Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.48 no.4 Ciudad de México oct./dic. 2004
Investigación
Synthesis and Multinuclear (1H, 13C, 31P, 119Sn) NMR Study of Trimethyl- and Triphenyl-tin (IV) with Cyclic Dithiophosphate Ligands
Patricia García y García,a* Marcela López-Cardoso,a María del Carmen Pérez-Redondo,a Patricia Martínez-Salas,a Ave María Cotero-Villegas,a and Raymundo Cea-Olivaresb
a Centro de Investigaciones Químicas, Universidad Autónoma del Estado de Morelos, Avenida Universidad 1001, Chamilpa. Cuernavaca, 62210, Morelos, México. E-mail: pgarcia@ciq.unam.mx
b Instituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, México 04510, D.F., México.
Recibido el 29 de septiembre del 2004.
Aceptado el 7 de diciembre del 2004.
Abstract
We report the synthesis and characterization of six new trimethyl- and triphenyl-tin dithiophosphates. Compounds 1-6 were characterized by IR, EM-EI, and multinuclear NMR (1H, 13C, 19P and 119Sn). The six compounds show a monomeric behavior with five-coordinated tin as well as a biconective monometallic behavior of the ligands. Compounds 1-4 show, in solution, a conformational equilibrium between an equal population of the two chair conformations of the dioxaphosphinane ring and a rapid exchange of the exocyclic sulfur atoms, resulting from the chelated situation of the tin atom.
Key words: Dithiophosphates, triorgantin-dithiophosphates, Cyclics dithiophosphates.
Resumen
En este trabajo se informa la síntesis y caracterización estructural de seis nuevos ditiofosfatos de trimetil- y trifenil-estaño (IV). Los compuestos 1-6 fueron caracterizados por IR, EM IE y RMN multinuclear, (1H, 13C, 19P y 119Sn). Los seis compuestos manifiestan un comportamiento monomérico, con una pentacoordinación del átomo de estaño y un comportamiento monometálico biconectivo de los ligantes. Los compuestos 1-4 presentan en solución una rápida interconversión entre igual población de las dos conformaciones silla del anillo dioxafosfinano y un rápido intercambio de los átomos de azufre exocíclicos, resultado de la quelatación del átomo de estaño con el ligante.
Palabras clave: ditiofosfatos, triorgano-estaño-ditiofosfatos, ditiofosfatos cíclicos.
Dedicated to the memory of Dr. Raymundo Cruz Almanza.
Introduction
The dithiophosphates (RO)2PS2- and dithiophosphinates R2PS2- ligands, show a broad diversity of coordination patterns, basically due to the ability to involve the sulfur atoms in primary or secondary (intra or intermolecular) bonds as well as the metal tendency to modify its coordination number, which can be influenced by the nature of the dithio-ligands, the type of the organic groups on the phosphorus atom and the substituents attached to the main group metal [1-3]. Most of the Tin (IV) dialkyl dithiophosphates [2] exhibit an anisobidentate or isobidentate coordination, except in the cases of Ph3SnS2P(OEt)2 [4] and O(CH2CH2S)2Sn(nBu)S2P(OCH2)2CEt2 [5] that display a monodentate coordination. In the last years, considerable attention has been directed to the synthesis of O,O'-alkylene-dithiophosphates which incorporate five and six membered 1,3,2-dioxaphospholanes or 1,3,2-dioxaphosphinane rings [3], with a view to study the effect of the ring size and the nature of the substituents on the bonding modes. Two structures of tin(IV)- 2,2-dithio-1,3,2-dioxaphospholanes are known: Ph3SnS2P(OCMe2)2 [6] and Me2Sn[S2P(OCMe2)2]2 [7] both displaying an anisobidentate coordination, the first one shows an intermediate geometry between tetrahedral and trigonal bipyramidal, whereas the second one corresponds to the octahedral one. We have reported the structure of two tin(IV) 2,2-dithio-1,3,2-dioxaphosphinanes X(CH2CH2S)2Sn(nBu)S2P (OCH2)2 CEt2 (X= O, S) [5]. The coordination of the Sn atom is trigonal bipyramidal in the first case and intermediate between trigonal bipyramidal and a bicapped tetrahedral arrangement in the second one.
