Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista de la Sociedad Química de México
versión impresa ISSN 0583-7693
Rev. Soc. Quím. Méx vol.48 no.1 Ciudad de México ene./mar. 2004
Investigación
Furanoeremophilane Derivatives from Psacalium beamanii
Ana L. Pérez-Castorena,1* Amira Arciniegas,1 José Luis Villaseñor,2 Alfonso Romo de Vivar1
1 Instituto de Química.
2Instituto de Biología, Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán 04510, D.F., México. Tel.: (5255) 56-224412. Fax: (5255) 56-162217. E-mail: alperezc@servidor.unam.mx
Recibido el 24 de noviembre del 2003.
Aceptado el 26 de febrero del 2004.
Abstract
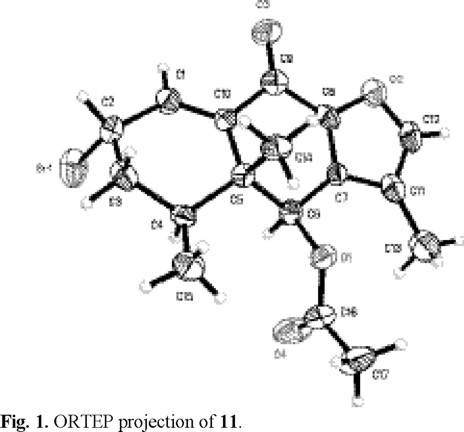
The phytochemical study of Psacalium beamanii afforded the phenylethanoid 1, three sterols, the furanoeremophilane derivatives 2-10, and maturone acetate (12), which was isolated as a natural product for the first time. Its structure was established by means of spectroscopic data and confirmed by chemical correlation. The optical rotation of decompostin (4) was corrected and its identity was corroborated by the X ray analysis of its 2S-bromo derivative (11).
Keywords: Psacalium beamanii, Asteraceae, Senecioneae, Tussilagininae, furanoeremophilane derivatives.
Resumen
El estudio fitoquímico de Psacalium beamanii permitió el aislamiento del feniletanoide 1, tres esteroles, los furanoeremofilanos 2-10, y el acetato de maturona (12), el cual fue aislado por primera vez como producto natural. Su estructura se estableció por medio de sus datos espectroscópicos y se confirmó por correlación química. La rotación óptica de la decompostina (4) se corrigió y su identidad se corroboró por el análisis de rayos X de su derivado 2S-bromo (11).
Palabras clave: Psacalium beamanii, Asteraceae, Senecioneae, Tussilagininae, derivados de furanoeremofilano.
Dedicated to the memory of Dr. Raymundo Cruz Almanza
Introduction
The genus Psacalium (Asteraceae, Senecioneae, Tussilagininae) comprises about 47 species distributed from southwestern United States to Guatemala [1]. Previous chemical studies have shown that eremophilanes are the main secondary metabolites elaborated by species of this genus [2-5]. P. decompositum, P. palmeri, P. peltatum, P. sinuatum, P. radulifolium and Acourtia thurberi are grouped by the Tarahumara ethnia of northern Mexico, in the so called Matarique complex, and used for the treatment of rheumatism and diabetes, as well as renal, hepatic and gastrointestinal ailments [6]. Ethnomedical information has motivated several research projects such as the evaluation of the antihyperglycemic activity of P. decompositum and P. peltatum extracts [4,7], and the antimicrobial activity of P. radulifolium extracts [5]. Thus, in order to continue with the phytochemical research of Mexican plants of the Senecioneae tribe [8], we studied Psacalium beamanii H. Rob. In the present study, we report the isolation of a phenylethanoid (1), three sterols, and nine furanoeremophilane derivatives (2-10). Additionally, the characterization of maturone acetate (12), isolated as a natural product, for the first time, is also described.
Results and discussion
The phytochemical study of Psacalium beamanii afforded the known compounds β-sitosterol, stigmasterol, β-sitosterol glucoside [9], the phenylethanoid icariside D2 (1) [10], the furanoeremophilane derivatives maturinone (2) [11], maturone (3) [2], decompostin (4) [12], maturinin (5) [2,13], maturin acetate (6) [2,13], cacalonol (7/8) [14] and peroxycacalonol (9/10) [14]. The steroidal compounds were identified by comparison with authentic samples, and compounds 1-10 by comparison of their physical and spectroscopic features with those described in literature.
