Introduction
Carnation (Dianthus caryophyllus L.) is one of the most popular cut flowers in the world, and after rose (Rosa spp.), is the second best-selling cut flower in the world (Jawaharlal, Ganga, Padmadevi, Jegadeeswari, & Karthikeyan, 2009; Villanova et al., 2017). In 2014, this cut flower ranked 14th among the 25 best-selling ornamental plants in a Dutch auction with a turnover of 25 million euros (Niyokuri, Nyalala, & Mwangi, 2017).
Carnation’s short postharvest life is one of the main challenges facing the cut flower industry, since it only lives for 7 days after harvest (Amini, Arab, Rahemi, Rahimi, & Daraei-Garmakhni, 2016; Teixeira-da Silva, 2003). Aging and quality of cut flowers are affected by various factors such as water stress, microorganism activity and carbohydrate deficiency (Amini et al., 2016; Hunter, Yi, Xu, & Reid, 2004). Various chemical compounds such as 8-hydroxyquinoline sulfate (8-HQS), silver thiosulfate (STS), 1-methylcyclopropene (1-MCP), ethanol, methanol, and others delay aging and extend vase life (Farokhzad, Khalighi, Mostofi, & Naderi, 2005; Muraleedharan, 2020).
One of the most commonly used commercial salts for the preservation of cut flowers is 8-HQS, which has a strong bactericidal effect (Dong, Seaton, & Singh, 2017). Pun, Rowarth, Barnes, and Heyes (2001) reported that, in cut carnation flowers, sucrose treatment with 8-HQS prevented microbial activity and extended flower lifetime after harvest. In an experiment on cut rose flowers, it was found that 8-HQS increased vase life (Liao, Lin, Huang, Chen, & Cheng, 2000).
Alcohols are among the preservatives for cut flowers and inhibitors of ethylene synthesis, by preventing the synthesis of 1-amino-cyclopropanol-1-carboxylic acid (ACC) (van Doorn, 2002). In Eustoma grandiflora, the use of 2 % ethanol with 2.5 % sucrose had the greatest effect on increasing the vase flowering life of this cut flower (Farokhzad et al., 2005). It has been shown that 4 % ethanol in a vase solution is an effective ingredient in postharvest preservation of Dianthus and that it prevents ethylene activity (Pun et al., 2001). This treatment significantly increased vase life by 10 days. Petridou, Voyiatzi, and Voyiatzis (2001) and Yaghoubi-Kiaseh and Yadegari (2016) observed an approximately 5-day increase in vase life in chrysanthemum and Alstroemeria due to the use of 2 % ethanol.
Despite the role of the aforementioned chemicals in prolonging postharvest life, there is a problem with their use, as most of them are harmful to human health and that of other organisms. For this reason, it is necessary to identify and use natural compounds to maintain the postharvest quality of cut flowers (Amini et al., 2016; Teixeira-da Silva, 2003).
Essential oils are effective, safe and degradable natural compounds produced by some plants (Solgi, Kafi, Taghavi, & Naderi, 2009; Hashemabadi, Abedini-Aboksari, Sedaghathoor, & Kaviani, 2016). Due to high concentrations of phenol compounds, these compounds have antimicrobial properties which reduce the amount of bacteria in the vase solution and stem base, preventing vessel obstruction (Bounatirou et al., 2007; Solgi & Ghorbanpour, 2014). They also have antioxidant properties (Solgi et al., 2009).
Recent experiments have shown that the use of herbal essences improves quality and vase life due to their antimicrobial and antioxidant properties (Bounatirou et al., 2007; Hashemabadi et al., 2016). Oraee, Asgharzadeh, Kiani, and Oraee (2011) found that the use of thymol prolongs the vase life of Gerbera by 5 days compared to the control. Jalili-Marandi, Hassani, Abdollahi, and Hanafi (2011) stated that the effect of essential oils of Carum copticum and Saturega hortensis can be attributed to their anti-bacterial property, which reduced bacterial replication in the rose flower vessels. Considering the above, the objective of this study was to compare the effect of the essential oil of geranium, caraway and dill against chemical 8-HQS treatments and alcohol on postharvest life, bacterial control and some qualitative characteristics of cut Dianthus caryophyllus L. cv ‘Yellow Candy’ flowers.
Materials and methods
Cut Dianthus caryophyllus L. cv ‘Yellow Candy’ flowers were obtained from a greenhouse located in Tehran, Iran, and transferred to the postharvest laboratory at Islamic Azad University, Rasht, Iran, immediately after harvest. Five flowering stems, cut to 40 cm in height, were placed in 500 mL vases, which were placed in a room with a temperature of 20 ± 2 °C and relative humidity of 65 ± 5 %. Light intensity in the room was 15-20 µm·s-1·m-2 with a light period of 12 h, which was supplied from a white fluorescent light source.
The experiment was carried out using a completely randomized design with 14 treatments: 8-HQS at three concentrations (100, 200 and 400 mg·L-1), geranium, caraway and dill essential oils at three concentrations (50, 100 and 150 mg·L-1), a control solution with alcohol (2 %) and a control solution with distilled water (Table 1). The trial was conducted in triplicate, which generated 42 experimental plots with five cut flowers each (210 flowers in total). Distilled water was utilized to prepare essential oils from geranium, caraway and dill; the compounds were analyzed by gas chromatography/mass spectrometry (GC/MS) (Tables 2, 3 and 4).
