Introduction
Tomatillo (Physalis ixocarpa Brot. ex Horm.) is the seventh most cultivated vegetable in Mexico, accounting for 4.8 % of national vegetable production. The main producing states, in order of importance, are Sinaloa, Zacatecas, Jalisco, Puebla and Michoacán, with a share of 55 % (Servicio de Información Agroalimentaria y Pesquera [SIAP], 2021). According to the National Seed Inspection and Certification Service (SNICS, 2021), there are 16 registered and improved by selection varieties. However, given the drastic environmental conditions and the demand in the national and international market for this vegetable, varieties with better agronomic characteristics and resistance to phytopathogens are required.
The most suitable genotypic methods for the generation of improved varieties are stratified visual mass selection (SVMS), half-sib family selection (HSFS) and combined half-sib selection (CHSS) (Peña-Lomelí & Márquez, 1990). This is due to the fact that plants show gametophytic self-incompatibility (Kant-Pandey, 1957), which prevents the formation of inbred lines for hybrid formation.
One strategy to generate hybrids in tomatillo is to cross populations with a certain degree of inbreeding, generated through fraternal or plant-to-plant crosses in high-yielding elite families, until a hybrid combination between superior-yielding parents is found. However, commercial production of hybrid seed requires propagation and maintenance of the genotype of the parents throughout generations (Santiaguillo-Hernández, Cervantes-Santana, & Peña-Lomelí, 2004). This may be possible through vegetative propagation by in vitro culture (van Groenendael & de Kroon, 1990; Manzo-González et al., 1998).
One of the purposes of in vitro culture in breeding programs is the clonal propagation of parental lines for hybrid seed production (Dore, 1987). One method of propagation in in vitro culture is through enhanced axillary branching, using stem tips and side shoots as explants. The advantage of this type of micropropagation is that very little callus is formed, and the degree of abnormality and genetic variability is reduced, which guarantees the genetic stability of propagated plants (George & Debergh, 2008).
Regarding in vitro culture of Physalis ixocarpa, different sources have been used as explants. Ramírez-Malagón and Ochoa-Alejo (1991) evaluated the morphogenic response of hypocotyl explants grown in Murashige and Skoog (1962) medium (MS), in combination with benzyladenine (BA), naphthaleneacetic acid (NAA) and 2,4-dichlorophenoxyacetic acid (2,4-D). Shoot and root induction was performed with a combination of cytokinins and auxins. Manzo-González et al. (1998) used as explants stem, leaf, petiole and axillary bud segments of the Rendidora, Salamanca and Tamazula varieties, and used MS culture medium supplemented with 2,4-dichlorophenoxyacetic acid (2,4-D), naphthaleneacetic acid (NAA), benzyladenine (BA) and indolacetic acid (IAA).
Andrade-Rodríguez, López-Peralta, González-Hernández, García-Velázquez, and Peña-Lomelí (2005) studied the in vitro induction and elongation capacity, in vivo rooting, acclimatization and chromosomal stability of plants from 10 Physalis ixocarpa Brot. varieties, including two wild ones. In addition, in vitro regenerated plants were brought to the acclimatization stage in a greenhouse using sterile substrate composed of black soil and leaves (1:3 ratio) in pots covered with 1 L transparent plastic cups.
It is essential to evaluate the different tomatillo varieties to know their regeneration response in in vitro culture, as well as to adapt the culture medium to the genotypes of agronomic interest for a breeding program; this is because in vitro regeneration is mainly determined by the genotype of the plants. This influence has been observed in the response to organogenesis of four cultivars of Dianthus caryophyllus L. (Kallak, Reidla, Hilpus, & Virumäe, 1997) and in embryogenesis induction in microspore culture of 50 different genotypes of Capsicum annuum L., using growth regulators and activated charcoal (Cheng, Ma, Jiao, Quiao, & Li, 2013).
