavy metal can be taken up by roots actively and can be accumulated in different plant parts (Ahmad et al., 2012). It is more toxic due to its high mobility into water (Pinto et al., 2004). The main sources by which it may enters into the environment are mineral fertilizers, metal-working industries, and mining units (Schutzendubel et al., 2001; Hirve and Bafna, 2013). Cadmium induces oxidative stress in plants either by increasing or decreasing antioxidative molecules (Malecka et al., 2001), inhibits the plumule and radical growth in rice (Jun-yu et al., 2008) and root growth is more affected than shoot growth in wheat (An et al., 2004). Plant membrane is the first target of heavy metal toxicity, which may directly affect the nucleus or react with the hormones that are in aerial plants parts (Laspina et al., 2005; Hirva and Bafna, 2013).
Wheat is the second most important crop after rice as it fulfills the protein and caloric requirement of the world's one third population (Skovmand and Reynolds, 2000). During 2011-2012, 696 million Mg wheat were produced, but its yield is stagnant during the last 50 years due to biotic and abiotic stresses. According to Sial et al. (2009), 40 % more wheat would be required up to 2025 to feed the rapidly growing population. In Pakistan, studies were conducted on Cd toxicity in wheat (Jiang et al., 2001; Peralta-Videa et al., 2002; Khan et al., 2006), but still there is a need to check its effects under several conditions. The present study was aimed to evaluate the effects of cadmium toxicity on germination and growth of two wheat cultivars under hydroponics conditions.
MATERIALS AND METHODS
The experiment was carried out at the Plant Physiology laboratory, Department of Botany PMAS-Arid Agriculture University Rawalpindi, Pakistan. Two wheat cultivars, Galaxy and NARC-11 were selected to evaluate the effect of three CdCl2•5H2O concentrations on seed germination and seedling growth.
Seeds of Galaxy and NARC-11 cultivars were obtained from the National Agriculture Research Centre (NARC) Islamabad, Pakistan. Before germination, viable and healthy seeds were surface sterilized with 5 % sodium hypochloride for 3 min and then rinse with distilled water for three times.
Cadmium chloride (CdCl2•5H2O) of high purity (98 % Sigma-Aldrich). Concentrations of 150 μM, 200 μM and 250 μM were applied on wheat cultivars along with control (0 Cd). The germination evaluation was conducted in autoclaved Petri plates. Ten seeds of each wheat cultivar were utilized. Three mL of each Cd concentration solution was added 1 d after starting the experiment, and afterwards every 2 d for 7 d. The following variables were calculated:
Germination:

Promptness index:
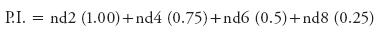
In this equation, nd2, nd4, nd6 and nd8 are the number of seedlings emergence at 2, 4, 6 and 8 d.
Germination stress tolerance index:
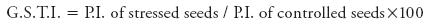
Seedling vigour (S.V.I.):
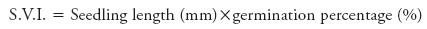
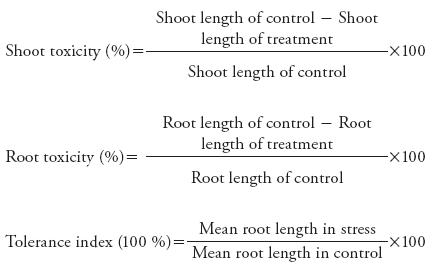
The experiment of hydroponics was arranged in complete randomized design (CRD) with three replicates. Firstly, 20 surface sterilized seeds of both cultivars were sown in a sand tray. Then at two leaf stage after 8 d of germination, the seedlings were shifted into the Hoagland's solution. The pH of solution was maintained between 5.5-6.5. 150 μM, 200 μM and 250 μM CdCl2•5H2O solution, about 150 mL, were applied in each hydroponic container along with nutrients every 7 d. The plants were kept in growth room at room 25 °C. After 28 d plants were harvested and germination percentage, seed vigour index, tolerance index, root and shoot length, fresh and dry weight and turgid weight of the plant, chlorophyll and carotenoid contents, and membrane stability index, were measured. The shoot and root toxicity, and tolerance index were calculated by the following formula:
For relative water contents the leaves were weighed to get fresh weight and were soaked in water for 24 h (turgid weight). After that leaves were oven dried at 65 °C for 2 d to obtain dry
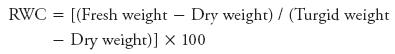
To measure the membrane stability index (M.S.I) the leaf disc of the each plant sample was taken into 10 mL distilled water and heated in water bath at 40 °C for 30 min and its electrical conductivity (EC) was measured (C1). Then the same sample was heated at 100 °C for 10 min and its EC was registered (C2). The M.S.I was calculated by the following formula:
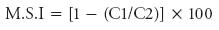
Chlorophyll a, b, total and carotenoids were measured according to the following formulas:

Data analysis was carried out by using Statistics software 9.0 versions. The experimental data was expressed as means and standard errors (SE) with three replicates. Mean significant difference among treatment was detected by ANOVA.