Table 1 Concentrations of essential oils and chemical treatments.
| Symbol | Treatments |
|---|---|
| Control | 500 mL distilled water |
| A2 % | Alcohol (2 %) |
| 8-HQS100 | 8-Hydroxyquinoline sulfate (100 mg·L-1) |
| 8-HQS200 | 8-Hydroxyquinoline sulfate (200 mg·L-1) |
| 8-HQS400 | 8-Hydroxyquinoline sulfate (400 mg·L-1) |
| DEO50 | Dill essential oil (50 mg·L-1) |
| DEO100 | Dill essential oil (100 mg·L-1) |
| DEO150 | Dill essential oil (150 mg·L-1) |
| GEO50 | Geranium essential oil (50 mg·L-1) |
| GEO100 | Geranium essential oil (100 mg·L-1) |
| GEO150 | Geranium essential oil (150 mg·L-1) |
| CEO50 | Caraway essential oil (50 mg·L-1) |
| CEO100 | Caraway essential oil (100 mg·L-1) |
| CEO150 | Caraway essential oil (150 mg·L-1) |
Table 2 Analysis of geranium essential oil.
| Number | Compounds | Percentage | KI index |
|---|---|---|---|
| 1 | Spathulenol | 0.67 | 1656 |
| 2 | 6-octen-1-ol,3,7-dimethyl | 0.15 | 1543 |
| 3 | Alpha-pinene | 0.12 | 955 |
| 4 | Beta-citronellol | 2.90 | 1358 |
| 5 | 1H-cycloprop[e]azulene | 0.12 | 1459 |
| 6 | 1H-cyclopropa[a]naphthalene | 0.10 | 1597 |
| 7 | Beta-bourbonene | 0.94 | 1447 |
| 8 | Beta-cubebene | 0.78 | 1534 |
| 9 | Cadina-1,4-diene | 0.15 | 1590 |
| 10 | cis-2,6-dimethyl-2,6-octadiene | 4.81 | 2019 |
| 11 | Germacrene-D | 2.87 | 1549 |
| 12 | cis-rose oxide | 0.81 | 1128 |
| 13 | Delta cadinene | 0.42 | 1495 |
| 14 | Epizonarene | 0.84 | 1583 |
| 15 | 6-octen-1-ol,3,7-dimethyl-(R) | 0.16 | 1266 |
| 16 | Cycloundecatriene-4,7,10 | 1.64 | 1520 |
| 17 | Gamma-elemene | 1.17 | 1647 |
| 18 | Delta-cadinene naphthalene | 0.44 | 1563 |
| 19 | Citral | 0.61 | 1305 |
| 20 | Naphthalene | 1.20 | 1610 |
| 21 | 3,7-guaiadiene | 0.32 | 1495 |
| 22 | Geraniol | 13.03 | 1293 |
| 23 | Linalool | 1.60 | 1114 |
| 24 | Cyclohexanone | 5.50 | 1202 |
| 25 | Butanoic acid | 4.70 | 2064 |
| 26 | 6-octen-1-ol | 8.50 | 1455 |
| 27 | Alpha-amorphene | 1.77 | 1528 |
| 28 | Geranyl tiglate | 3.24 | 1202 |
| 29 | Isoaromadendrene epoxide | 0.19 | 1743 |
| 30 | Caryophyllene oxide | 2.32 | 1668 |
| 31 | Geranyl propionate | 0.26 | 1965 |
| 32 | L-(-)-methyl | 0.10 | 1222 |
| 33 | 1,6-octadien-3-ol,3,7-dimethyl | 0.65 | 1275 |
| 34 | 1,6-octadien-3-ol,3,7-dimethyl (R) | 7.93 | 1293 |
| 35 | E-citral 2,6-octadienal, 3,7 | 0.67 | 1305 |
| 36 | Alpha-copaene | 1.10 | 1427 |
| 37 | 4,7,10-cycloundecatriene | 1.64 | 1520 |
| 38 | 1,2 benzenedicarboxylic acid | 0.32 | 2006 |
| 39 | Citronella | 0.51 | 1167 |
| 40 | Trans-rose oxide | 0.30 | 1149 |
| 41 | Alpha-amorphene | 0.77 | 1528 |
Table 3 Analysis of caraway essential oil.
| Number | Compounds | Percentage | KI index |
|---|---|---|---|
| 1 | α-Phellandrene | 0.27 | 950 |
| 2 | Alpha-pinene | 0.74 | 883 |
| 3 | Sabinene | 0.75 | 1006 |
| 4 | Beta-pinene | 1.32 | 1003 |
| 5 | Beta-myrcene | 0.56 | 1076 |
| 6 | α-Terpinene | 0.25 | 1207 |
| 7 | p-Cymene | 7.11 | 1249 |
| 8 | Limonene | 3.53 | 1269 |
| 9 | 1,8 cineol | 0.10 | 1282 |
| 10 | Gamma-terpinene | 21.86 | 1416 |
| 11 | α-Terpineol | 0.38 | 1534 |
| 12 | trans-sabinene hydrate | 0.14 | 1618 |
| 13 | Linalool | 0.10 | 1608 |
| 14 | 4-terpineol | 0.86 | 2035 |
| 15 | Thymol | 0.10 | 2081 |
| 16 | Cyclopentane | 2.20 | 2108 |
| 17 | Methyl-3-phenyl-2-propenal | 26.05 | 2073 |
| 18 | Felandral | 0.17 | 1913 |
| 19 | α-Thujenal | 11.66 | 2065 |
| 20 | Phenyl-1-butanol-4 | 20.72 | 2095 |
| 21 | Cyclohexane-1,4-dimethanol | 0.10 | 1272 |
Table 4 Analysis of dill essential oil.