In this work, we report the synthesis and characterization of six new trimethyl- and triphenyl-tin(IV) dithiophosphates, in order to know the coordination mode of the tin atom, as well as the conformational behavior of the three 2,2-dithio-1,3,2-dioxaphosphinane ligands.
Results and discussion
Dithiophosphoric acids (5,5-diethyl-2-mercapto-2-thiono-1,3,2-dioxaphosphinane, 5-methyl-5-n-propyl-2-mercapto-2-thiono-3,2-dioxaphosphinane and 4,6-dimethyl-2-mercapto-2-thiono-1,3,2-dioxaphosphinane) were prepared by the reaction of phosphorus pentasulphide with the corresponding diols (2,2-diethyl-1,3-propanediol, 2-methyl-2-propyl-1,3-propanediol and 2,4-pentanediol) in 1:2 molar ratio in dry benzene, according to the method proposed by Chauhan [8]. The sodium salts were obtained by treatment with ethanolic solution of sodium hydroxide.
Stoichiometric amount of each one of the sodium salts of the cyclic dithiphosphates reacted with chloro trimethyl-tin and chloro triphenyl-tin in ethanol according to Figure 1. Compounds 1-6 are solid, air stable compounds, soluble in organic solvents, and were characterized by elemental analyses, mass spectrometry, infrared and multinuclear magnetic resonance 1H, 13C, 31P, 119Sn. The IR spectra exhibit absorption bands characteristic of the phosphorodithioate ligand, the assignment of such bands was done by comparison whit the spectra of the starting materials and literature data [3,9,10]. The bands in the region 1058-1083 cm-1 are due to the stretching vibrations ν[(P)-O-C] and 960-1014 cm-1 for ν [P-O(C )]. Two strong absorption bands in the regions 681-697 cm-1 y 499-526 cm-1 were assigned to the stretching vibration (νP=S) y (νP-S) respectively [3,9], or to the stretching vibration phosphorus-sulfur, νasim(PS2) y νsim(PS2) [10]. The organotin compounds 1-6 show a displacement to higher frequencies with respect to free acid but lower in relation to the sodium salts of the ligand. The absorption band between 930 to 965 cm-1 is due to the dioxaphosphinane ring [3,9,11]. The spectra also exhibit absorption bands characteristic of the symmetrical and asymmetrical methyl or phenyl groups on the tin atom.
Electron impact mass spectra (70eV) display, with exception of compound 2, the molecular ion, showing a similar pattern of fragmentation, since in all cases the base peak corresponds to the fragment result of the loss of a methyl LSnMe2+ or phenyl LSnPh2+ (L= Ligand) group, and the presence of fragments due to Me3Sn+, MeSn+, in compounds 1, 3, 5 and Ph3Sn+, PhSn+ in 2, 4, 6. No fragments were found at higher mass than the molecular mass, situation that is consistent with a monomeric nature of the obtained compounds.
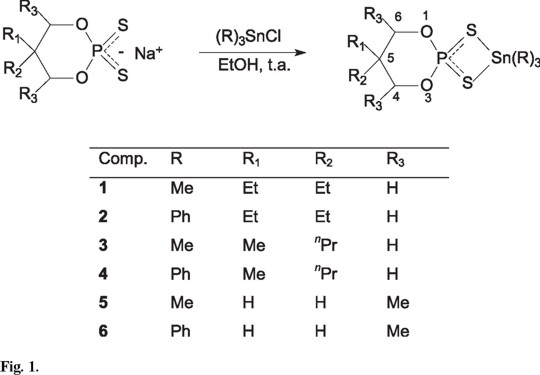
The 31P NMR spectral data show the presence of a single peak in compounds 1-4, whereas compounds 5-6 display two signals in 4:1 ratio, indicative of the presence of the one and two species respectively. Glidewell [12] has proposed that the difference in the phosphorus chemical shift between the appropriate free acid and its metallic derivatives is a strong evidence of the coordination mode. Upfield shifts or values smaller to 82 ppm are associated with a monodentate coordination (a), Figure 2, while downfield shifts in the range 82-101 ppm were consistent with the presence of bidentate ligands [chelate (b) or bridge (c)] and higher chemical shifts than 101 ppm, the behavior is indicative of the ionic type. The compounds containing triphenyltin display chemical shifts in the range of 86.11 to 87.09 and the trimethyltin ones in the range of 90.91 to 91.81 ppm, these data suggest that, in solution the six compounds present a isobidentate coordination of the ligand (monometallic biconective) and a five-coordination of the tin atom (the free acids display chemical shifts in the range of 76.9 to 78.7 ppm).
