Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista latinoamericana de química
versión impresa ISSN 0370-5943
Rev. latinoam. quím vol.40 no.1 Naucalpan de Juárez abr. 2012
Chemical composition and antibacterial activity of the essential oil of Monticalia Imbricatifolia Schultz (Asteraceae)
Diolimar Buitragoa*, Judith Velascob, Tulia Díazc y Antonio Moralesa
a Instituto de Investigaciones. Facultad de Farmacia y Bioanálisis. Universidad de Los Andes. Mérida-Venezuela.
b Departamento de Microbiología y Parasitología. Facultad de Farmacia y Bioanálisis. Universidad de Los Andes. Mérida-Venezuela.
c Departamento de Bioanálisis Clínico. Facultad de Farmacia y Bioanálisis. Universidad de Los Andes. Mérida-Venezuela. Corresponding author: diolbui@ula.ve.
Received June 2012
Accepted September 2012
Abstract
Essential oil from fresh aerial parts of Monticalia imbricatifolia Schultz., was isolated by hydrodistillation and analyzed by GC/MS. A yield of 0.18% was obtained. Eleven components were identified by comparison of their mass spectra with the Wiley GC-MS Library data and the retention indices (RI) calculated for every compound. The major components were monoterpenes: α-phellandrene (33.89%), β-phellandrene (19.28%), α-pinene (16.81%) y β-pinene (10.97%). Antibacterial activity of the essential oil of this species was evaluated against Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Klebsiella pneumoniae (ATCC 23357), using the disc diffusion agar method. The results showed the oil is highly effective against all tested bacteria, with minimal inhibitory concentration (MIC) values ranging from 20 to 60 µg/ml.
Key words: Monticalia imbricatifolia, Asteraceae, essential oil, antibacterial activity.
Resumen
El aceite esencial de las partes aéreas frescas de Monticalia imbricatifolia Schultz., fue aislado por hidrodestilación y analizado por CG/EM. El rendimiento obtenido fue de 0.18%. Once componentes fueron identificados por comparación de sus espectros de masas con los de la Librería Wiley GC-MS y los índices de retención (IR) fueron calculados para cada compuesto. Los componentes mayoritarios fueron los monoterpenos: α-felandreno (33.89%), β-felandreno (19.28%), α-pineno (16.81%) y β-pineno (10.97%). La actividad antibacteriana del aceite esencial de esta especie se evaluó contra Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) y Klebsiella pneumoniae (ATCC 23357), por el método de difusión en agar con discos. Los resultados demostraron que el aceite fue altamente efectivo contra todas las bacterias probadas, con un intervalo de concentración inhibitoria mínima (CIM) de 20-60 µg/ml.
Palabras clave: Monticalia imbricatifolia, Asteraceae, aceite esencial, actividad antibacteriana.
Introduction
The Asteraceae family comprised about 1500 genera and 25000 species, distributed worldwide (Badillo, 1997). The genus Monticalia belongs to the family Asteraceae, tribe Senecioneae (Aristiguieta, 1964). From Venezuela, 29 species of this genus have been reported (Badillo, 2001). Species of this genus have been used in traditional medicine as antiallergic, hypotensive and to treat alopecia (Gil et al., 2003). Nieves et al., 2010, reported that essential oil of M. imbricatifolia presents low repellent activity against the bites of Lutzomyia migonei in laboratory conditions. Previous investigations of the essential oil of different species of Monticalia have reported a variety of compounds, such as α-pinene, β-pinene, α-longipinene, δ-3-carene, cyperene and β-phellandrene found in M. andicola (Baldovino et al., 2009); 1-nonane, α-pinene, germacrene D and β-cedrene reported in M. greenmaniana (Cárdenas et al., 2012), both species have showed antibacterial activity. In the present study, the composition of the essential oil of Monticalia imbricatifolia Schultz, is reported as well as their antibacterial activity. This species had previously been known as Senecio imbricatifolius (Schultz-Bip-ex Wedd) and is characterized by being a woody, small, branched plant, with tiny leaves, sessile, alternate, imbricate, directly attached to the stem (Badillo, 1997).
Materials and methods
Plant Material
Aerial parts of M. imbricatifolia, were collected in the way to Piñango Paramo, Mérida State, Venezuela at 2320 m above sea level. Voucher specimen (DBB017) has been deposited in the Faculty of Pharmacy and Bioanalysis MERF Herbarium, University of Los Andes, Venezuela.
Isolation of Essential Oil
Fresh leaves (1105 g) were cut into small pieces and subjected to hydrodistillation for 3 h, using a Clevenger-type apparatus. The oil was dried over anhydrous sodium sulphate and stored at 4 ºC.