| Number | Compounds | Percentage | KI index |
|---|---|---|---|
| 1 | Linalool | 52.23 | 1096 |
| 2 | Alpha-pinene | 19.96 | 1210 |
| 3 | Limonene | 4.83 | 1032 |
| 4 | p-Cymene | 4.72 | 1089 |
| 5 | Gamma-terpinene | 4.59 | 1055 |
| 6 | α-Terpinene | 4.01 | 1123 |
| 7 | p-Cymene | 1.52 | 1210 |
| 8 | Limonene | 1.10 | 1099 |
| 9 | 1,8 Cineol | 0.92 | 938 |
| 10 | Gamma-terpinene | 0.72 | 1024 |
| 11 | α-Terpineol | 0.40 | 1325 |
| 12 | Trans-sabinene hydrate | 0.35 | 1245 |
| 13 | α-Terpinene | 0.27 | 1015 |
After drying the samples, 50 g of the samples were extracted using the water distillation method and Clevenger apparatus. The extraction time of essential oil was the same for all samples, which was 3 h. After dehydration with sodium sulfate, the percentage and amount of essential oil were determined. A GC/MS gas chromatograph 5773 connected to a mass spectrometer equipped with an HPS column with a length of 30 cm, an inner diameter of 250 μm and a thickness of 25 mm of stationary phase layer was used to identify the essential oil compounds. The oven temperature increased from 45 to 250 °C at 5 °C·min-1 and then reached 280 at 20 °C·min-1. Helium gas with ionization energy of 70 electron volts was used. The obtained spectra were identified by comparison with the mass spectra of standard compounds.
GC-MS analysis was carried out on a GC-HP-6890 with an HP-5MS automatic injector and a non-polar Elite-5 fused silica capillary column (30 m, 0.35 mm (i.d.). Mass spectra were obtained by EI at 70 Ev. Oven temperature was 60 °C for 3 min and increased to 220 °C at the rate of 7 °C min. Injection volume was 0.5 µL at 1:200 split, and injector and detector temperature was 220 °C. The gas speed in column was 1 mm·min-1 and the type of carrier gas was He (99.999 %). Given the retention time of each combination, the components of the essential oils were identified by comparison of their mass spectra with those of digital library (Adams, 2007) and confirmed by their retention indices with data published in the literature.
The variables considered were vase life, solution absorption, stem end bacteria population, dry matter percentage, leaf chlorophyll content, petal carotenoids and malondialdehyde (MDA) content. In addition, peroxidase (POD) and catalase (CAT) activity was evaluated.
Vase life
The vase life of cut flowers from the beginning of treatment to flower aging (wilting and discoloration of petals) was evaluated daily. The average life of flowers was considered as their vase life until the first sign of wilting. Of the five flowers, three were used to measure pigments and enzymes, and two were used to assess vase life.
Absorption of the solution
This parameter was calculated using the following formula:
Four pots containing 500 mL of vase solution (without flowers) were placed in different parts of the room. At the end of the experiment (the end of the vase life of the last flower), the volume reduction of all four vessels was measured with a graduated cylinder and averaged.
Bacterial counting at the stem end
Bacterial sampling at the stem end was performed 24 h after the start of the test. Bacteria were counted using the method of Liu et al. (2009). The stem was cut off about 2 cm from the bottom. The samples were washed 3 times with deionized water until the germ level at the surface was reduced. Then the samples were completely pulverized and diluted with 0.9 % saline solution. Then 0.1 mL of the above solution was placed on nutrient agar and the colonies were counted 24 h after incubation at 37 °C.
Percentage of dry matter
At the end of vase life, the fresh weight (FW) of the flowers was measured; after that, the flowers were dried at 105 °C for 24 h. To ensure complete drying, cut flowers were weighed and then their dry matter percentage was calculated according to the following formula:
Leaf chlorophyll content
On the last day of vase life of the control treatment, a cut flower from each plot was extracted to measure chlorophyll and total chlorophyll content was calculated based on the following equation (Mazumdar & Majumder, 2003):
Chlorophyll a = 9.93(A663) - 0.777(A645)
Chlorophyll b = 22.9(A645) - 4.86(A633)
Total chlorophyll = chlorophyll a + chlorophyll b
where A is light absorbance at a wavelength of 663 and 645 nm.
Petal carotenoids
Petal carotenoid content was determined by extracting the petals with 80 % acetone and using the Mazumdar and Majumder (2003) method. They were then measured by means of a spectrophotometer at a wavelength of 665, 660, and 645 nm, and using the following formula the carotenoid value of petals was obtained. Petal carotenoids were measured as soon as the first signs of wilting were observed.
Malondialdehyde (MDA)
The concentration of MDA was measured using the Heath and Parker (1986) method. First, 1,000 μL 20 % TCA containing 0.5 % TBA was added to 500 μL of the extract. The resulting mixture was placed in a boiling water bath at 95 °C for 30 min and then immediately cooled in ice. The samples were then centrifuged at 10,500 g for 10 min. The red material containing MDA-TBA was measured at 532 nm with a spectrophotometer and the absorption of other specific pigments was read at 600 nm, after which this value was reduced. The concentration of MDA was expressed in nmol·g-1 FW.
Peroxidase enzyme activity (POD)
To measure the activity of POD (nmol·g-1 FW), the petals were isolated as soon as the first signs of wilting were observed and the enzyme was measured using the In, Motomura, Inamoto, Doi, and Mori (2007) method.