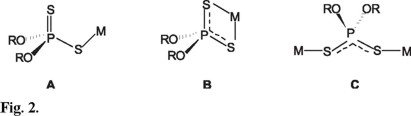
The 1H NMR spectrum of compounds 1-6 exhibit characteristic signals for the dioxaphosphorinane ring. As well as the free acid and some metallic derivatives as As[S2P(OCH2) CEt2]3 [13], Me2Te[S2P(OCH2)CEt2]2 [14] and X(CH2CH2S)2 Sn(nBu) S2P(OCH2)2CEt2 (X= O, S) [5] 1 and 2, show only one double signal for the methylene group (CH2OP) of the 1,3,2-dioxaphosphinane ring at 4.08 and 4.05 ppm respectively, due to the coupling with the phosphorus atom, with coupling constants of 3J(POCH) 15.66 y 16.2 Hz, that indicate the equivalence of the hydrogen of the methylene groups, which is explained by the degeneration of a system A2B2X that would be expected for such protons to a A4X system, derived from a rapid interconversion between equal populations of the two chair conformations of the dioxaphosphinane ring and a rapid interchange between the two exocyclic sulfur atoms. Compounds 3 and 4, like the free acid, show an equilibrium between two chair conformations, Figure 3, showing in this case the signals of the diastereotopics protons of the fragment CH2OP at 4.07 and 4.01 ppm, with coupling constants 3J(POCH) = 15.6 and 16.2 Hz, Jgem = 11.2 and 11.1 Hz respectively. It is interesting to note, that the coupling constants 3J(31P-O-C-1H) of compounds 1-4 correspond to the average value for the sum of coupling constants 3Jgauche and 3Jtrans of the chair conformation of the dioxaphosphinane ring in the methyl derivative [5], compound that also display the non-equivalence of the groups R1 and R2, in contrast with the tin derivatives 1-4.
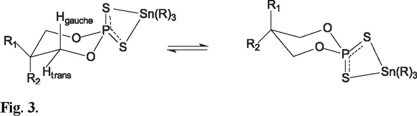
Compounds 5-6, Figure 4, display at high field, the signal corresponding to the cis equatorials methyls C-4 and C-6 as a double of double at 1.36 and 1.33 ppm respectively, with coupling constants J(H-H) of 6.2 and 6.4 Hz and 4J(P-O-C-C-H) of 1.8 and 2 Hz, respectively. A multiple signal at 4.69 and 4.62 ppm, corresponding to the axial protons C-4 and C-6, with coupling constants that are consistent with a chair conformation of the dioxaphosphinane ring, with values of 10.8 and 10.5 Hz, and a coupling situation of the trans-diaxial disposition of the hydrogen of C-4 and C-6 with the hydrogen of the C-5 and values of the coupling constants, 2.2 and 2.0 Hz, that correspond to the coupling of hydrogen in C-4 and C-6 with the equatorial hydrogen of C-5, according to the Karplus relationship [15]. Additionally, the signals in the range 1.95 -1.57 ppm are assigned to the geminal hydrogens at C-5. It was also observed a small proportion of the product with the methyl groups in trans disposition, and this was confirmed by 31P NMR.
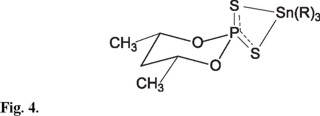
13C NMR, spectra data for compounds 1-6 show for the dioxaphosphinane ring, two double signals due to the phosphorus-carbon coupling, with chemical shifts between 76.32 - 73.25 for C-4 and C-6 and to upfield shift in the range 41.2-35.21 ppm for C-5. The coupling constant for compounds 1-4 are in the intervals 2J(31P-O-13C) 9.2 - 8.6 Hz and 3J(31P-O-C-13C) 6.0 - 5.2 Hz respectively, this last value is close to 6.2 Hz, in accord with the Karplus relationship [15] for these compounds when the angle is equal to zero, which confirms the equilibrium between a equal population of chair conformers. Whereas compounds 5-6 display coupling constant values phosphorus-carbon 3J(POCC) for C-5 of 4.12 and 4.87 Hz, that are the expected ones for the chair conformation of the dioxaphosphinane ring with an angle of θ C(5)-C(4)-O-P of 60° and the equatorial methyls on C-4 and C-6 display values of 10.05 and 10.25 Hz, respectively, that correspond to the angle of θ CH3-C(4)-O-P or CH3-C(6)-O-P of 180° [8.5-10 Hz].