Cacalonol and peroxycacalonol were optically inactive, suggesting the presence of 1:1 mixtures of the corresponding enantiomers (7/8 and 9/10 respectively). Compound 4, identified as decompostin by means of its mp and spectral data, showed a specific rotation ([α]D29-68°) with opposite sign to that reported in literature [12]. Its 2α-bromo derivative 11 exhibited an [α]D29-426°, which also differed from that reported for the same compound ([α]D-41.4°) [12]. In order to clarify this ambiguity, an x-ray crystallographic analysis of the 2S-bromo derivative (Fig 1) was performed, thus confirming the structure and absolute configuration of decompostin (4) and showing that the reported specific rotations of 4 and 11 were mistaken.
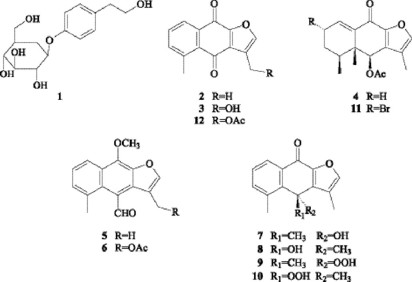
In addition to the above mentioned, modified furanoeremophilane 12 was isolated as a crystalline yellow solid, mp 150-1º. Its molecular formula C16H12O5 deduced from HREIMS (m/z 284.068, [M]+) as well as its UV (λmax 246, 293 and 350 nm) and IR (νmax 1670, 1585and 1543 cm-1) spectra suggested an aromatic structure with a quinoid system. This was corroborated by the two singlet signals at δ 183.54 and 173.62 (C-6 and C-9 respectively) observed in the 13C NMR spectrum. The presence of an acetate group was deduced by its characteristic signals (δH 2.14, δC 20.85 and δC 170.56) observed in the 1H and 13C NMR spectra. The remaining signals in both spectra showed a structural relationship with maturone (3). The main difference observed in 1H NMR spectrum of 12 was the downfield shift of the C-13 methylene signal (δ 5.38), in agreement with the presence of an acetate group bonded to this carbon atom. The structure and identity of 12 were confirmed when maturin acetate (6) was transformed into 12 by Jones oxidation. Maturone acetate was reported as an acetylation product of maturone [2]. Nevertheless, this is the first time that 12 is isolated as a natural product and fully characterized by 1H, 13C, COSY, HETCOR, and COLOC NMR spectral data.
Experimental
General Experimental Procedures
Melting points were determined on a Fisher Jones melting point apparatus and are uncorrected. Optical rotations were determined on a JASCO DIP-360 digital polarimeter. IR spectra were recorded on a Nicolet Magna-IR 750 spectrometer. EIMS data were determined on a JEOL JMS-AX505HA mass spectrometer at 70 eV. 1H NMR and 13C NMR data were obtained on a Varian Unity 300 instrument. Chemical shifts were referred to TMS (δ 0). Standard Varian programs were used for COSY spectra at 300 MHz. HETCOR experiments were obtained for 1JCH = 140 Hz at 75 MHz. COLOC experiments were obtained for nJCH = 9 Hz at 75 MHz. Vacuum column chromatographies (VCCs) were performed using Sil G 60 E. Merck, 70-230 mesh.
Plant Material
Psacalium beamanii H. Rob. was collected in Ixtlán, Oaxaca, México, June 1997. A voucher specimen (MEXU 775149) was deposited at the Herbario Nacional, Instituto de Biología, UNAM, México.