RESULTS AND DISCUSSION
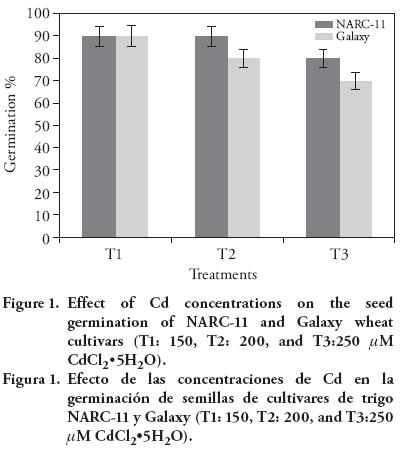
Cadmiun phytotoxicity had a pronounced effect on the germination and seedling growth of the T. aestivum L. cv. NARC-11 and Galaxy. There was a drastic effect on both cultivars as compared to the control and response also varied with concentrations in both cultivars. The inhibitory effect of Cd was observed on seed germination of NARC-11 and Galaxy which was dependent on the dose of heavy metal.
Seed germination was reduced as the concentration of Cd increased. The maximum germination inhibition (30 %) was observed in Galaxy at 250 μM Cd treatment, in contrast with NARC-11 it was 20 %. The 150 μM Cd treatment had similar effect in both cultivars. NARC-11 showed more tolerance than Galaxy in germination (Figure 1). The inhibition of germination with increased Cd concentration may be due to the hindrance of metal ions in water absorption. Similar results were also presented by Amirjani (2012) in wheat. The difference in seed germination might be due to the genetic variability in both cultivars. The same response was observed in Pb rice stressed (Zhang et al., 2005). The stress tolerance at germination level was significantly decreased at 250 μM Cd concentration (37 % and 41 % in NARC-11 and Galaxy). With 150 μM there was maximum tolerance (83 % in NARC-11 and 82 % in Galaxy) (Figure 2). The same findings were shown by Aydinalp and Marinova (2009).
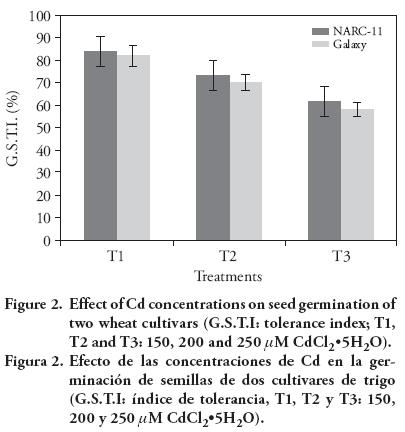
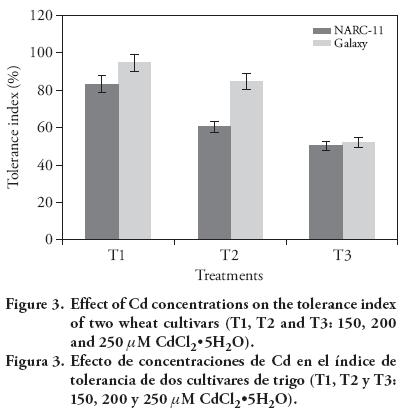
The tolerance index was 83 %, 70 % and 50 % in NARC-11, whereas in Galaxy it was 94 %, 84 % and 52 %, for 150 μM, 200 μM and 250 μM. The response of wheat cultivars varied with Cd concentration (Figure 3). The reason of low tolerance with increased Cd concentration may be due to the physiological changes with growth stages (Khan et al., 2006). The higher tolerance index was shown by Galaxy at 200 μM (84 %), as compared to NARC-11 (70 %), but with 250 μM there was no significant difference between cultivars. A same pattern of Cd effect on tolerance indices was observed by Ahmad et al. (2012) in wheat.