The 119Sn-NMR spectrum of compounds 1-6 show chemical shifts in the range of 118.9 to 108.96 and -77.9 to -84.15 ppm for the trimethyl-tin and triphenyl-tin derivatives, respectively. These last values are close to the interval proposed by Otera [16] for a five-coordination, in agreement with the isobidentate behavior of the ligand, also suggested by 31P-NMR. In all the cases were observed upfield shifts between 30 and 55 ppm with respect to chlorotrimethyl-tin (165.7) and chlorotriphenyl-tin (-44.7), situation that suggests the change of tetra-coordination to penta-coordination of the tin atom.
Experimental
All reagents were commercial grade and were used as received. Dithiophosphoric acids were obtained using the published procedures [8]. The sodium salts of cyclic dithiophosphates were obtained with ethanolic solution of sodium hydroxide. Solvents were dried by standard methods before use. Elemental analyses (C, H) were performed by Galbraith Laboratories. Inc. (Knoxville, TN). IR spectra were recorded as KBr pellets using Bruker and MIDAC Prospect spectrometers. 1H, 13C, 31P and 119Sn NMR spectra were obtained in a JEOL Eclipse+ 300 and Varian Gemini 200 spectrometers operating at (299.949, 199.99), 75.57, 121.65 and 112.06 MHz, respectively, using CDCl3 as solvent. The chemical shifts are relative to internal Me4Si (1H, 13C), external H3PO4 (85%) (31P) and Me4Sn (119Sn) for the indicated nuclei.
General procedure
Stoichometric amounts of trimethyl-tin and triphenyl-tin chlrorides and the sodium salts of the dithiophosphates in ethanol were stirred for 24 h. The reaction mixture was filtered to remove the resulting NaCl, the clear filtrate was evaporated at low pressure and the resulting powder was recrystallized from a CH2Cl2-hexane mixture.
Trimethyl-tin (5,5-diethyl-2-thiono-1,3,2-dioxaphosphinane-2-thioate)
(CH3)3Sn[S2P(OCH2)2CEt2] 1. White solid, stable on exposure to air, mp 119-20°C. Yield (250 mg, 64 %). Anal. Calc. for C10H23O2S2PSn: C, 30.88 %; H, 5.95 %. Found C, 31.08 %; H, 6.01 %. EI-MS (70eV): m/z 375 [(C7H14O2P S2Sn(CH3)2+; (M+ - Me), 100%]; 359 (C7H14O2PS2SnCH3+ <10%), 344 (C7H14O2S2PSn+, <10%); 259 (Me3SnS2P+, 26%); 229 (Me3SnS2+, 8%); 165 (Me3Sn+, 13%); 135 (MeSn+, 5%). IR (KBr): 2965m, 2922m, 2876m, (νC-H); 1462m (δ CH2); 1066m [ν(P)-O-C]; 994f, [ν(P-O-(C )], 933m [dioxaphosphinane ring] 683 (νsym PS2), 523f cm-1 (νasym PS2 ). 1H NMR (CDCl3): δ 4.08 (d, 3J(31P-O-C-1H) = 15.66 Hz, 4H) (CH2OP); 1.44 (c, J= 7.41 Hz, 4H) CH2CH3, 0.81 (t, J= 7.41 Hz, 6H) CH2CH3; 0.69 (s, 9H) CH3Sn. 13C-NMR (CDCl3): δ 74.24 [d, 2J(31P-O-13C = 9.2 Hz)] CH2OP; 37.38 [d 3J(31P-O-CH2-13C) = 5.7 Hz] CCH2OP; 22.94 CH2CH3; 7.14 CH2CH3; -1.57 CH3Sn. 31P NMR (CDCl3): δ 91.35. 119Sn NMR (CDCl3): δ 118.88.