The acclimation stage is the most critical for plants obtained in vitro, as it determines the success of total plant survival to transplanting under greenhouse or field conditions. The stomata of in vitro-grown plants are unable to close when first removed from in vitro culture, resulting in excessive evapotranspiration after transplantation under ex vitro conditions (Drew, Kavanagh, & Maynard, 1992). This causes plants in ex vitro conditions to wilt and possibly even die from water loss. To avoid this, a suitable transitional environment applied to acclimatization is provided over a period of one to several weeks (Grout & Millam, 1985). In this transitional environment, the relative humidity should be maintained in a range of 70 to 100 % by means of a misting system (Teixeira-da Silva et al., 2017).
Based on the above, the present work was carried out with the objective of evaluating the response of select families from three tomatillo varieties to in vitro culture, as well as establishing and evaluating the protocol for acclimatization of plants from in vitro culture, and studying the phenology of the plants obtained by this means.
Materials and methods
The work consisted of two stages, and was carried out in the Tissue Culture Laboratory of the Plant Science Department and in the greenhouses of the Experimental Agricultural Field of the Universidad Autónoma Chapingo. In the first stage, axillary buds and apices were obtained from select plants cloned in advance and acclimatized in a greenhouse, which were established under aseptic conditions for micropropagation and evaluation in the Tissue Culture Laboratory. In the second stage, the clones obtained were transferred to a greenhouse for acclimatization in a plastic tunnel with constant relative humidity, after which they were transplanted in the greenhouse into pots with tezontle irrigated with Steiner's (1984) nutrient solution at 100 %.
Botanical seeds were established from 12 maternal half-sib families (MHSF) derived from three generations of fraternal crosses of the Diamante, Tecozautla 04, Manzano Tepetlixpa and Morado San Miguel varieties, three families for each variety. The families were provided by the Tomatillo Breeding Program of the Universidad Autónoma Chapingo. The 12 original families were obtained through a mass selection cycle and a family selection one (Peña-Lomelí & Márquez, 1990). The 12 select families were sown in spring 2017 in 200-cavity polystyrene trays with peat moss as substrate. After five weeks, they were transplanted into 54 pots per family with two plants per pot, which were kept in a greenhouse. Twenty days after transplanting, a selection was made based on the best individual per pot. Data on number of flowers, number of fruits, vigor and health were collected from all plants, which were only used as criteria to select the 10 best individuals per family.
From the best individuals of each of the 12 MHSF established by seed, axillary buds and stem apices were taken for disinfestation, micropropagation in the Tissue Culture Laboratory, and subsequent acclimatization and transplanting in the greenhouse. From these cloned plants, axillary buds and apices of healthy plants of the Tecozautla 04, Manzano Tepetlixpa and Morado San Miguel varieties were collected in December 2017. Of the original clones, due to unfavorable responses in in vitro culture, only clones of the varieties Tecozautla (one from family 2 and one from family 3), Morado San Miguel (two different clones from family 2, two different clones from family 3 and one clone from family 1) and Manzano Tepetlixpa (one clone from family 2) were obtained. That is, eight clonal families were studied, which was due to the fact that not all of the original families had a favorable response to in vitro culture.
The bud disinfection treatment consisted of washing vigorously with detergent and Tween 20, carefully separating apices and buds. The explants were then immersed in a solution of alcohol diluted in 70 % water for 3 min, then transferred to a solution of sodium hypochlorite (Cloralex®) diluted in 7% water for 15 min, then rinsed three times in sterile water and, finally, in vitro seeding was performed.
For multiplication of apices and buds, culture medium with 100 % Murashige and Skoog (1962) inorganic salts was used, supplemented with 0.4 mg·L-1 thiamine, 60 mg·L-1 L-cysteine, 100 mg·L-1 myo-inositol, 0.5 mg·L-1 nicotinic acid, 0.5 mg·L-1 pantothenic acid, 3 % sucrose and 7 g·L-1 agar. The culture medium was adjusted to a pH of 5.7 ± 0.1 and poured into clear glass canning jars with a 460 mL volume (78.3 mm in diameter and 134.95 mm in height) and a twist-off screw cap. Fifty mL of culture medium were added to each jar. The jars, scalpels, scissors, crystals and water were sterilized in an autoclave for 20 min at 121 ° C and 1.5 kg·cm-2 pressure. Subsequently, with the explants disinfected and the materials sterilized, the explants were seeded in a laminar flow hood. Once the buds and apices were seeded in the jars, they were transferred to the laboratory culture area for 30 days, with 16 h of light and 3,000 µmol⋅m-2·s-1 illumination.