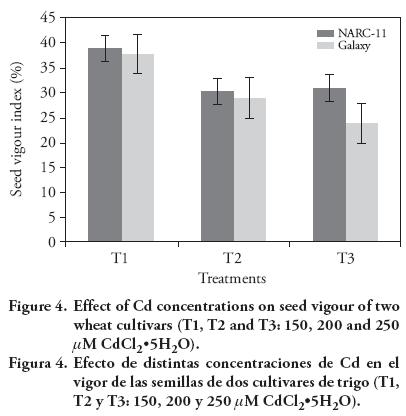
The results showed that the seed vigour of Galaxy was more affected than NARC-11 by Cd 150 μM; but with Cd 250 μM the maximum dedrease was for Galaxy (30.15 %) than for NARC-11 (23.8 %) (Figure 4). Similar results of Cd effect were reported by Shaikh et al. (2013). The seed vigour and germination may decrease due to the increased breakdown of reserved food in embryo. These findings were reported by Titov et al. (1996), Jun-yu et al. (2008), and Raziuddin et al. (2011). The P.I. decreased as the Cd concentration increased. Maximum P.I. was shown at 150 μM (14 and 13.25 in NARC-11 and Galaxy). At 250 mM the P.I. of Galaxy was more negatively affected (9.0) than NARC-11 (10.0) (Figure 5).
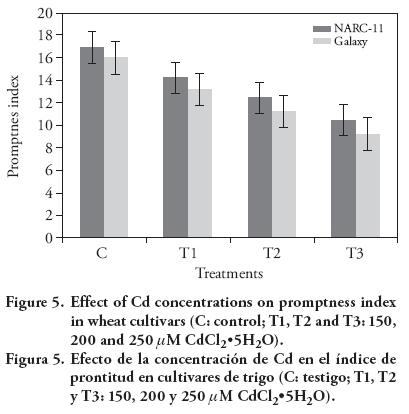
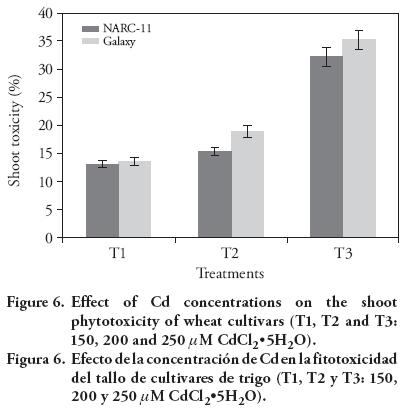
Shoot and root growth was adversely affected by the increased Cd concentrations in medium. Shoot toxicity was more pronounced in Galaxy (35 %, 19 % and 13.55 % at 250, 200 and 150 μM) than in NARC-11 (32 %, 15 % and 13 %) (Figure 6 and 7). The results show that the roots of NARC-11 show more resistance to Cd than Galaxy. These findings are similar with those obtained by Shaikh et al. (2013).
