Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.48 no.4 Ciudad de México oct./dic. 2004
Investigación
Anti-inflammatory Activity of Some Extracts and Isolates from Leonotis nepetaefolia on TPA-induced Edema Model
Hortensia Parra-Delgado, Gabriela García Ruiz, Antonio Nieto Camacho, and Mariano Martínez-Vázquez*
Instituto de Química, Universidad Nacional Autónoma de México, Ciudad Universitaria, Coyoacán 04510, México, D. F., Tel +(52) 55 5622-4403. E-mail: marvaz@servidor.unam.mx
Recibido el 18 de octubre del 2004.
Aceptado el 30 de noviembre del 2004.
Abstract
Several extracts of aerial parts of Leonotis nepetaefolia showed anti-inflammatory activity on TPA-induced edema model. The chromatography of the extracts led to the isolation of stigmasterol and leonotinin. Although the presence of leonotinin is in agreement with previous phytochemical studies of this species, this is the first time that its anti-inflammatory activity is determined.
Key words: Leonotis nepetaefolia, leonotinin, stigmasterol, diterpenes, labdanes, anti-inflammatory activity.
Resumen
Diversos extractos de las partes aéreas de Leonotis nepetaefolia mostraron poseer propiedades antiinflamatorias en el modelo de edema inducido por TPA en oreja de ratón. La cromatografía de los extractos permitió el aislamiento de estigmasterol y leonotinina. La presencia de leonotinina está de acuerdo con estudios previos de esta especie. Sin embargo, es la primera ocasión que se informa la actividad antiinflamatoria de este compuesto.
Palabras clave: Leonotis nepetaefolia, leonotinina, estigmasterol, diterpenos, labdanos, actividad antiinflamatoria.
Dedicated to the memory of Dr. Raymundo Cruz Almanza.
Introduction
The genus Leonotis (Lamiaceae) is formed by fifteen tropical species [1]. The most abundant species of this genus is the African L. nepetaefolia, a very abundant weed in abandoned agricultural land not only in our country but also in Africa, the West Indies and South America [2]. Previous studies have attributed a variety of salutary physiological effects to this species [1, 3-4]. A tea made from its leaves is used to treat coughs, fever, stomach ache, and akin ailments. It is also used to treat kidney diseases, rheumatism and dysmenorrhea. In India, the ash of the inflorescence is used to treat burns. Also the antibacterial activity of the MeOH and EtOAc extracts of the plant against Pseudomonas aeruginosa have been reported [1]. Chemical studies of this plant have led to the isolation of labdanoid diterpenoids, coumarins and iridoids [1, 4-10].
As a part our ongoing systematic studies, looking for bioactive compounds from Mexican species [11-13], we report in this paper a chemical study and the anti-inflammatory activity of extracts and isolates from L. nepetaefolia.
Results and Discussion
Flowers, leaves, and stems of L. nepetaefolia were studied separately. The hexane, EtOAc and methanol extracts of each limb were obtained. The anti-inflammatory activity of each extract was determined using the TPA-induced edema test. It is a screening to evaluate the ability of test compounds or extracts to prevent an inflammatory reaction in response to the edemogen 12-O-tetradecanoylphorbol-13-acetate (TPA)[13].
All the tested extracts showed, in some degree, anti-inflammatory activity in the TPA-induced edema test in mice (Table 1). However, the highest activity was obtained with the EtOAc extract of leaves (65.75%), flowers (69.06%) and stems (72.93%).
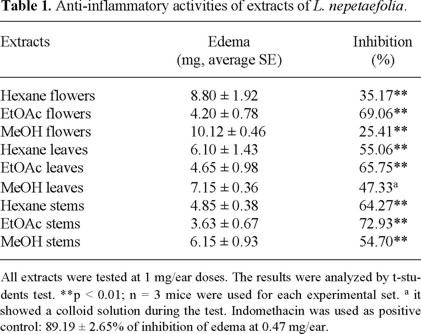
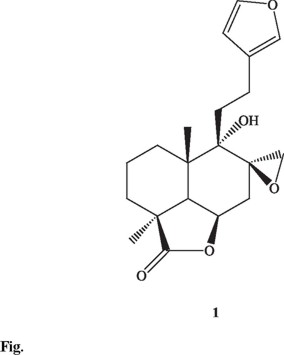
In order to isolate the possible involved components, all the extracts were chromatographed. Although several lab-danoid diterpenoids, coumarins and iridoids have been isolated from this species, only stigmasterol and leonotinin (1) were isolated in this opportunity. The identity of each compound was achieved by comparison of its physical and spectroscopic data with those previously reported [6, 14-15].