Extraction and Isolation
Dried and ground roots (129 g) were extracted successively with hexane and MeOH at room temperature. Hexane extract (4.47 g) was analyzed by VCC eluting with an hexane/EtOAc gradient system. Fractions eluted with hexane were combined and purified by means of VCC (hexane/EtOAc 99:1) to yield maturinone (2, 11.1 mg) [11], mp 169-72°. Fractions eluted with hexane/EtOAc 90:10 afforded maturone acetate (12, 44 mg) [2], mp 150-1°. The mother liquors of the last compound and fractions eluted with hexane/EtOAc 85:15 were combined and submitted to VCC (hexane/EtOAc 95:5) to yield decompostin (4, 49.4 mg) [12], mp 195-6°, [α]D29 -68° (c 0.456, CHCl3), and 12 (37.9 mg). Decompostin (4, 19 mg) was transformed into 2S-bromodecompostin (11, 6 mg) as already described [12], mp 188-192° dec, [α]D29 -426° (c 0.2, CHCl3). MeOH extract (28 g) was purified by VCC eluting with an hexane/Me2CO gradient system. Fractions eluted with hexane were analyzed by VCC (hexane/EtOAc 99:1) affording maturinin (5, 18.1 mg) [2, 13], mp 94-6°, 2 (4.4 mg) and a mixture of β-sitosterol/stigmasterol (25 mg), mp 134-6º. Fractions eluted with hexane/Me2CO 90:10 were submitted to VCC (hexane/EtOAc 95:5) to yield 4 (123.6 mg) and maturin acetate (6, 125.5 mg) [2, 13], mp 84-6°. Fractions eluted with hexane/Me2CO 80:20 were purified by VCC (hexane/EtOAc 85:15) affording cacalonol (7/8, 11.4 mg) [14], mp 205-7°, [α]D29 0º (c 0.26, CHCl3) and maturone (3, 3 mg) [2], mp 164-70°. Dried and ground aerial parts (476.2 g) were extracted with hexane and MeOH. Hexane extract (12.7 g) was submitted to VCC eluting with an hexane/Me2CO gradient system. Fractions eluted with hexane/Me2CO 95:5 afforded a mixture of β-sitosterol/stigmasterol (25 mg). Fractions eluted with hexane/Me2CO 90:10 yielded 7/8 (33.4 mg) and peroxycacalonol (9/10, 34.7 mg) [14], mp 204-6° dec., [α]D29 0º (c 0.185, CHCl3). The MeOH extract (100 g) was analyzed by VCC eluting with an hexane/Me2CO gradient system. Fractions eluted with hexane/Me2CO 95:5 were purified by VCC (hexane/EtOAc 90:10) yielding a mixture of β-sitosterol/stigmasterol (28 mg), and 12 (10.6 mg). Fractions eluted with hexane/Me2CO 90:10 and 85:15 were combined and purified by VCC (hexane/EtOAc 85:15) affording 12 (2.1 mg), 7/8 (31.6 mg), and 3 (17.5 mg). Fractions eluted with hexane/Me2CO 50:50 and 40:50 were combined and analyzed by VCC (CHCl3/MeOH 95:5) to give β-sitosterol glucopyranoside (75.6 mg) [9], mp 285-90º. Fractions eluted with hexane/Me2CO 30:70 were treated with charcoal/MeOH and submitted to VCC (CHCl3/MeOH 80:20) to yield icariside D2 (1, 148.3 mg) [10], mp 153-5°.
Maturone acetate (12). Yellow crystals from hexane/EtOAc, mp 150-1° [2]. UV (MeOH): λmax (log ε) 204 (4.25), 246 (4.45), 293 (3.83), 350 nm (3.71). IR (CHCl3):νmax 1744, 1670, 1585, 1543 cm-1. EIMS m/z (rel. int.) 284 [M]+ (10), 242 [M-42]+ (15), 224 [M-HOAc]+ (100), 196 [224-28]+ (5), 168 [196-28]+ (16), 139 [168-29]+ (20), 43 [C2H3O]+ (63). HREIMS m/z [M]+ 284.0681 (calcd for C16H12O5 284.0685). 1H-NMR (300 MHz, CDCl3): δ 8.17 (1H, br d, J = 6.6 Hz, H-1), 7.61 (1H, t, J = 7.5 Hz, H-2), 7.54 (1H, br d, J = 7.2 Hz, H-3), 7.76 (1H, s, H-12), 5.38 (2H, s, H-13), 2.81 (3H, s, H-15), 2.14 (3H, s, Ac). 13C-NMR (75 MHz, CDCl3): δ 125.99 (C-1), 132.94 (C-2), 138.49 (C-3), 142.29 (C-4), 130.55 (C-5), 183.54 (C-6), 128.70 (C-7), 152.17 (C-8), 173.62 (C-9), 134.05 (C-10), 121.61 (C-11), 147.10 (C-12), 56.47 (C-13), 23.12 (C-15), 170.56 (C=O, Ac), 20.85 (CH3, Ac).
Oxidation of maturin acetate (6). A solution of 6 (27.8 mg) in Me2CO (2 ml) at 3° was oxidized with Jones reactive in the usual manner. The residue purified by VCC (hexane/EtOAc 80:20) gave maturone acetate (12, 16.4 mg), mp 149-51°.