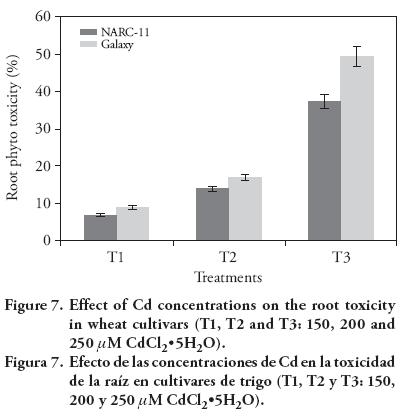
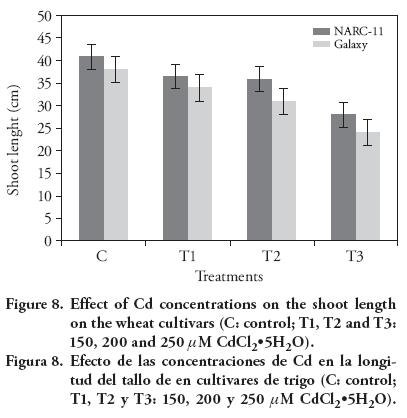
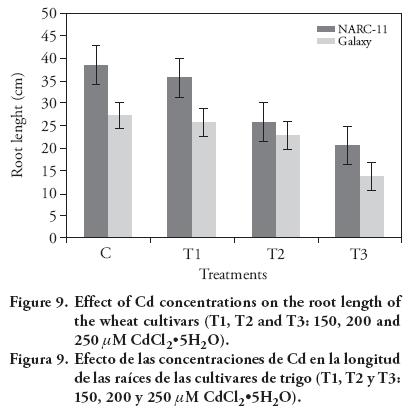
Gradual decrease in the plant root and shoot length with Cd concentration was observed. Galaxy seedling growth exhibited more susceptibility to Cd then NARC-11. The results showed that Cd (250 μM) significantly reduced the shoot length up to 13 % and 9 % in NARC-11 and Galaxy (Figure 8), whereas NARC-11 and Galaxy showed a decrease of 12.5 % and 9.5 %, respectively, in root length, as compared to control (Figure 9). According to the Rascio et al. (2008), root length was reduced under Cd stress because it changes the morphogenesis of root. Similar results were reported by Ahmad et al. (2012) and Amirjani (2012) in wheat. Root, shoot and seedling length are the most sensitive morphology of plants and are directly correlated to the effect of heavy metal (Correa et al., 2006; Ahmad et al., 2008; Jun-yu et al., 2008). Roots are more affected by Cd; however Liu et al. (2005) reported that root and shoot are similarly affected but since Cd is absorbed first by roots, they showed more retardation. Similar results were reported by Krantev et al. (2008), Yadav (2010) and Rascio and Navari-Izzo (2011), who stated that the Cd stress inhibits the lateral root formation, decrease root and shoot length and also cause chlorosis.
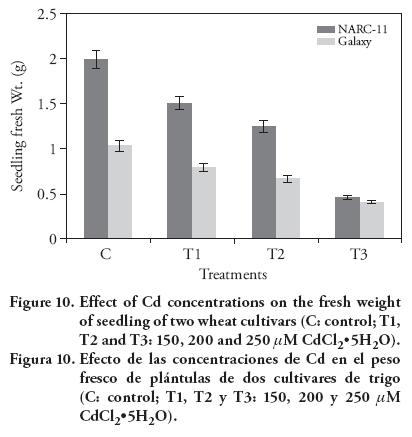
The results showed that high Cd concentrations decreased seedling fresh and dry weights, as compared to the control (Figure 10). Similar findings were reported by Ashraf et al. (2011) and Ahmad et al. (2012). The decrease in the fresh and dry weight was due to the decreased metabolism because of the Cd interaction with enzymes and biochemical reactions. Overall plant growth was decreased with Cd (Oncel et al., 2000; Shafi et al., 2010).