Catalase activity (CAT)
The activity of this enzyme was measured using the Chance and Maehly (1955) method with some changes. Measuring the activity of the CAT enzyme (µg·g-1 FW) was performed as soon as the first signs of wilting were observed by measuring the hydrogen peroxide destruction with a spectrophotometer (Aplle-PD-330V) at 240 nm.
Results
Figure 1 shows the comparison of carnation flower condition at the beginning and end of the experiment.
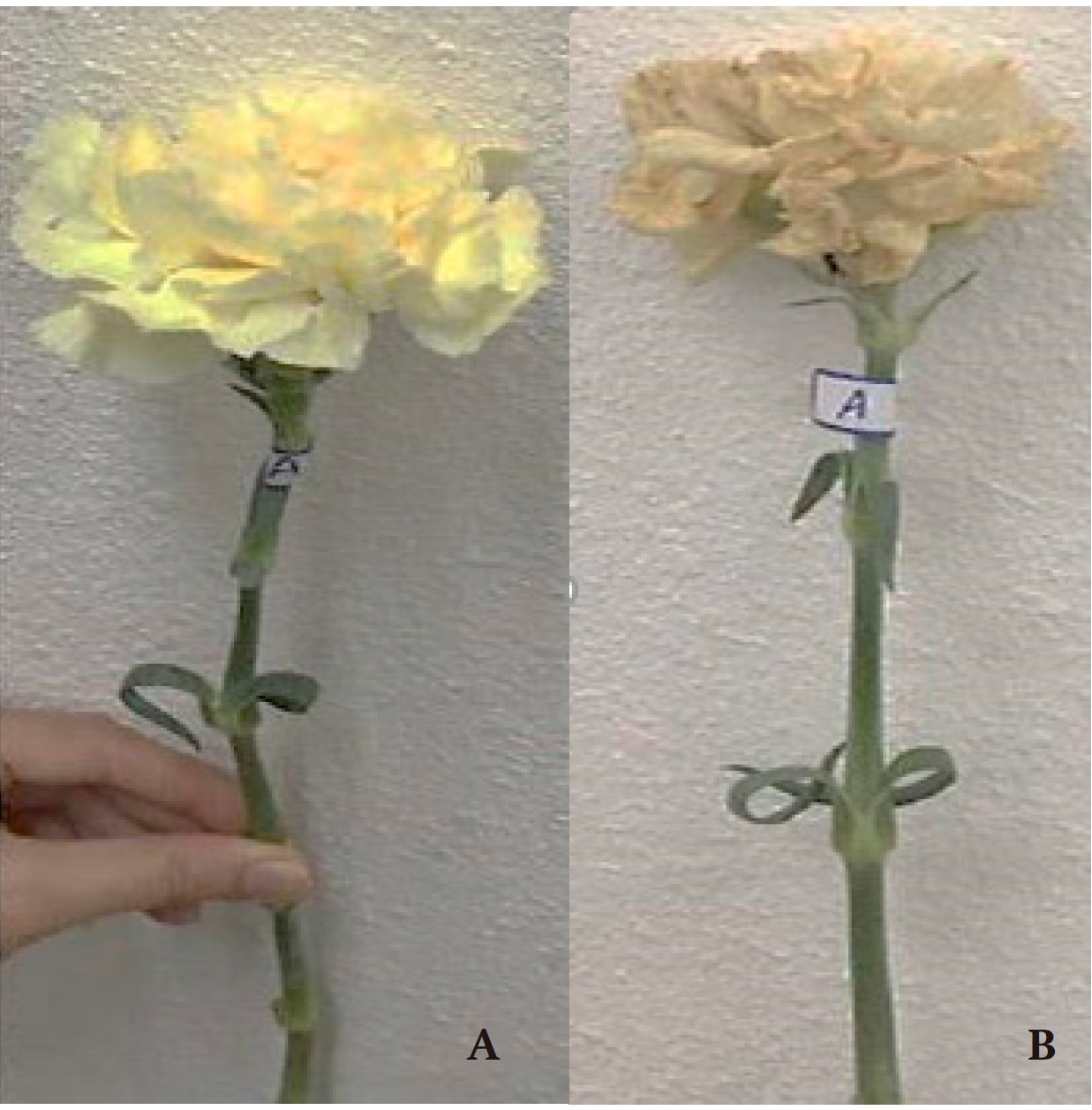
Figure 1 Comparison of cut flower of Dianthus caryophyllus L. cv ‘Yellow Candy’: A) first day of the experiment and B) last day of the experiment.
Vase life
The results of analysis of variance showed that there was a significant difference between the effect of treatments and most of the measured parameters (Table 5). Flower longevity increased in all treatments compared to the control (Table 6). The longest vase life (15.72 days) was observed in cut flowers treated with 2 % alcohol. In herbal essential oil treatments, 100 mg·L-1 dill essential oil (15.43 days), 50 mg·L-1 geranium essential oil (15.11 days), and 100 mg·L-1 caraway essential oil (14.51 days) had the best performance. Also, the 8-HQS treatment with a concentration of 400 mg·L-1 with an average of 14.82 days had a greater effect on vase life compared to the other concentrations of herbal essential oils (Table 6). Although there is no significant difference between these treatments, 50 mg·L-1 geranium essential oil is recommended due to the fact that this compound has less active ingredient and is more economical.