Triphenyl-tin (5,5-diethyl-2 thiono-1,3,2-dioxaphosphinane 2-thioate)
Ph3Sn[S2P(OCH2)2CEt2] 2. White solid, stable on exposure to air, mp 96-98°C. Yield (235 mg, 67 %). Anal. Calc. for C25H29O2S2PSn: C, 52.2 %; H, 5.081 %. Found C, 51.69 %; H, 5.089 %. EI-MS (70 eV): M+. 576 (7%); m/z 499 (M+ - Ph, 100%) 421 [(C7H14O2S2PSnPh)+, 5%]; 385 (Ph3SnS+, 6%), 351 (Ph3Sn+, 15%); 229 (PhSnS+, 34%); 197 (PhSn+, 15%). IR (KBr): 3064m, 2979m, 2941m, 2879m, (νC-H); 1460m (δ CH2-); 1068m [ν(P)-O-C]; 995, [ν(P-O-(C )], 935 [dioxaphosphinane ring] 686 (νsym PS2), 507m cm-1 (νasym PS2 ). 1H-NMR (CDCl3): δ 4.05 (d, 3J(31P-O-C-1H) = 16.2 Hz, 4H) (CH2OP); 1.40 (c, J = 7.41 Hz, 4H) CH2CH3, 0.80 (t, J= 7.41 Hz, 6H) CH2CH3; 7.73 (sa, 6H) [H-Ar (o-H)]; 7.44 (m, 9H) [m-H and p-H]. 13C NMR (CDCl3): δ 74.60 [d, 2J(31P-O-13C = 8.6 Hz)] CH2OP; 37.30 [d 3J(31P-O-CH2-13C) = 5.2 Hz] CCH2OP; 22.94 CH2CH3; 7.13 CH2CH3; 137.91 [C-Ar (o- C)]; 136.94 (p-C) 130.13 (m-C), 128.98 (i-C). 31P NMR (CDCl3): δ 86.37. 119Sn NMR (CDCl3): δ -77.9.
Trimethyl-tin (5-methyl-5-propyl-2-thiono-1,3,2-dioxaphosphinane-2-tioate)
(CH3)3Sn[S2P(OCH2)2CMe(nPr)] 3. Pale yellow solid, stable on exposure to air, mp 52°C. Yield (59 mg, 16 %). Anal. Calc. for C10H23O2S2PSn: C, 30.88 %; H, 5.96 %. Found C, 31.96 %; H, 5.95 %. EI-MS (70 eV): M+ 390 (16); m/z 375 [(C7H14O2P S2Sn(CH3)2+; (M+ - Me), 100%]; 359 (C7H14O2PS2SnMe+, 5%), 344 (C7H14O2S2PSn+, 15%); 259 (Me3SnS2P+, 12%); 165 (Me3Sn+, 40%); 135 (MeSn+, 10%). IR (KBr): 2958m, 2928m, 2871m, 2869m, (νC-H); 1466m (δ CH2); 1058m [ν(P)-O-C]; 989, [ν(P-O-(C )], 960m [dioxaphosphinane ring] 681 (νsym PS2), 501 cm-1 (νasym PS2). 1HNMR (CDCl3): δ 4.07 (m, 3J(31P-O-C-1H) = 15.66 Hz, Jgem(31P-O-C-1H) = 11.2 Hz, 4H) (CH2OP); 1.38 (m, 4H) CH2CH2CH3; 1.31 (m, 4H) CH2CH2CH3; 0.94 (t, J= 7.2 Hz, 3H) CH2CH2CH3; 0.94 (s, 9H) CH3Sn. 13C NMR (CDCl3): δ 76.31 [d, 2J(31P-O-13C = 8.9Hz)] CH2OP; 35.21 [d, 3J(31P-O-CH2-13C) = 6 Hz); 36.92 CH2CH2CH3; 16.52 CH2CH2CH3; 14.85 CH2CH2CH3; 18.94 CH3; -1.377 CH3Sn. 31P NMR (CDCl3): δ 90.91. 119Sn NMR (CDCl3): δ 113.019.
Triphenyl-tin (5-methyl-5-propyl-2-thiono-1,3,2-dioxaphosphinane-2-thioate).