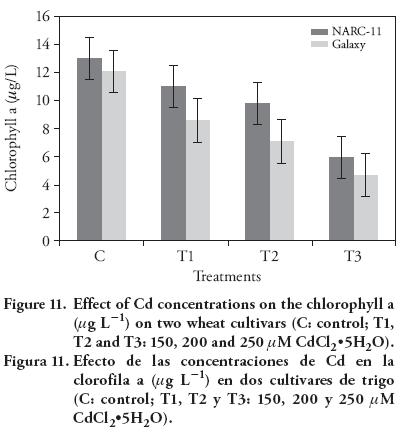
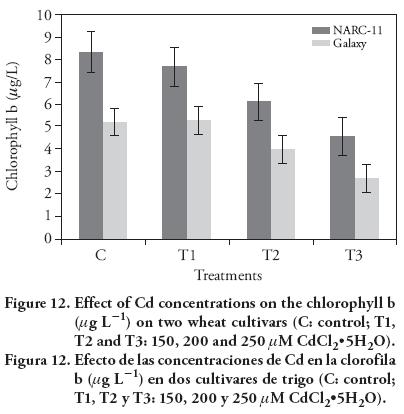
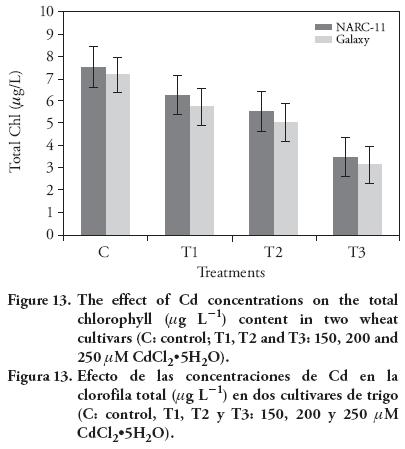
Results of Cd stress on chlorophyll contents showed a significant decrease in both wheat varieties, as compared to the control. The chlorophyll contents decreased as the Cd level increased. The chlorophyll a, b and total chlorophyll drastically decreased in Galaxy with the higher Cd concentration, as compared to the control (Figure 11-13) (12). At maximum Cd concentration, total chlorophyll content decreased at same level in both cultivars (10 %), as compared to control. In wheat, long and short term Cd exposure have inhibitory effect on the photosynthesis (Moussa and El-Gamal, 2010). The Cd at 200 μM and 250 μM caused leave chlorosis. Similar findings were reported by Stiborova et al. (1986), Oncel et al. (2000), Muthuchelian et al. (2001), Wu et al. (2003), Amirjani (2012), and Bheemareddy (2013). This effect was more pronounced in Galaxy than NARC-11. The treatment affected more pigments on leaf surface than in the mesophyll. In leaves, Cd ions cause hindrance and division of chloroplast (Baryla et al., 2001). The negative effect of Cd ions on the structure and functioning of chloroplast in T. aestivum L. was also reported by Atal et al. (1991). Besides, Cd have drastic effect on the PSII specially electron transport chain and on the oxygen evolving complex (OEC) because it replace the Ca+/Mn bonding in OEC (Sigfridsson et al., 2004).
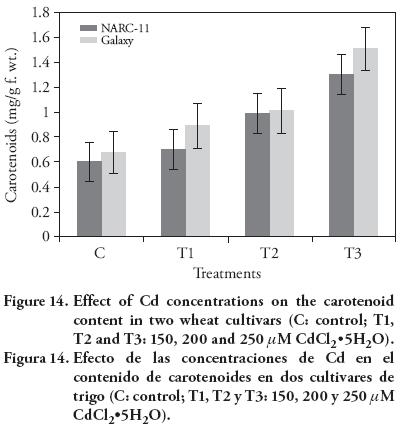
In our study, a gradual increase in carotenoids content was observed with rise of Cd concentrations. There was more appearance of carotenoid pigments in Galaxy cultivar than the NARC-11. The maximum carotene content was found at 250 μM (Figure 14). These findings contrasted with results of Amirjani (2012). But these findings are similar to those of Bhatti et al. (2013) showing the increase in carotenoids as Pb concentration increased. The carotenoid pigment is a secondary metabolite and a light-harvesting pigment which prevents the cellular structures from damage and also protects the membranes destabilization due to reactive oxygen species (ROS) damage. So, as the Cd level increased, plants produce more carotenoids to cope up with the Cd stress (Singh et al., 2006).
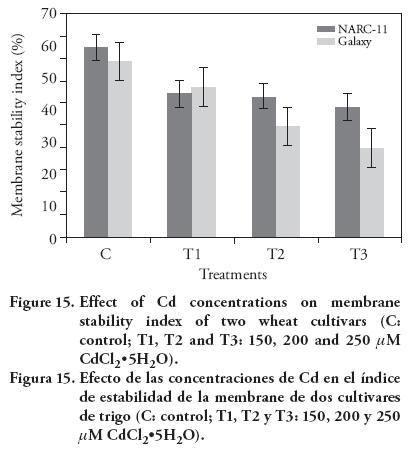
Our results showed that high Cd concentration significantly affects the membrane stability. The membrane stability drastically decreased as the Cd concentration increased in NARC-11 (35 % and 28 %) as compared to Galaxy (44 % and 41 %) at 200 μM and 250 μM. Besides, membrane stability index decreased 18.5 % and 27 % at 250 μM as compared to control (Figure 15). Popova et al. (2008) also showed that the Cd stress significantly changes the membrane stability and it's functioning.