For acclimatization, peat moss was used as substrate, which was sterilized and deposited in 240 mL Styrofoam cups. At this stage, the seedlings were removed from the in vitro culture vessels and rinsed with water; data were then collected and the seedlings were transplanted into the cups. Afterwards, the cups were placed in a mini-greenhouse with a misting system comprising eight irrigations per day, with a 1 h interval between irrigations of 5 min. With this misting system, a relative humidity of 60 to 100 % and an average temperature of 25 °C were maintained. The entire acclimatization system remained active for 15 days.
Once the seedlings were acclimatized, they were transplanted in the greenhouse in 18 L black polyethylene pots, with tezontle as substrate. The pots were drip irrigated with 100 % Steiner solution (Steiner, 1984). Data were collected every two weeks.
To assess the response to micropropagation through rooting in the laboratory, a completely randomized experimental design was used. The experimental unit consisted of three plants per vessel, with four replications per family. Data were recorded for in vitro plant height (cm), stem vigor (ordinal scale 1, 2, 3), callus presence (binomial proportion), health (binomial proportion), root length (cm), and number of leaves, roots, stems and buds.
To evaluate acclimatization, a randomized complete block design with four replications was established, where the experimental unit consisted of seven plants. Data on plant height (cm) and number of leaves, buds and flowers were recorded, and the percentage of acclimatization was determined. To study the phenology of the clones transplanted in the greenhouse, a randomized complete block design with four replications was used. The experimental unit was seven plants, and the data recorded were plant height and number of leaves, flowers and set fruits.
With the data obtained, analysis of variance and Tukey's mean comparison tests (P ≤ 0.05) were performed for the variables in the three stages, except for seedling vigor (where the Kruskall-Wallis test was used), and for callus presence and health (where the comparison of binomial proportions was made; P ≤ 0.05).
Results and discussion
The in vitro plant height of the eight clonal families showed a slight difference among them, with the greatest heights in families 2 from Tecozautla 04 and Manzano Tepetlixpa, and family 3a from Morado San Miguel. The lowest values were from family 1 of Morado San Miguel and family 3 of Tecozautla 04 (Table 1). Plants from family 2b of the Morado San Miguel variety presented greater height, as did plants from clonal family 2 of the Tecozautla 04 variety (Figures 1a and 1b).
Table 1 Comparison of means of plant height, root length and number of leaves, roots, stems, and buds of plants of tomatillo (Physalis ixocarpa Brot. ex Horm.) plants in vitro.