Gas Chromatography-Mass Spectrometry
The oil was analyzed by GC-MS on an Hewlett Packard GC-MS system, Model 5973, fitted with a 30 m long cross-linked 5 % phenylmethyl siloxane (HP-5MS, Hewlett Packard, USA) fused-silica column (0.25 mm diam, film thickness 0.25 mm). The initial oven temperature was 60°C; it was then heated to 280°C at 4°C/min, and the final temperature was maintained for 20 min. The injector and detector temperatures were 200°C and 230°C, respectively. The carrier gas was helium, adjusted to a linear velocity of 34 m/s, the ionization energy 70 eV, and the scan range 40-500 amu at 3.9 scans/s. A Hewlett-Packard ALS injector was used with split ratio 1:100. The injected volume was 1.0 ml of a 2% dilu tion of oil in n-heptane. The identification of the oil components was based using a Wiley MS Data Library (6th edn), reference mass spectra from published sources, and retention indices (RI) (Adams, 1995).
Microbiological Analysis Bacterial Strains
The microorganisms used were Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 29212), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (ATCC 23357) and Pseudomonas aeruginosa (ATCC 27853).
Antimicrobial Method
The antimicrobial activity was carried out according to the disc diffusion assay described by Velasco et al., 2007. The strains were maintained in agar at room temperature. Each bacterial inoculum (2.5 ml) was incubated in Müeller-Hinton broth at 37ºC for 18 hours. The bacterial inoculum was diluted in sterile 0.85% saline to obtain turbidity visually comparable to a McFarland Nº 0.5 standard (106-8 CFU/ml). Every inoculum was spread over plates containing Müeller-Hinton agar and a paper filter disc (6 mm) saturated with 10 µl of essential oil. The plates were left for 30 min at room temperature and then incubated at 37 ºC for 24 h. The inhibitory zone around the disc was measured and expressed in mm. A positive control was also assayed to check the sensitivity of the tested microorganisms using the following antibiotics: Ampicillin-sulbactam® (10 mg/10 mg), Vancomycin® (30 mg), Netilmicin® (30 mg), Aztreonam® (30 mg) and Cefoperazone® (75 mg) (Table 2).

The minimal inhibitory concentration (MIC) was determined only with microorganisms that displayed inhibitory zones. MIC was determined by dilution of the essential oil in dimethyl sulphoxide (DMSO) pipetting 10 µl of each dilution onto a filter paper disc. Dilutions of the oil within a concentration range of 10-120 µg/ml were also carried out. MIC was defined as the lowest concentration that inhibited the visible bacterial growth (CLSI, 2012). A negative control was also included in the test using a filter paper disc saturated with DMSO (10 µl) to check possible activity of this solvent against the bacteria assayed. The experiments were repeated at least twice.
Results and discussion
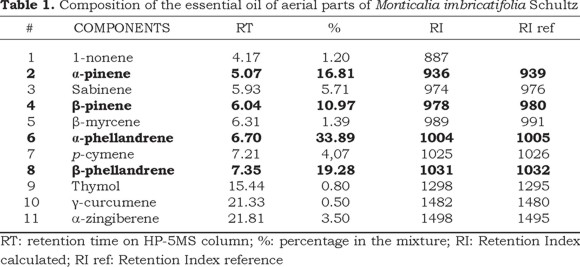
The essential oil from aerial parts of M. imbricatifolia yielded 0.18 %. GC/MS analyses showed the presence of 11 components. Table 1 shows the identified components that constitute the 96.92 % of the oil. The major compounds of the oil were α-phellandrene (33.89%), β-phellandrene (19.28%), α-pinene (16.81%) and β-pinene (10.97%), these compounds belong to the monoterpene group.
These results were compared to the oil composition of M. andicola (Baldovino et al., 2009) where the main components were α-pinene, β-pinene and β-phellandrene like M. imbricatifolia but in different proportions; whereas for M. greenmaniana only α-pinene was present in major quantity (Cárdenas et al., 2012).
Antibacterial activity of essential oil of M. imbricatifolia was evaluated against Gram- positive and Gram-negative bacteria (Table 2). The results show that the oil inhibited the development of all the bacteria included in the study, with MIC values of 20-60 µg/ml for the Gram-positive bacteria and 30-50 µg/ml for Gram-negative bacteria. These results demonstrated a broad spectrum of antibacterial activity of the essential oil, reflected in the values of the MIC to low doses, comparable to the concentration of the reference antibiotics.