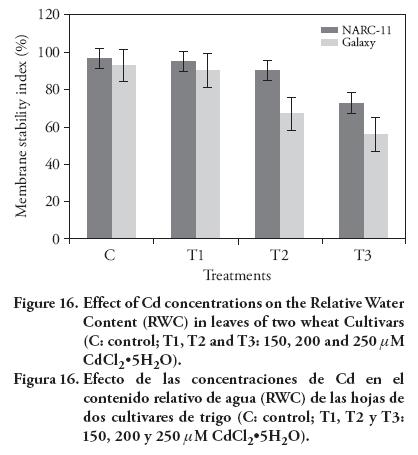
The relative water content decreased as the Cd level increased. No significant effect on relative water contents was observed with 150 μM, as compared with controls (Figure 16). These results are contrary to those presented by Malar et al. (2014) with Pb on wheat. This might be due to the fact that there was less water absorption by roots due to the interference of Cd ions in root zone. Besides, roots also prevent the Cd ions movement into the leaves to protect the phytotoxicity.
CONCLUSION
Results of this research showed cadmium dose dependent inhibition of seed germination and seedling growth. Gradual decrease in root length, shoot length and seedling growth was observed at different concentrations of Cd on cultivars NARC-11 and Galaxy under hydroponic conditions. Cadmium adversely affects morphological and physiological characters of wheat. Cultivar NARC-11 shows more tolerance than Galaxy.
LITERATURE CITED
Ahmad, I., M. J. Akhtar, Z. A. Zahir, and A. Jamil. 2012. Effect of cadmium on seedling growth of four wheat (Triticum aestivum L.) cultivars. Middle-East J. Sci. Res.14: 142-154. [ Links ]
Ahmad, M. S. A., M. Hussain, S. Ijaz, and A. K. Alvi. 2008. Photosynthesis performance of two mung bean (Vigna radiate (L.) Wilczek) cultivars under lead and copper application. Int. J. Agric. Biol. 10: 167-176. [ Links ]
Amirjani, M. R. 2012. Effects of cadmium on wheat growth and some physiological factors. Int. J. Forest. Soil Eros. 2: 50-58. [ Links ]
An, Y. J., Y. M. Kim, T. I. Kwon, and S.W. Jeong. 2004. Combined effect of copper, cadmium and lead upon Cucumis sativus growth and bioaccumulation. Sci. Total Environ. 326: 85-93. [ Links ]
Anjum, S. A., X. Xie, L. Wang, and M. F. Saleem. 2011. Morphological, physiological and biochemical responses of plants to drought stress. Afri. J. Agri. Res. 6: 2026-2032. [ Links ]
Ashraf, M. Y., R. Sadiq, M. Hussain, M. Ashraf, and M. S. A. Ahmad. 2011. Toxic effect of nickel (Ni) on growth and metabolism in germinating seeds of sunflower (Helianthus annuus L.). Biol. Trace Elem. Res. 143: 1695-1703. [ Links ]
Atal, N., P. P. Saradhi, and P. Mohanty. 1991. Inhibition of the chloroplast photochemical reactions by treatment of wheat seedlings with low concentrations of cadmium: analysis of electron transport activities and changes in florescence yield. Plant Cell Physiol. 32: 943-951. [ Links ]
Aydinalp, C., and S. Marinova. 2009. The effect of Heavy metals on seed germination and plant growth on alfalfa plant (Medicago sativa). Bulgarian J. Agric. Sci. 15: 347-350. [ Links ]
Baryla, A., P. Carrier, F. Franck, C. Coulomb, C. Sahut, and M. Havaux. 2001. Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: causes and consequences for photosynthesis and growth. Planta 212: 696-709. [ Links ]
Bhatti, K. H., S. Anwar, K. Nawaz, K. Hussain, K. H. Siddiqi, R. U. Sharif, A. Talat, and A. Khalid. 2013. Effect of heavy metal (Pb) stress of different concentration on wheat (Triticum aestivum L.). Middle-East J. Sci. Res. 4: 148-154. [ Links ]
Bheemareddy, V. S. 2013. Impact of cadmium phytotoxicity on photosynthetic rate and chlorophyll content in Triticum aestivum L. DWR 225 variety. Middle-East J. Sci. Res. 17: 1209-1212. [ Links ]