X-ray crystallographic data of 11. Colorless crystal obtained from CHCl3/Et2O; crystal size 0.206 × 0.144 × 0.052 mm; crystal data: C17H19BrO4, mol. wt = 367.23, monoclinic, space group P21, a = 7.242 (1) Å, b = 8.321 (1) Å, c = 14.131 (1) Å, β = 103.535 (2)°, V = 827.9 (2) Å3, Z = 2, Dc = 1.473 Mg/m3, F (000) = 376, T = 293 (2) K, µ = 2.497 mm-1, λ (Mo Kα) = 0.71073 Å, 6805 measured intensities (2.86 < θ < 25.03°), -8 ≤ h ≤ 8, -9 ≤ k ≤ 9, -16 ≤ l ≤16, collected Bruker Smart Apex CCD diffractometer. Refinement by the full matrix-least squares on F2 method was performed using 2907 reflections (> 2σ (I)) with 203 parameters. The largest difference peak was 0.497. The final R-factor was 4.04 % and wR2 was 5.6 %. Flack's parameter = -0.05 (1). Crystallographic data have been deposited at the Cambridge Crystallographic Data Center (CCDC 232110), 12 Union Road, Cambridge, CB2 1EZ, UK.
Acknowledgement
We are indebted to Rubén Toscano, Rubén Gaviño, María Isabel Chávez, Héctor Ríos, Angeles Peña, Rocío Patiño, Luis Velasco, Javier Pérez, and Gabriela Salcedo for technical assistance.
References
1. Barkley, T. M.; Clark, B. L.; Funston, A. M. Proceedings of the International Compositae Conference, Kew 1994. Hind, D. J. N., Ed., Royal Botanical Gardens: Kew, 1996; Vol. 1. p. 613-620. [ Links ]
2. Correa, J.; Romo, J. Tetrahedron 1966, 22, 685-691. [ Links ]
3. Joseph-Nathan, P.; Negrete, M. C.; González, M. P. Phytochemistry 1970, 9, 1623-1628. [ Links ]
4. Inman, W. D.; Luo, J.; Joland, S. D.; King, S. T.; Cooper, R. J. Nat. Prod. 1999, 62, 1088-1092. [ Links ]
5. Garduño-Ramírez, M. L.; Trejo, A.; Navarro, V.; Bye, R.; Linares, E.; Delgado, G. J. Nat. Prod. 2001, 64, 432-435. [ Links ]
6. Bye, R.; Linares, E.; Estrada, E., in: Phytochemistry of Medicinal Plants, Arnason, J. T.; Mata, R.; Romeo, J. T. Ed., Plenum Press, New York, 1995, 65. [ Links ]
7. Alarcón-Aguilar, F. J.; Jiménez-Estrada, M.; Reyes-Chilpa, R.; González-Paredes, B.; Contreras-Weber, C. C.; Román-Ramos, R. J. Ethnopharmac. 2000, 69, 207-215. [ Links ]
8. Pérez-Castorena, A. L.; Arciniegas, A.; Ramírez A. M. T.; Villaseñor, J. L.; Romo de Vivar, A. Planta Med. 2002, 66, 645-647. [ Links ]
9. Voutquenne, L.; Lavaud, C.; Massiot, G.; Sevenet, T., Hadi, H. A. Phytochemistry 1999, 50, 63-69. [ Links ]
10. Miyase, T.; Ueno, A.; Takizawa, N.; Kobayashi, H., Oguchi, H. Phytochemistry 1989, 28, 3483-3485. [ Links ]
11. Brown, P. M.; Thomson, R. H. J. Chem. Soc. C 1969, 1184-1186. [ Links ]
12. Rodríguez-Hahn, L.; Guzmán, A.; Romo, J. Tetrahedron 1968, 24, 477-483. [ Links ]
13. Bohlmann, F.; Zdero, C.; Grenz, M. Chem. Ber. 1977, 110, 474-486. [ Links ]
14. Naya, K.; Miyoshi, Y.; Mori, H.; Takai, K.; Nakanishi, M. Chem. Lett. 1976, 73-76. [ Links ]
Nota
Contribution 2465 of the Instituto de Química, UNAM.