| Variety | Family | PH | NL | RL | NR | NS | NB |
|---|---|---|---|---|---|---|---|
| Morado San Miguel | 1 | 4.16 bz | 7.3 b | 1.86 c | 9.6 b | 1.6 a | 1.4 ab |
| Morado San Miguel | 2 a | 8.26 ab | 5.5 b | 15.76 a | 29.9 b | 1.1 ab | 0.4 c |
| Morado San Miguel | 2 b | 8.26 ab | 7.3 b | 7.27 bc | 26.0 b | 1.0 b | 0.3 c |
| Morado San Miguel | 3 a | 9.04 a | 5.4 b | 11.66 ab | 65.5 a | 0.9 b | 0.9 bc |
| Morado San Miguel | 3 b | 6.53 ab | 8.8 b | 5.51 bc | 34.7 b | 1.4 ab | 0.4 c |
| Manzano Tepetlixpa | 2 | 9.79 a | 8.3 b | 6.58 bc | 14.5 b | 1.0 b | 0.0 c |
| Tecozautla 04 | 2 | 10.5 a | 14.7 a | 10.63 ab | 22.1 b | 1.1 ab | 0.0 c |
| Tecozautla 04 | 3 | 4.45 b | 5.3 b | 8.53 bc | 20.5 b | 1.2 ab | 2.1 a |
| LSD | 4.38 | 4.9 | 6.88 | 27.6 | 0.6 | 1.0 |
PH = plant height (cm); NH = number of leaves; RL = root length (cm); NR = number of roots; NT = number of stems; NB = number of buds. LSD = least significant difference. zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
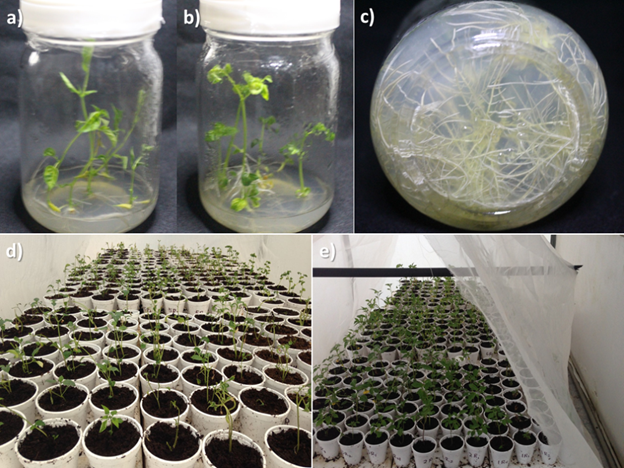
Figure 1 Plants of the clonal families grown in vitro. a) Plant from family 2b of Morado San Miguel, b) plant from family 3 of Tecozautla 04, c) root system of family 3 of Tecozautla 04, d) plants grown in vitro at the beginning of acclimatization and e) plants grown in vitro after 15 days of acclimatization.
For number of leaves, family 2 from the Tecozautla 04 variety had the greatest quantity in relation to the other families, among which there were no significant differences in this variable (Table 1). On the other hand, the longest root length was obtained by family 2a from Morado San Miguel, and the lowest value was obtained by family 1 from the same variety (Table 1). Family 3a, of the same variety, presented the greatest root development, and in the other families, the number of roots was not significantly different (Table 1). The root system of the plants developed to a greater or lesser extent depending on the family and variety. Plants from clonal family 3 of the Tecozautla 04 variety showed vigorous root development (Figure 1c).
The highest number of stems developed per explant corresponded to family 1 from Morado San Miguel; however, there was no significant difference in relation to families 2a and 3b of the same variety, and to both families from Tecozautla 04. Families 3a and 2b from Morado San Miguel, and family 2 from Manzano Tepetlixpa, presented the lowest number of stems (Table 1).
Family 3 from the Tecozautla 04 variety had the highest number of buds, but without significant difference in relation to family 1 from Morado San Miguel. The other families presented a lower number of buds, with no significant differences among them (Table 1).
The plants that developed the greatest stem vigor corresponded to family 2 from Tecozautla, with no significant difference with family 2 from Manzano Tepetlixpa. Families 1 from the Morado San Miguel variety and 3 from Tecozautla 04 developed the least stem vigor (Table 2).
Table 2 Kruskall-Wallis test for stem vigor and comparison of binomial proportions for callus presence and health in tomatillo (Physalis ixocarpa Brot. ex Horm.) plants regenerated in vitro.
| Variety | Family | R(x) | Callus | Health |
|---|---|---|---|---|
| Morado San Miguel | 1 | 23.08 ey | 0.667 abz | 0.417 ab |
| Morado San Miguel | 2 a | 49.21 c | 0.167 cd | 0.583 a |
| Morado San Miguel | 2 b | 47.00 cd | 0.333 bc | 0.250 abc |
| Morado San Miguel | 3 a | 53.23 bc | 0.909 a | 0.182 bcd |
| Morado San Miguel | 3 b | 41.67 cd | 0.833 a | 0.083 cd |
| Manzano Tepetlixpa | 2 | 66.96 ab | 0.750 a | 0.000 d |
| Tecozautla 04 | 2 | 71.83 a | 0.000 d | 0.000 d |
| Tecozautla 04 | 3 | 31.46 de | 0.333 bc | 0.167 bcd |
| Tc | 34.43 ** |
Tc = Kruskall-Wallis test statistic; R(x) = average rank for stem vigor; Callus = binomial proportion of callus presence; Health = binomial proportion of healthy plants. yRanks with the same letter are not different (Kruskall-Wallis, P ≤ 0.05). zProportions with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05). **Significant with P ≤ 0.01.