Correa, A. X. R., L. R. Rorig, M. A. Verdinelli, S. Cotelle, J. F. Ferard, and C. M. Radetski. 2006. Cadmium phytotoxicity: Quantitative sensitivity relationships between classical endpoints and antioxidative enzyme biomarkers. Sci. Total Environ. 357: 120-127. [ Links ]
Hirve, M., and A. Bafna. 2013. Effect of Cadmium exposures on growth and biochemical parameters of Vigna radiata seedlings. Int. J. Environ. Sci. 4: 315-322. [ Links ]
Jiang, W., D. Liu, and W. Hou. 2001. Hyperaccumulation of cadmium by roots, bulbs and shoots of garlic. Biores. Technol. 76: 9-13. [ Links ]
Jun-yu, H., R. Yan-fang, Z. Cheng, and J. De-an. 2008. Effects of cadmium stress on seed germination, seedling growth and seed amylase activities in rice (Oryza sativa). Rice Sci. 15: 319-325. [ Links ]
Khan, N. A., I. Ahmad, S. Singh, and R. Nazar. 2006. Variation in growth, photosynthesis and yield of five Wheat cultivars exposed to cadmium stress. World J. Agri. Sci. 2: 223-226. [ Links ]
Krantev, A., R. Yordanova, T. Janda, G. Szalai, and L. Popova. 2008. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 165: 920-931. [ Links ]
Laspina, N. V., M. D. Groppa, M. L. Tomaro, and M. P. Benavides. 2005. Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci. 169: 323-330. [ Links ]
Liu, X., S. Zhang, X. Shan, and Y. G. Zhu. 2005. Toxicity of arsenate and arsenate on germination, seedling growth and amylolytic activity of wheat. Chemosphere 61: 293-301. [ Links ]
Malar, S., S. S. Vikram, P. J. C. Favas, and V. C. Perumal. 2014. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths Eichhornia crassipes (Mart.). Bot. Studies. 55: 54. [ Links ]
Malecka, A., W. Jarmuszkiewics, and B. Tomaszewska. 2001. Antioxidative defense to lead stress in subcellular compartments of pea root cells. Acta Biochim. Polon. 48: 687-698. [ Links ]
Montenegro, G., C. Fredes, E. Mejias, C. Bonomelli, y L. Olivares. 2009. Content of heavy metals in soils near a Chilean copper mining tailing. Agrociencia 43: 427-435. [ Links ]
Moussa, H., and S. El-Gamal. 2010. Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol. Planta. 54: 315-320. [ Links ]
Muthuchelian, N. K., M. Bertamini, and N. Nedunchezhian. 2001. Triacontanol can protect Erythina variegate from Cd toxicity. J. Plant Physiol. 158: 1487-1490. [ Links ]
Oncel, I., S. Keles, and A. S. Ustun. 2000. Effect of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Pollut. 107: 315-320. [ Links ]
Peralta-Videa, J. R., J. L. Gardea-Torresdey, E. Gomez, K. J. Tiermann, J. G. Parsons, and G. Carrillo. 2002. Effect of mixed cadmium, copper, nickel and zinc at different pHs upon alfalfa growth and heavy metal uptake. Environ. Pollut. 119: 291-301. [ Links ]
Pinto. A. P., A. M. Mota, A. de Varennes, and F. C. Pinto. 2004. Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Sci. Total Environ. 326-329. [ Links ]
Popova, L., L. Malenkov, R. Yordanova, A. Krantev, G. Szalai, and T. Janda. 2008. Salicylic acid protects photosynthesis against cadmium toxicity in pea plants. Gen. Applied Plant Physiol. 34: 133-144. [ Links ]
Rascio, N., and F. Navari-Izzo. 2011. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 180: 169-181. [ Links ]