Table 5 Analysis of variance for the effect of different treatments on the measured traits of cut carnation (Dianthus caryophyllus L. cv ‘Yellow Candy’) flowers.
| Source of variations | DF | Vase life | Solution absorption | Bacteria in the stem end | Dry matter | Chlorophyll content | Petal carotenoid | Malondialdehyde | Peroxidase | Catalase |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | 13 | 8.92* | 1.72** | 1.85** | 30.28** | 10.20** | 2.22** | 5.52** | 0.10** | 0.20** |
| Error | 28 | 3.52 | 0.57 | 3.48 | 9.48 | 0.16 | 0.08 | 1.22 | 0.01 | 0.51 |
| CV (%) | 14.05 | 22.07 | 22.53 | 9.46 | 0.18 | 0.23 | 5.38 | 9.34 | 15.49 |
DF = degrees of freedom; CV = coefficient of variation; *, ** = significant at P ≤ 0.05 and P ≤ 0.01, respectively.
Table 6 Means comparison for the effect of different treatments on the measured traits of cut carnation (Dianthus caryophyllus L. cv ‘Yellow Candy’) flowers.
| Treatments | Vase life (day) | Solution absorption (mL·g-1 FW) | Bacteria in the vase solution (log10 CFU·mg-1) | Dry matter (%) | Chlorophyll content (mg·g-1 FW) | Petal carotenoid (µg·g-1 FW) | Malondialdehyde (nmol·g-1 FW) | Peroxidase (nmol·g-1 FW) | Catalase (µg·g-1 FW) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 9.73 ± 1.75 ez | 1.07 ± 0.30 e | 888.30 ± 132.28 a | 30.61 ± 2.56 cdef | 6.68 ± 0.01 g | 2.67 ± 0.01 m | 24.02 ± 1.33 a | 0.96 ± 0.11 e | 0.90 ± 0.14 e |
| A2 % | 15.72 ± 1.52 a | 2.26 ± 1.04 a | 776.60 ± 152.75 a | 36.05 ± 3.73 ab | 7.27 ± 0.01 e | 3.98 ± 0.01 e | 20.76 ± 0.23 b | 1.02 ± 0.05 d | 1.67 ± 0.01 ab |
| 8-HQS100 | 13.74 ± 0.28 abcd | 2.06 ± 0.73 ab | 88.30 ± 26.45 ef | 29.62 ± 3.97 ef | 6.12 ± 0.01 h | 4.96 ± 0.01 b | 20.12 ± 1.63 bc | 1.15 ± 0.01 cd | 1.64 ± 0.17 ab |
| 8-HQS200 | 13.55 ± 1.32 abcd | 1.52 ± 0.34 bcde | 70.00 ± 29.88 f | 38.16 ± 7.34 a | 8.44 ± 0.01 c | 4.49 ± 0.01 c | 20.25 ± 0.39 bc | 1.27 ± 0.02 bc | 1.32 ± 0.15 bcd |
| 8-HQS400 | 14.82 ± 0.76 abc | 2.02 ± 0.68 ab | 73.33 ± 26.45 f | 36.55 ± 6.21 ab | 11.30 ± 0.02 a | 3.34 ± 0.01 j | 21.29 ± 0.83 b | 0.91 ± 0.01 c | 1.09 ± 0.11 de |
| D.E.O50 | 12.08 ± 1.11 cde | 1.53 ± 0.59 bcde | 220.00 ± 25.16 cd | 32.13 ± 1.30 bcde | 5.95 ± 0.01 i | 3.55 ± 0.01 i | 20.24 ± 0.55 bc | 1.45 ± 0.53 ab | 1.14 ± 0.09 cde |
| D.E.O100 | 15.43 ± 3.77 ab | 2.18 ± 0.84 a | 173.30 ± 15.27 cde | 34.90 ± 7.12 abc | 9.95 ± 0.02 b | 3.71 ± 0.01 g | 19.73 ± 2.07 bcd | 1.15 ± 0.12 cd | 1.78 ± 0.40 a |
| D.E.O150 | 12.56 ± 3.58 bcde | 1.79 ± 0.40 abcd | 153.30 ± 25.16 def | 34.78 ± 2.10 abcd | 4.12 ± 0.01 i | 2.27 ± 0.01 n | 21.17 ± 1.23 b | 1.34 ± 0.27 ab | 1.65 ± 0.32 ab |
| G.E.O50 | 15.11 ± 3.46 abc | 1.88 ± 1.21 abc | 206.60 ± 28.88 cd | 32.87 ± 4.35 bcde | 7.44 ± 0.01d | 4.32 ± 0.01 d | 20.95 ± 1.73 b | 0.99 ± 0.22 de | 1.58 ± 0.04 ab |
| G.E.O100 | 12.48 ± 1.73 bcde | 1.65 ± 0.78 de | 266.60 ± 45.09 c | 29.68 ± 4.00 def | 6.72 ± 0.01 f | 3.68 ± 0.01 h | 19.88 ± 2.36 bcd | 1.06 ± 0.16 de | 1.58 ± 0.26 ab |
| G.E.O150 | 12.31 ± 3.17 bcde | 1.26 ± 0.82 cde | 156.60 ± 36.05 def | 32.01 ± 2.17 bcde | 6.66 ± 0.01 g | 3.86 ± 0.01 f | 21.11 ± 0.93 b | 1.05 ± 0.25 de | 1.57 ± 0.42 ab |
| C.E.O50 | 12.64 ± 2.38 bcde | 1.48 ± 0.47 bcde | 166.60 ± 191.39 def | 30.61 ± 2.62 cdef | 5.88 ± 0.01 j | 3.29 ± 0.01 k | 18.23 ± 0.77 d | 1.07 ± 0.32 de | 1.50 ± 0.02 abc |
| C.E.O100 | 14.51 ± 1.80 abc | 1.71 ± 0.68 abcd | 210.00 ± 11.87 cd | 30.80 ± 8.25 cdef | 4.91 ± 0.01 k | 5.54 ± 0.01 a | 18.68 ± 1.57 cd | 1.34 ± 0.34 ab | 1.65 ± 0.21 ab |
| C.E.O150 | 11.10 ± 2.29 de | 1.44 ± 1.04 bcde | 226.60 ± 25.16 cd | 26.81 ± 3.84 f | 6.12 ± 0.01 h | 3.23 ± 0.01 l | 20.86 ± 1.08 b | 1.51 ± 0.13 a | 1.34 ± 0.12 bcd |
z Means with the same letters within each column do not differ statistically (LSD, P ≤ 0.05).