The presence of leonotinin in L. nepetaefolia is in agreement with previous phytochemical reports of this species [6, 8].
The anti-inflammatory activity of stigmasterol is well-documented [16] then its presence in this species could account for the anti-inflammatory activity. On the other hand, Table 2 shows the results of the evaluation of the anti-inflammatory activity of 1. Though 1 have been frequently isolated from Leonotis genus, to our knowledge this is the first time that its anti-inflammatory activity is determined.
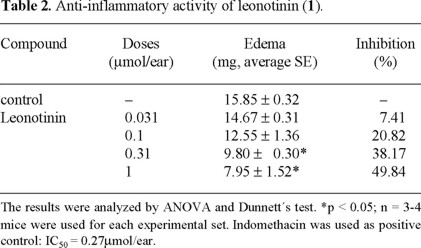
It is known that phorbol esters, like TPA induce skin inflammation, and a hyperproliferative response with an infiltration of neutrophils [11, 17]. It is also known that TPA stimulates phospholipase A2 (PLA2) and that consequently a release of arachidonic acid and prostaglandins occurs [11, 18]. On the other hand, several studies had reported that some labdane-type diterpenes inhibit PLA2 enzyme [19, 20], which is importantly involved in the inflammation process. In addition, it has been reported that ethanol extracts of L. intermedia, and L. leonorus showed a high inhibitory activity for prostaglandin synthesis [21]. Taking into account, it can be hypothesized that the anti-inflammatory activity of 1 could be due to an inhibition effect in the prostaglandin syntheses.
Materials and Methods
Plant material. L. nepetaefolia was collected in Cardonal, Hidalgo in 2004 and flowers, leaves and stems were separated. A voucher specimen was deposited in the Herbario Nacional (MEXU- 1147583).
Flowers (39.48 g), leaves (674.21 g ) and stems (1179.72 g) were treated separately. Plant material was exhaustively extracted with n-hexane, EtOAc and MeOH, successively. Then from the flowers, 0.86 g of the hexane extract (17.82% dry weight), 5.79 g of EtOAc extract (15.05% dry weight) and 8.01 g of methanol extract (20.81% dry weight) were obtained, while from the leaves, 3.77 g (0.55% dry weight), 17.25g (2.55% dry weight) and 35.03 g (5.19% dry weight) were obtained, and from the stems 2.90 g (0.24% dry weight), 8.70g (0.73% dry weight) and 5.169 g (0.43% dry weight) were obtained respectively.
Although all the extracts were cromatographed using an open column packed with Si-gel (G- Altech, 0.2-0.5 mm, ASTM) in a 1:30 proportion to the extract and eluted with solvent mixtures of increasing polarity starting with hexane and ending with methanol, only those where pure compounds were isolated are described.
From the hexane extract of the leaves a total of 45 fractions of 200 mL each were collected. Fractions showing similar TLC data were combined, affording six pools (F1-F6): F1 (eluted with hexane 100%), F2 (eluted with hexane-EtOAc 9:1), F3 (eluted with hexane-EtOAc 7:3), F4 (eluted with hexane-EtOAc 6:4), F5 (fractions eluted with hexane-EtOAc 4:6), F6 (eluted with MeOH). Stigmasterol (14 mg) was isolated from F2 and leonotinin (1; 12.4 mg; [α ] +41, [α ]lit +60) [6] from F5.
Similar chromatographic procedures led the isolation of stigmasterol from the hexane extracts of flowers (14.1 mg) and stems (34.6 mg), and also from the EtOAc extracts of stems (32.2 mg) and leaves (11.8 mg).
The identities of isolated compounds were achieved by comparison of their physical and spectroscopic data with those previously reported [6, 14-15].
Animals. Male CD-1 mice, weighting 20-25g each were used. Instituto de Fisiología Celular, Universidad Nacional Autónoma de México provided the experimental animals. All animals were held under standard laboratory conditions (temperature 27 ± 1 °C). They were fed laboratory diet and water ad libitum. All experiments were carried out using 3-4 animals per group.
TPA-induced edema model. Effects of the test substances on TPA-induced ear edema in mice were studied as described by De Young [17] with slight modifications [22]. The extracts and leonotinin were applied topically. A solution of TPA (2.5µg) in EtOH (10 µL was applied topically to both faces (5 µL each face) of the right ear of the mice, 10 min after the test substances were applied (10µL each face). The left ear received ethanol (10µL) first, and 20 µL of the respective solvent subsequently.