Rascio, N., F. V. Dalla, N. L. Rocca, R. Barbato, C. Pagliano, M. Raviolo, C. Gonnelli, and R. Gabbrielli. 2008. Metal accumulation and damage in rice (cv. Vialone nano) seedlings exposed to cadmium. Environ. Exp. Bot. 62: 267-278. [ Links ]
Raziuddin, F., G. Hassan, M. Akmal, S. S. Shah, F. Mohammad. M. Shafi, J. Bakht, and W. Zhou. 2011. Effect of cadmium and salinity on growth and photosynthesis parameters of brassica species. Pak. J. Bot. 43: 333-340. [ Links ]
Rodriguez, M. E., Canaless, and O. B. Hidalago. 2005. Molecular aspects of abiotic stress in plants. Biotecnologia Aplicada. 22: 1-10. [ Links ]
Schutzendubel, A. P. Schwanz, T. Teichmann, K. Gross, R. Langenfeld-Heyser, D. L. Godbold, and A. Polle. 2001. Cadmium-Induced Changes in Antioxidative Systems, Hydrogen Peroxide Content, and Differentiation in Scots Pine Roots. Plant Physiol. 127: 897-891. [ Links ]
Shafi, M., G. P. Zhang, J. Bakht, M. A. Khan, E. Islam, M. K. Dawood, and Raziuddin. 2010. Effect of cadmium and salinity on root morphology of Wheat. Pak. J. Bot. 42: 2747-2754. [ Links ]
Shaikh, I. R., S. R. Parveen, A. S. Rafique, and A. S. Alamgir. 2013. Phytotoxic effects of Heavy metals (Cr, Cd, Mn and Zn) on wheat (Triticum aestivum L.) seed germination and seedlings growth in black cotton soil of Nanded, India Res. J. Chem. Sci. 3: 14-23. [ Links ]
Sial, M. A., M. U. Dahot, M. A. Arain, G. S. Markhand, S. M. Mangrio, M. H. Naqvi, K. A. Laghari, and A. A. Mirbahar. 2009. Effect of water stress on yield and yield components of semi-dwarf bread wheat (Triticum aestivum L.). Pak. J. Bot. 41: 1303-1310. [ Links ]
Sigfridsson, K. G. V., G. Bernat, F. Mamedov, and S. Styring. 2004. Molecular interference of Cd2+ with photosystem II. Bioch. Biophys. Acta. (BBA)-Bioenergetics. 1659: 19-31. [ Links ]
Singh, S., S. F. Eapen, and Dsouza, 2006. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant Bacopa monnieri L. Chemosphere 62: 233-246. [ Links ]
Skovmand, B., and M. P. Reynolds. 2000. Increasing yield potential for marginal areas by exploring genetic resources collection. The eleventh regional wheat workshop for, eastern, central and southern Africa, Addis Ababa, Ehiopia. 18-22 September: 67-77. [ Links ]
Stiborova, M., M. Doubravova, A. Brezinova, and A. Fredrich. 1986. Effect of heavy metal ions on growth and biochemical characteristics of photosynthesis of Barley. Photosynthetica 20: 418-425. [ Links ]
Stobrawa, K., and G. Lorence-Plucinska. 2007. Changes in carbohydrate metabolism in fine roots of the native European black poplar (Populus nigra L.) in heavy metal-polluted environment. Sci. Total Environ. 373: 157-165. [ Links ]
Titov, A. F., V. V. Talanova, and N. P. Boeva. 1996. Growth responses of barley and wheat seedlings to lead and cadmium. Biol. Plan. 38: 431-436. [ Links ]
Weatherly, P. E. 1950. Studies in water relations in cotton plants. The field measurement of water deficit in leaves. New Phytol. 49: 81-87. [ Links ]
Wu, F., G. Zhang, and P. Dominy. 2003. Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 50: 67-78. [ Links ]
Yadav, S. K. 2010. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South Afri. J. Bot. 76: 167-179. [ Links ]
Zhang, S., J. Hu, Z. H. Chen, J. F. Chen, Y. Y. Zheng, and W. J. Song. 2005. Effects of Pb pollution on seed vigour of three rice varieties. Rice Sci. 12: 197-202. [ Links ]