Solution uptake
The means comparison of the data showed that all treatments had better performance compared to the control (Table 6). Flowers treated with 2 % alcohol and 100 mg·L-1 dill essential oil with an average of 2.26 and 2.18 mL·g-1 FW had the most solution uptake, respectively (Table 6). However, the essential oils of geranium (50 mg·L-1), dill (150 mg·L-1) and cumin (100 mg·L-1) had no significant difference with the best treatments. In addition, flowers treated with 100 and 400 mg·L-1 8-HQS also showed no significant difference in solution uptake compared to the other treatments (Table 6).
Bacterial populations of stem
All treatments reduced the bacterial population compared to the control. The best treatments were those of 200 and 400 mg·L-1 8-HQS, with a mean of 70 and 73.33 log10 CFU·mg·L-1, respectively. Among the essential oils, dill and geranium, at a concentration of 150 mg·L-1, had the lowest bacterial population (153.30 and 156.6 log10 CFU·mg·L-1, respectively); however, the treatment with 50 mg·L-1 caraway oil showed no significant difference with the previous ones (166.6 log10 CFU·mg·L-1) (Table 6).
Percentage of dry matter
Caraway essential oil (150 mg·L-1) with an average of 26.81 % had the lowest amount of dry weight compared to the control. Plants treated with 200 mg·L-1 8-HQS with an average of 38.16 % had the highest dry matter, although plants treated with 2 % alcohol, 400 mg·L-1 8-HQS, and 100 and 150 mg·L-1 dill essential oil did not show significant difference (Table 6).
Total chlorophyll
Different treatments had a significant effect on total chlorophyll content in cut flowers at the 1 % level (Table 5). Flowers treated with 400 mg·L-1 8-HQS (11.30 mg·g-1 FW) and 150 mg·L-1 dill essential oil (12.6 mg·g-1 FW) had the highest and the lowest leaf chlorophyll content, respectively (Table 6).
Carotenoids of petals
Different treatments had a significant effect on carotenoids in petals (Table 5). According to the means comparison of the data, the carotenoid value of petal was increased in all treatments except for 150 mg·L-1 dill essential oil, which showed the lowest amount compared to the control (2.27 µg·g-1 FW) (Table 6). The application of 100 mg·L-1 caraway essential oil had the highest carotenoid content of petals (54.5 µg·g-1 FW). The treatments with the longest vase life also showed the highest values in carotenoids in petals (Table 5).
Malondialdehyde (MDA)
Analysis of variance showed that there was a significant difference in the amount of MDA in cut flowers (P < 0.01) (Table 5). Means comparison showed that the control flowers had the highest amount of MDA (24.22 nmol·g-1 FW) and caraway essential oil treatment with the concentration of 50 mg·L-1 had the lowest amount (18.23 nmol·g-1 FW). However, the use of essential oils of dill, geranium and caraway with a concentration of 100 mg·L-1 did not show any significant difference (Table 6).
Peroxidase enzyme activity (POD)
According to means comparison, the highest activity of the POD (1.51 nmol·g-1 FW) was observed with 150 mg·L-1 caraway essential oil. Dill essential oil (50 and 150 mg·L-1), 100 mg·L-1 cumin and 200 mg·L-1 8-HQS had no significant difference with the superior treatment. The lowest amount of enzyme activity was observed in the control and 400 mg·L-1 8-HQS (0.96 and 0.91 nmol·g-1 FW, respectively) (Table 6). The effect of different treatments on peroxidase activity of cut flowers was significant at the 1 % level (Table 5).
Catalase enzyme activity (CAT)
All treatments had a significant effect on catalase activity of cut flowers (Table 5). Control flowers had the least amount of catalase enzyme (0.92 µg·g-1 FW). The 50 mg·L-1 dill essential oil and 400 mg·L-1 8-HQS did not differ significantly compared with control flowers (Table 6). Dill essential oil (100 mg·L-1) had the greatest effect on catalase activity (1.78 µg·g-1 FW), but did not have a significant difference with other treatments (Table 6).
Discussion
Carnation stems treated with alcohol (2 %) had the longest vase life compared to the other treatments. The use of alcohol in a vase solution as a disinfectant and anti-ethylene improves water conduction and reduces vessel obstruction (Farokhzad et al., 2005). Alcohol-treated flowers had more water content than the control, indicating improved water transfer in the stem vessel of the flower and their vase life increased significantly compared to the control. On the other hand, alcohol prevents the transfer of carbohydrates from petals to the ovary, respiratory carbohydrates remain in the petals and they are used for petal metabolism (Podd & van Staden, 1998; Sharif-Hossain, Boyce, & Osman, 2007). By opening the vessels and controlling the microbial population, the water status of the petals improves and the percentage of plant dry matter increases due to the presence of sugar in the water. Therefore, enough sugar replaces the sugar consumed during respiration.