Explants from families 3a and 3b of Morado San Miguel variety, and 2 of Manzano Tepetlixpa, developed more callus, while families 2 of Tecozautla 04 and 2a of Morado San Miguel showed less callus. Regarding health, plants from families 1, 2a and 2b of Morado San Miguel presented more disease problems. Families 2 of Manzano Tepetlixpa, 2 of Tecozautla 04, and 3b and 3a of Morado San Miguel had no health problems (Table 2).
The response to the number of roots increased successfully in vitro with the 100 % MS medium without the addition of growth regulators, and varied depending on the genotype. This coincides with what was reported by Manzo-González et al. (1998), who were able to induce root formation with 100 % MS medium and without growth regulators in the Rendidora, Salamanca and Tamazula varieties of tomatillo, where they obtained different numbers of roots among varieties. Andrade-Rodríguez et al. (2005), when rooting in vivo 10 varieties of tomatillo from in vitro culture in 100 % MS without the addition of growth regulators, obtained different numbers and lengths of roots according to the genotype used.
The differentiated responses to in vitro culture obtained among the clonal families of the three varieties were a function of the particular genotype of each one. This was demonstrated by Andrade-Rodriguez et al. (2005), who observed that the in vitro propagation capacity of tomatillo varied according to the genotype of the variety used. Muktadir, Habib, Mian, and Akhond (2016) also found different responses in in vitro culture of five varieties of Solanum melongena L. with respect to the number of shoots regenerated per explant and their rooting.