Four hours later the mice were killed by cervical dislocation. A 7-mm diameter plug was removed from each ear. The swelling was assessed as the difference in weight between right and left ear plugs. Data were expressed as the standard error of the mean (SEM) of 3-4 mice. Inhibition of edema (EI, %) was calculated by the equation: EI (%) = 100 - [B × 100 / A], with A = edema induced by TPA alone, and B = edema induced by TPA plus sample. All the extracts were tested at 1 mg / ear doses while leonotinin was evaluated at several doses. The results were analyzed by ANOVA. Statistical comparisons were made between control group and the experimental group using a t student and Dunnett's test for extracts and leonotinin respectively.
Acknowledgments
The authors would like to thank Nieves Zavala, Hector Ríos, Rocío Patiño, Angeles Peña, Elizabeth Huerta, Eréndira García, Gabriela Salcedo and Javier Pérez for their technical support.
References
1. Boalino, D. M.; Tinto, W. F. Heterocycles 2004, 63, 383-387. [ Links ]
2. Takeda, T.; Narukawa, Y.; Hada, N. Chem. Pharm. Bull. 1999, 47, 284-286. [ Links ]
3. Borunda, M. E.; Ochurte, C.; Castañeda, G.; Peralta, B. Flora Medicinal Indígena de México, Tomo II, ED Instituto Nacional Indigenista, México, 1994, p. 574. [ Links ]
4. White, J. D.; Manchand, P. S. J. Org. Chem. 1973, 38, 720-728. [ Links ]
5. Narukawa, Y.; Shimizu, N.; Takeda, T. Nat. Med. 2001, 55, 79-82. [ Links ]
6. Purushothaman, K. K.; Vasanth, S.; Conolly, J. D. J. Chem. Soc., Perkin Trans. I 1974, 2661-2663. [ Links ]
7. Dahl, A. R.; Norman, A. D. J. Am. Chem. Soc. 1970, 92, 5527-5528. [ Links ]
8. Blount, J. F.; Manchand, P. S. J. Chem. Soc. Perkin I, 1980, 264-268. [ Links ]
9. Sivaraman, J.; Subramanian, K.; Vasanth, S. Acta Cryst. 1996, C52, 2043-2045. [ Links ]
10. Govindasamy, L.; Rajakannan, V.; Velmurugan, D.; Banumathi, S.; Vasanth, S. Crystal Res. and Technol. 2002, 37, 896-909. [ Links ]
11. Argumedo, R.; Parra-Delgado, H.; Ramírez, T.; Nieto, A.; Martínez-Vázquez, M. Rev. Soc. Quím. Méx. 2003, 47, 167-172. [ Links ]
12. Martínez-Vázquez, M.; García-Argáez, A. N. Phytochemical studies and biological evaluations of some Mexican plants. In Recent Res. Devel. Phytochem. ED Research Signpost, India, 2001, 5, pp59-85. [ Links ]
13. Oviedo-Chávez, I.; Ramírez-Apan, T.; Soto-Hernández, M.; Martínez-Vázquez, M. Phytomedicine 2004, 11, 436-445. [ Links ]
14. Hussein, A.; Meyer, M. J.; Rodríguez, B. Magnet. Res. Chem. 2003, 41, 147-151. [ Links ]
15. Thompson, M. J.; Dutky, S. R.; Patterson, G. W.; Gooden, E. L. Phytochem. 1972, 11, 1781-1790. [ Links ]
16. Akihisa, T.; Yasukawa, K. Antitumor-promoting and anti-inflammatory activities of triterpenoids and sterols from plants and fungi. In Stud. Nat. Prod. Chem. ED Elsevier Sc. 2001, 25, 43-87. [ Links ]
17. De Young, L. M.; Kheifets, J. B.; Ballaron, S. L.; Young, J. M. Agents Actions 1989, 26, 335-341. [ Links ]
18. Carlson, R. P.; O'Neil-Davis, L.; Chang, J.; Lewis, A. J. Agents Actions 1985, 17, 197-204. [ Links ]
19. Demetzos, C.; Dimas, K. S. Labdane-type diterpenes: chemistry and biological activity. In Stud. Nat. Prod. Chem. ED Elsevier Sc. 2001, 25, 235-292. [ Links ]
20. Singh, M.; Pal, M.; Sharma, R. P. Planta Med. 1999, 65, 2-8. [ Links ]
21. Jager, A. K.; Hutchings, A.; Van Staden, J. J. Ethnopharm. 1996, 52, 95-100. [ Links ]
22. García-Argáez, A.; Ramírez-Apan, T. O.; Parra-Delgado, H.; Velásquez, G.; Martínez-Vázquez, M. Planta Med. 2000, 66, 279-281. [ Links ]