Increasing the vase life with the use of essential oils is due to the antimicrobial and anti-bacterial properties found in essential oils (Blokhina, Virolainen, & Fagerstedt, 2003). The positive effects of various plant essential oils for increasing the longevity of cut flowers have been reported (Amini et al., 2016; Kavosiv, Mirzakhani, & Hakimi, 2013; Mirdehghan & Aghamolayi, 2016; Mallahi, Ramezaniana, Saharkhiz, Javanmardi, & Iraji, 2018).
In a vase solution, microorganisms cause stem obstruction and accelerate the aging of petals (de Witte, Harkema, & van Doorn, 2014). Microorganisms and their toxic products restrict water uptake by blocking the ends of the stem (Liu et al., 2009). Water balance is an important factor in maintaining the quality and longevity of cut flowers and the inability to absorb water is the main cause of senescence. Disinfectants in the vase solution prevent the growth of microbes, protecting the vessels from obstruction and promoting healthy flowering (Kim & Lee, 2002). Shanan (2012) found that application of herbal essential oils improved water absorption in cut rose flowers by preventing vessel obstruction. The above results are similar to the results of this study.
Treatment with 8-HQS increased water absorption by acidifying the environment and preventing vessel obstruction (Li et al., 2017). In this regard, Zadeh-Bagheri, Namayandeh, Soulati, and Javanmardi (2011) stated that the use of chemical disinfectants such as 8-HQS led to an increase in soluble absorption in cut flowers. Several factors play a role in increasing the vase life of cut flowers, which include reducing the number of microorganisms, especially bacteria (Hashemabadi et al., 2016).
The 8-HQS antimicrobial properties can inhibit the growth of bacteria, thereby increasing the absorption of the vase solution by the plant and extending the life of the cut flowers (Kavosiv et al., 2013; van Doorn, 1997). The results of this study also emphasize the importance of the use of non-toxic compounds such as anti-bacterial herbal essential oil in preservative solutions to reduce bacterial populations at the stem end, obstruction, and limitations in water absorption. Adding herbal extracts to the preservative solution prevents the growth and activity of microbes and vessel obstruction due to the antimicrobial properties of these compounds and, as a result, water absorption is increased without interruption.
Also, the activity of enzymes such as CAT and POD is controlled due to the antioxidant properties of plant essential oils, which are associated with the aging of cut flowers (Shanan, 2012; Hashemabadi et al., 2016). These conditions induce freshness and prolonged longevity of cut flowers.
The results of this study indicate that all treatments significantly reduced the bacterial population as compared to the control except for 2 % alcohol. The antimicrobial, anti-bacterial and anti-fungal properties of the herbal essential oils depend on chemical compounds (alcohols, phenols, aldehydes, ketones, etc.). As the amount of these phenolic and alcoholic compounds increased so did these properties.
Microorganisms cause the production of internal ethylene and toxic substances, stem obstruction and accelerated aging of petals. The growth of microbes in the vase solution decreases hydraulic conductivity in the stem of cut flowers (Shanan, 2012). The use of antimicrobial agents such as herbal essential oils and chemical antimicrobial compounds in preservative solutions is effective in preventing the growth of microbes and increasing vase life (Kavosiv et al., 2013; Mallahi et al., 2018).
Based on Tables 2, 3 and 4, the highest percentage of effective components of essential oils in geranium, dill and caraway is with geraniol, linalool and methyl-3-phenyl-2-propenal, respectively. Research has shown that most of these compounds, which have alcohol bases and other active ingredients that were present in low percentages in the essential oils of these plants, have antimicrobial and antioxidant properties (Alviano et al., 2005; Nazzaro, Fratianni, Coppola, & de Feo, 2017; Walsh, Livinghouse, Goeres, Mettler, & Stewart, 2019). Terpene compounds have also been shown to limit oxidative damage caused by the accumulation of reactive oxygen species (Ross, 2005).
Our results are consistent with those of Hejazi and El-Kot (2009) on the effect of herbal essential oils on reducing the number of microbes and increasing the durability of cut gladiolus flowers. Solgi et al. (2009) reported that herbal essential oils disrupt the respiratory chain of bacteria and reduce their populations in vase solution and cut flowers. The use of chemical antimicrobial compounds and antimicrobial properties of plant essential oils prevent vascular occlusion, therefore, compared to the control, the absorption of the preservative solution is increased and because this solution contains sucrose, the dry weight increases that it will be effective on the dry matter. The results of this study show that water absorption was increased in all treatments compared to the control.
The percentage of dry matter in the treated flowers had better performance compared to the control flowers. After harvesting cut flowers, the main metabolic process in the plant is cellular respiration, which leads to a decrease in carbohydrates stores in the plant and leads the plant to aging. Antimicrobial compounds prevent vascular occlusion by controlling the bacterial population, and the flow of solution and sucrose in it remains open. As a result, the carbohydrates lost in the process of cellular respiration are compensated and aging is delayed. Hejazi and El-Kot (2009) and Mohammadi, Mostofi, and Basirat (2009) reported the same results in dry matter by using disinfectants in a vase cut flower solution. Since leaves in cut carnation flowers show a favorable condition at the end of their life, they cannot be the criterion for ending vase life.
According to the results of this study, 8-HQS had a better and more favorable effect on cut flowers. In the case of dill treatments (100 mg·L-1) and geranium (50 mg·L-1), which had the longest vase life, chlorophyll content did not decrease and was more than that of 100 mg·L-1 caraway essential oil. The most important effect of essential oils is the maintenance of chlorophyll due to their antioxidant properties. The ability to prevent chlorophyll degradation and severe depletion is due to the activation of the cells and the increase in glucose production. Increasing the glucose content by regulating the osmotic pressure and respiration reduces the loss of chlorophyll (Andersen, Williams, & Serek, 2004).