Ph3Sn[S2P(OCH2)2CMe(nPr)] 4. White solid, stable on exposure to air, mp 96-98°C. Yield (235 mg, 67 %). Anal. Calc. for C25H29O2S2PSn: C, 52.19 %; H, 5.08 %. Found C, 53.69.69 %; H, 4.92 %. EI-MS (70eV): M+. 576 (7%); m/z 499 (M+ -Ph, 100%); 421 [(C7H14O2S2PSnPh)+, 5%]; 351 (Ph3Sn+, 65%); 229 (PhSnS+, 22%); 197 (PhSn+, 16%). IR (KBr): 3065m, 2965m, 2922m, 2876m, (νC-H); 1466m (δ CH2); 1074m [ν(P)-O-C]; 1014, [ν(P-O-(C )], 930 [dioxaphosphinane ring] 681 (νsym PS2), 501m cm-1 (νasym PS2 ). 1H NMR (CDCl3): δ 4.03 (m, 3J(31P-O-C-1H) = 16.2 Hz, Jgem(31P-O-C-1H) = 11.2Hz, 4H) (CH2OP); 1.25 (m, 4H) CH2CH2CH3; 1.25 (m, 4H) CH2CH2CH3; 0.94 (t, J= 7.2 Hz, 3H) CH2CH2CH3; 0.94 (s, 3H) CH3; 7.40 (m, 6H) [H-Ar (o-H)]; 7.71 (m, 9H) [m and p-H]. 13C-NMR (CDCl3): δ 75.94 [d, 2J(31P-O-13C = 9.1 Hz)] CH2OP; 35.28 [d, 3J(31P-O-CH2-13C) = 5.7Hz); 36.81 CH2CH2CH3; 16.47 CH2CH2CH3; 14.81 CH2CH2CH3; 18.86 CH3; 137.01 [C-Ar (o-C)]; 136.29 (p-C) 130.20 (m-C), 128.90 (i-C). 31P NMR (CDCl3): δ 86.112. 119Sn NMR (CDCl3): δ 15.52.
Trimethyl-tin (4,6-dimethyl-2-thiono-1,3,2-dioxaphosphinane-2-thioate).
(CH3)3Sn[S2P(OCHMe)2CH2] 5. Pale yellow solid, stable on exposure to air, mp 137-39 °C. Yield (271 mg, 75 %). Anal. Calc. for C8H19O2S2PSn: C, 26.613 %; H, 5.304 %. Found C, 26.88 %; H, 5.602 %. EI-MS (70eV): M+ 361 (6%); m/z 347 [(C5H10O2PS2Sn(CH3)2+; (M+ - Me), 100%]; 332 (C5H10O2PS2SnMe+, 30%), 165 (Me3Sn+, 60%); 135 (MeSn+, 23%). IR (KBr): 2974m, 2923m, 2860m, (νC-H); 1447m (δ CH2); 1083m [ν(P)-O-C]; 960, [ν(P-O-(C )], 960m [dioxaphosphinane ring] 696 (νsym PS2), 499 cm-1 (νasym PS2). 1H NMR (CDCl3): δ 4.69 (m, 2H) CHaxOP; 1.95 (td, J= 2, 13.8 1H) C-5, CHax; 1.67 (sa, 1H) C-5, CHec; 1.36 (dd, J= 1.8, 6.2 Hz, 6H), CH3ec en C-4 y C-6; 0.71 (s, 9H) CH3Sn. 13C-NMR (CDCl3): δ 73.25 [d, 2J(31P-O-13C = 9.0 Hz)] CHOP, C-4 y C-6; 41.2 [d, 3J(31P-O-CH2-13C) = 4.87 Hz] CCH2OP, C-5; 22.67 [d, 3J(31P-O-CH-13C) = 10.05 Hz] CH3CHOP; -1.065 CH3Sn. 31P NMR (CDCl3): δ 91.81 y 89.84 (4:1). 119Sn NMR (CDCl3): δ 108.96.
Triphenyl-tin (4,6-dimethyl-2-thiono-1,3,2-dioxaphosphinane-2-thiolate.