At the beginning of acclimatization, family 1 from Morado San Miguel presented the lowest height compared to the other families, among which there were no significant differences (Table 3). After two weeks under acclimatization, families 1 and 3b from the Morado San Miguel variety and 2 from Tecozautla 04 showed the lowest height (Figure 2a). In general, the clonal families with the greatest height at the beginning of acclimatization maintained it during the entire period evaluated.
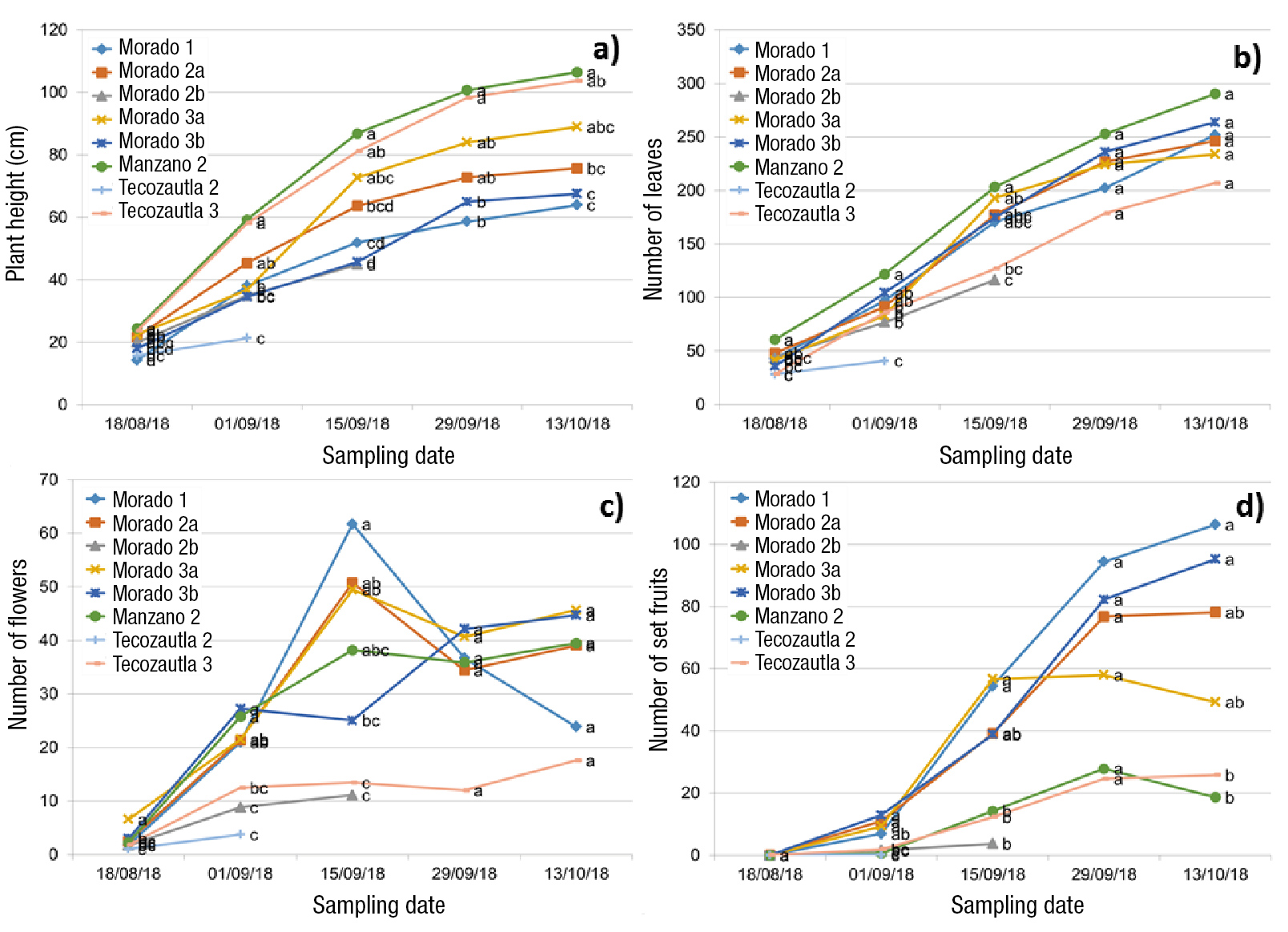
Figure 2 Comparison of means between clonal families of three varieties of tomatillo (Physalis ixocarpa Brot. ex Horm.) under a greenhouse for 75 days after acclimatization. a) Plant height, b) number of leaves, c) number of flowers and d) number of set fruits. Means with the same letter, for each date and variable, do not differ statistically (Tukey, P ≤ 0.05).
Regarding the number of leaves, at the beginning of acclimatization, family 3b from Morado San Miguel had the most leaves, and family 3 from Tecozautla had the lowest number of leaves (Table 3). After acclimatization, the highest number of leaves corresponded to family 1 from Morado San Miguel, and the lowest number of leaves was for family 2 from the Tecozautla 04 variety (Figure 2b).
Table 3 Comparison of means of plant height and number of leaves, flowers, buds and stems taken from tomatillo (Physalis ixocarpa Brot. ex Horm.) plants regenerated in vitro for acclimatization.