Results obtained by Babarabie, Zarei, and Varasteh (2016) showed that the leaf chlorophyll content of Alstroemeria was increased with the use of essential oil compounds. Abdul-Wasea (2012) showed that the use of 8-HQS to delay chlorophyll degradation was the most effective treatment compared to the control. Researchers believe that the reason for the superiority of these antimicrobial compounds could be the inhibition of chlorophyllase activity during treatment.
Carotenoids are tetra-terpene compounds that are responsible for maintaining chlorophyll from optical oxidation, light absorption and transferring energy to chlorophyll a (Ross, 2005). They are also known to support non-photosynthetic pigments that can take extra energy from short wavelengths and convert individual oxygen to triple oxygen and show antioxidant activity by producing oxygen-rich radicals (Inze & Montagu, 2000; Howltt & Pogson, 2006). The results of previous studies have shown that the use of antimicrobial compounds maintains and increases the amount of pigmentation during the post-harvest period (Zamani, Hadavi, Kazemi, & Hekmati, 2011). In the present study, antimicrobial compounds in most treatments increased the amount of carotenoids in petals.
The active ingredients in plant essential oils such as limonene and carnosol increase carotenoid pigmentation (Grassmann, 2005; Proshkina et al., 2020). In this work, the amount of the pigments is higher in herbs treated with essential oils (due to the presence of phenolic compounds in essences), compared to 8-HQS. The superiority of the used compounds can be attributed to the ability of these compounds to reduce microbial load and improve water absorption. Because the color intensity of the flowers depends on the amount of carbohydrates in the tissues around the petals, it can be concluded that the antiseptic compounds prevent the removal of important pigmentation (especially carotenoids) by improving the absorption of water and sugar in the vase solution and protecting carotenoids against degradation and severe depletion which is in line with the views of researchers such as Asil and Karimi (2010). Also, during research, the use of 8-HQS in cut lily flowers increased the amount of flower dyeing (Han & Miller, 2003). In another study, it was found that herbal essential oil treatment showed the highest levels of carotenoids (Babarabie et al., 2017).
The important factor in lipid peroxidation is the free oxygen radicals. One of the products of peroxidation of membrane lipids is the MDA compound. The accumulation of MDA is a marker of cellular membrane degradation. The amount of this compound is considered as an indicator of physiological resistance and aging (Geng, Liu, Lu, Hu, & Okubo, 2009). According to the MDA results, the use of herbal essential oils and 8-HQS had a positive effect in most of the treatments and reduced MDA. In treatments with long vase life, MDA decreased considerably.
The phenolic compounds in plant essential oils are capable of removing reactive oxygen species, reducing membrane lipid oxidation and decreasing the concentration of MDA (Upadhyaya & Panda, 2004). Extending the vase life of cut chrysanthemum flowers and reducing MDA indicate membrane stability and increased longevity (Zamani et al., 2011). In this regard, Asil and Karimi (2010) reported that gladiolus flowers treated with 8-HQS and herbal essential oils had the least amount of MDA. With the onset of the first signs of aging, antioxidant enzymes such as POD increase in petals to counteract the destructive effects of active oxygen species. The POD enzyme reacts with hydrogen peroxide and converts it to water and oxygen (Hopkins et al., 2007).
The results of this study showed that POD has the main responsibility for neutralizing peroxide ions in cut carnation, as POD activity increased in all treatments increasing vase life compared to the control. The cause of the superiority of plant compounds can be attributed to their antioxidant properties, which increase the activity of antioxidant enzymes, and the enzymes such as CAT and POD contribute to the removal of free radicals in the plant system (Baily, Bogatek-Leszczynska, Come, & Corbineau 2002). The results are consistent with those of Kazemi and Ameri (2012) in terms of the effects of antimicrobial compounds on enhancement of enzyme activities and reduction of damage resulting from free radicals, thus increasing the vase life of cut carnations. The results showed that all treatments with long carnation vase life increased CAT activity compared to the control. The enzyme CAT, due to its antioxidant properties, neutralizes the generated radicals.
Since the free oxygen species obtained from the decomposition of hydrogen peroxide is one of the important factors in the early aging of petals, and because, on the other hand, CAT is an antioxidant and neutralizes the toxic oxygen release of hydrogen peroxide, the activity of this enzyme prevents the aging of petals (Mortazavi, Naderi, Khalighi, Babalar, & Allizadeh, 2007). In a study on carnation, the use of antimicrobial compounds increased the activity of enzymes and reduced the damage of free radicals and, consequently, increased the longevity of cut flowers (Kazemi & Ameri, 2012).
Conclusions
In conclusion, 2 % alcohol had the greatest effect on vase life compared to the other treatments. Also, high concentrations of 8-HQS increased the vase life. Herbal essences had a great influence on water absorption, carotenoids and dry matter content by preventing the accumulation of bacteria and vascular obstruction. Maximum vase life was obtained in cut flowers treated with 2 % alcohol, 100 mg·L-1 dill and 50 mg·L-1 geranium essential oils. Solution uptake and catalase activity were also at their highest with the 100 mg·L-1 dill essential oil solution. Therefore, 100 mg·L-1 dill essential oil was found to be the most effective eco-friendly treatment.











 texto en
texto en 