(Ph3)3Sn[S2P(OCHMe)2CH2] 6. White solid, stable on exposure to air, mp 91-96 °C. Yield (285 mg, 52 %). Anal. Calc. for C23H25O2PS2Sn: C, 50.478 %; H, 4.605 %. Found C, 50.97 %; H, 4.621 %. EI-MS (70eV): M+. 547 (5%); m/z 471 (M+ - Ph, 100%); 316 [(C5H10O2PS2SnPh)+, 22%]; 385 (Ph3SnS+, 12%); 351 (Ph3Sn+, 95%); 229 (PhSnS+, 33%); 197 (PhSn+, 58%). IR (KBr): 3060m, 2960m, 2878m, (νC-H); 1460m; 1083m [ν(P)-O-C]; 994, [ν(P-O-(C )], 965 [dioxaphosphinane ring] 697 (νsym PS2), 526m (νasym PS2 ) cm-1. 1H NMR (CDCl3): δ 4.62 (m, 2H) CHaxOP; 1.76 (td, J= 2, 13.6Hz, 1H) C-5, CHax; 1.57 (1H) C-5, CHec; 1.33 (dd, J= 1.8, 6.2 Hz, 6H), CH3ec.en C-4 y C-6; 7.40 (m, 6H) [H-Ar (o- H)]; 7.69 (m, 9H) [m and p-H]. 13C NMR (CDCl3): δ 73.25 [d, 2J(31P-O-13C = 8.9Hz)] CHOP, C-4 y C-6; 36.81 [d, 3J(31P-O-CH2-13C) = 4.87 Hz] CCH2OP, C-5; 22.11 [d, 3J(31P-O-CH-13C) = 10.0Hz] CH3CHOP; 136.85 [C-Ar (o-C)]; 136.12 (p-C) 129.97 (m-C), 128.85 (i-C). 31P NMR (CDCl3): δ 87.09 y 85.9. 119Sn NMR (CDCl3): δ -84.15.
References
1. a) Haiduc, I.; Sowerby, D.B.; Lu, S.F. Polyhedron 1995, 14, 3389-3472. [ Links ] b) Haiduc, I. Coord. Chem. Rev. 1997, 158, 325-358. [ Links ] c) Silvestru, C.; Haiduc, I. Coord. Chem. Rev. 1996, 147, 117-146. [ Links ]
2. Jain, V.K. Coord. Chem. Rev. 1994, 135/136, 809-843. [ Links ]
3. Chauhan, H.P.S. Coord. Chem. Rev. 1998, 173, 1-30. [ Links ]
4. Molloy, K.C.; Hossain, M.B.; Van der Helm, D.; Zuckerman, J.J.; Haiduc, I. Inorg. Chem. 1979, 18, 3507-3511. [ Links ]
5. García y García, P.; Toscano, R.A.; Cea-Olivares R. J. Organomet. Chem. 2000, 598, 160-166. [ Links ]
6. Preut, H.; Ngo, V.D.; Huber, F. Acta Cryst. 1987, C43, 164-165. [ Links ]
7. Preut, H.; Ngo, V.D.; Huber, F. Acta Cryst. 1986, C42, 809-811. [ Links ]
8. Chauhan, H.P.S.; Bhasin, C.P.; Srivastava, G.; Mehrotra, R.C. Phosphorus Sulfur, 1983, 15, 99-104. [ Links ]
9. Chauhan, H.P.S.; Srivastava, G. K.; Mehrotra, R.C. Polyhedron 1984, 3, 1337-1345.
10. Sowerby, D.B.; Haiduc, I.; Barbul-Rusu, A.; Salajan, M. Inorg. Chim. Acta. 1983, 68, 87-96. [ Links ]
11. Gupta, R.K.; Rai, A.K.; Mehrotra, R.C.; Jain, V.K. Inorg. Chem. Acta. 1984, 88, 201-207. [ Links ]
12. Glidewell, C. Inorg. Chem. Acta. 1977, 24, 255-260. [ Links ]
13. El Khadi, A.A.S.; Singh, Y.P.; Bohra, R.; Mehrotra, R.C.; Srivastava, G. Main Group Met. Chem. 1991, 14, 305-311. [ Links ]
14. Drake, J.E.; Khsrou, L.N.; Mislankar, A.G.; Ratnani, R. Can. J. Chem. 1994, 72, 1328-1337. [ Links ]
15. Samitov, Yu. Yu.; Karataeva, F. Kh.; Ovchinnkov V.V.; Cherkasov, R.A. J. Gen. Chem. 1986, 56, 1979-1991. [ Links ]
16. Otera, J. J. Organomet. Chem. 1981, 221, 57-61. [ Links ]