| Variety | Family | R(x) | Callus | Health |
|---|---|---|---|---|
| Morado San Miguel | 1 | 23.08 ey | 0.667 abz | 0.417 ab |
| Morado San Miguel | 2 a | 49.21 c | 0.167 cd | 0.583 a |
| Morado San Miguel | 2 b | 47.00 cd | 0.333 bc | 0.250 abc |
| Morado San Miguel | 3 a | 53.23 bc | 0.909 a | 0.182 bcd |
| Morado San Miguel | 3 b | 41.67 cd | 0.833 a | 0.083 cd |
| Manzano Tepetlixpa | 2 | 66.96 ab | 0.750 a | 0.000 d |
| Tecozautla 04 | 2 | 71.83 a | 0.000 d | 0.000 d |
| Tecozautla 04 | 3 | 31.46 de | 0.333 bc | 0.167 bcd |
| Tc | 34.43 ** |
PH = plant height (cm); NL = number of leaves; NF = number of flowers; NB = number of buds; NS = number of stems. LSD = least significant difference. zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
The highest number of buds was presented by families 3a and 2b of the Morado San Miguel variety, and the fewest buds were from families 1 and 3b of Morado San Miguel, 2 and 3 of Tecozautla 04, and 2 of Manzano Tepetlixpa (Table 3). After acclimatization, all families presented a number of buds that were not significantly different from each other (Figure 2c).
Regarding the number of flowers before acclimatization, family 2a from Morado San Miguel had the most flowers, and the only families that did not present flowers were 1 and 3b of the same variety (Table 3). After acclimatization, the family that developed the most flowers was family 3a from Morado San Miguel, and family 2 from Tecozautla 04 had the lowest number of flowers (Figure 2d). The family with the highest number of stems was family 1 from Morado San Miguel, although it only significantly surpassed family 3 from Tecozautla, which had the lowest value (Table 3).
At the beginning of the acclimatization stage, the plants showed excessive transpiration (Figure 1d) due to the anatomical conditions of the leaves from the in vitro culture. However, for all clonal families of the three varieties, 100 % survival was obtained, with excellent response to the established protocol and to the control of relative humidity by misting (Figure 1e). This coincides with the results of Andrade-Rodríguez et al. (2005), who obtained an equal survival percentage when acclimatizing eight improved and two wild varieties of Physalis ixocarpa Brot. Manzo-González et al. (1998) also achieved 100 % survival in the acclimatization of tomatillo plants of the Rendidora, Salamanca, and Tamazula varieties obtained in vitro.
The success achieved in acclimatization was due, firstly, to the induction of an acceptable functional root system in vitro in all clonal families (Table 1). A good root system is essential for guaranteeing a high percentage of survival in ex vitro conditions after transplanting (Teixeira-da Silva et al., 2017). This is because roots generated in vitro continue their growth during the ex vitro acclimatization process (Clapa, Fira, & Joshee, 2013; George & Debergh, 2008). Secondly, the maintenance of high relative humidity and its gradual decrease enhance the adaptation process of morphology and physiology, mainly of the leaves. This was demonstrated by Shekhawat, Kannan, Manokari, and Ravindran (2015), who by acclimatizing Passiflora foetida L. plants from in vitro culture, maintaining relative humidity at 100 % for the first two weeks and decreasing it for the subsequent two weeks achieved 100 % survival after acclimatization.
The phenological development of the cloned plants of the evaluated varieties showed a differentiated behavior. The Morado San Miguel variety had a prostrate or creeping growth, while Tecozautla 04 and Manzano Tepetlixpa had an erect growth. At the beginning of the transplant to the greenhouse, the plants from acclimatization showed good height (Figures 3a and 3b).

Figure 3 Acclimatized plants grown under greenhouse conditions. a) Plant from family 2b of Morado San Miguel, b) plant from family 2 of Manzano Tepetlixpa, c) flowers from family 1 of Morado San Miguel at 75 days after acclimatization, d) fruits from family 2b of Morado San Miguel, e) fruit from the Tecozautla 3 x Manzano Tepetlixpa 2 interclonal cross and f) fruit from the Morado San Miguel 1 x Morado San Miguel 2a interclonal cross.
For the plant height variable, family 2 from Manzano Tepetlixpa had the highest height, and families 1 and 3b from Morado San Miguel the lowest value during the evaluation (Figure 2a). In addition, it was observed that the increase in plant height in all families did not decrease during the evaluated phenological cycle. It should be noted that at the end of the cycle, families 2 from Tecozautla 04 and 2b from Morado San Miguel were eliminated due to the presence of phytopathogens.
Regarding the number of leaves, at the beginning of the evaluation, family 1 from the Morado San Miguel variety presented more leaves, and family 2 from Tecozautla 04 had the lowest number of leaves. However, at the end of the evaluation, the number of leaves among the families of the three varieties was not significantly different (Figure 2b). The increase in the number of leaves continued until the last weeks of the evaluation, a phenomenon that does not occur in plants from seed; this is in accordance with the four phenological phases of the tomatillo described by Cartujano, Fernández, and Jankiewics (1987). The increase was probably due to a greater juvenility of the plants obtained in vitro. It would be advisable to jointly study the phenology of plants from seed and cloned in vitro.
For the number of flowers, at the beginning of the evaluation, family 3a from Morado San Miguel presented the highest value, and family 2 from Tecozautla 04 the lowest. At the end of the evaluation, families 3 from Tecozautla 04 and 1 from Morado San Miguel had the lowest number of flowers in relation to the other families (Figures 2c and 3c). At the beginning of the cycle, the number of flowers increased until week 10, but from then on there was a decrease in the formation of buds and flowers, which is due to the continuous growth of generative organs that cause competition among them (Cartujano et al., 1987).
In relation to the variable number of set fruits, families 1 and 3b from the Morado San Miguel variety presented the highest values, while families from Tecozautla 04 and Manzano Tepetlixpa developed fewer set fruits (Figures 2d and 3d). This is mainly due to the growth habit, since creeping-type varieties have a greater number of set fruits than those with erect growth, so they are slightly more productive (Cartujano et al., 1987; Peña-Lomelí, Ponce-Valerio, Sánchez-del Castillo, & Magaña-Lira, 2014).
From the above, it can be affirmed that the evaluated variables and their expression are mainly due to the growth habit of each variety. Regarding the number of set fruits and flowers, the Morado San Miguel families expressed the highest value, this as a result of a higher level of branching present due to their creeping habit, which means that they produce more fruits, but of smaller size (Cartujano et al., 1987; Peña-Lomelí et al., 2014). Regarding height and the number of leaves and flowers, it can be confirmed that plants from in vitro culture have a lower value compared to plants from seed. Nevertheless, the life cycle of the plants obtained by in vitro propagation lasted about 98 days, from the in vitro culture in the laboratory to the development of the first fruits.
The propagation process included the stages of regeneration, rooting, acclimatization and development under greenhouse conditions, which, in a certain way, coincides with the life cycle of plants from seed (Mulato, Fernández, & Jankiewics, 1987). The first two stages took place in the laboratory. Regeneration lasted two weeks (Figures 1a and 1b), and rooting took two weeks (Figure 1c). Subsequently, the clones were taken to the greenhouse, where acclimatization lasted another two weeks (Figures 1d and 1e); at this stage, the first buds and flowers appeared. After transplanting under greenhouse conditions (Figure 3a), vegetative (Figure 3b) and reproductive (Figure 3c) development occurred during eight weeks, where pollination and fruit development comprised the last six weeks (Figure 3d).
The vigor expressed by the plants obtained in vitro, compared to those from seed, may be due to the anatomical and physiological changes they underwent, mainly in the leaves, in the transition stage from in vitro to ex vitro conditions, which causes slow development (Driver & Suttle, 1986). However, manually controlled pollination was performed (Peña-Lomelí, Magaña-Lira, Gámez-Torres, Mendoza-Celino, & Pérez-Grajales, 2018) for the cross between five clonal families, resulting in normal fruit set and interclonal hybrid seed production (Figure 3e and 3f).
Conclusions
The in vitro culture and acclimatization protocol was successfully established, in which a survival rate of 100 % was obtained in all the clonal families of the three varieties.
The response to in vitro culture of the clonal families depended on the genotype, with the Tecozautla 04 and Manzano Tepetlixpa varieties standing out, and the Morado San Miguel variety having a lower response.
The phenology of the clonal families showed normal development, similar to the phenology of seed plants, where the complete cycle from in vitro culture to seed production lasted 14 weeks.











 texto en
texto en 