There are few reports of antimicrobial activity of members of the genus Monticalia, Cárdenas et al., 2012, reported that leaf essential oil of M. greenmaniana showed broad spectrum of antibacterial activity against the important human pathogenic Gram-positive and Gram-negative bacteria Staphylococcus aureus (ATCC 25923), Enterococcus faecalis (ATCC 19433), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Klebsiella pneumoniae (ATCC 25955), with MIC values ranging from 75 to 6000 ppm. On the other hand, has been described antibacterial activity on the essential oil of M. andicola also proving a broad-spectrum (Baldovino et al., 2009).
The activity observed in the present study could be attributed to the major compounds belonging to the monoterpene group since, the literature refers an-tibacterial activity of the α-phellandrene, β-phellandrene, α-pinene and β-pinene in essentials oils of species of different genera (Erazo et al, 2006; Sonboli et al, 2006; Yan-qiu et al, 2008; Baldovino et al, 2009; Mora et al, 2011; Peña et al, 2012). The mechanism of action is not well known, in this regard, Trombetta et al., 2005, speculate that the antimicrobial effect of monoterpenes, may be due, at least partially, to a perturbation of the lipid fraction of bacterial plasma membranes, resulting in alterations of membrane permeability and in leakage of intracellular materials. Besides being related to physicochemical characteristic of the drugs (such as lipophilicity and water solubility), this effect appears to be dependent on the lipid composition and net surface charge of the bacterial membranes. Furthermore, the drugs might cross the cell membranes, penetrating the interior of the cell and interacting with intracellular sites critically.
The object of this bacteria study cause severe infectious processes in the human and currently, there is an increase in the resistance of these bacteria to the antibiotics of choice (Cornejo et al., 2007; Peña et al., 2007; Guzmán and Lozada, 2007, Cagnacci et al., 2008). At the global level the resistance has become a serious public health problem, in fact, there are resistant strains that cause a large number of diseases (González and Guzmán, 1999). In this sense, the essential oil of M. imbricatifolia represents a source of natural origin for the investigation of new metabolites with antibacterial activity of broad spectrum at low concentrations.
Conclusion
An analysis of the chemical composition of the essential oil of Monticalia imbricatifolia Schultz., revealed 11 compounds, the main compounds belong to the monoterpene group.
The oil showed a broad spectrum of antibacterial activity against the important human pathogenic Gram-positive and Gram-negative bacteria at low concentrations (MIC 20-60 µg/ml).
Acknowledgment
The authors would like to acknowledge to Dr. Pablo Meléndez Faculty of Pharmacy and Bioanalysis MERF Herbarium, University of Los Andes by the identification of the botanical material and Dr. Alfredo Usubillaga, Faculty of Pharmacy and Bioanalysis, University of Los Andes, Venezuela for helping in performing the analysis of GC/MS.
References
Adams, R. (1995). Identification of essential oils components by gas chromatography/mass spectroscopy. Allured Publishing Corporation, Carol Stream IL, USA pp: 469. [ Links ]
Aristeguieta, L (1964). Flora de Venezuela. Compositae. Instituto Botánico, Caracas-Venezuela pp: 361. [ Links ]
Badillo, V (1997). Los Géneros de las Compositae (Asteraceae) de Venezuela. Clave para su determinación, Ernstia 6 (4): 51-168. [ Links ]
Badillo, V (2001). Lista actualizada de las especies de la familia Compuestas (Asteraceae) de Venezuela, Ernstia 11 (3,4): 166-167. [ Links ]
Baldovino, S., Rojas, J., Rojas, LB., Lucena, M., Buitrago, A., Morales, A. (2009). Chemical composition and antibacterial activity of the essential oil of Monticalia andicola (Asteraceae) collected in Venezuela, Nat Prod Commun 4 (11): 1601-1604. [ Links ]
Cagnacci, S., Gualco, L., Roveta, S., Mannelli, S., Borgianni, L., Docquier, J D., Dodi, F., Centanaro, M., Debbia, E., Marchese, A., Rossolini, G M. (2008). Bloodstream infections caused by multidrug-resistant Klebsiella pneumoniae producing the carbapenem-hydrolysing VIM-1 metallo-beta-lactamase: first Italian outbreak. J Antimicrob Chemother 61(2): 296-300. [ Links ]
Cárdenas J, Rojas J, Rojas-Fermin L, Lucena M, Buitrago A. (2012). Essential oil composition and antibacterial activity of Monticalia greenmaniana (Asteraceae). Nat Prod Commun 7(2):243-244. [ Links ]
CLSI: Clinical and Laboratory Standars Institute (2012). Performance Standards for antimicrobial disk susceptibility tests; Approved Standard—Eleventh Edition. Document M02-A11. Wayne, PA: Clinical and Laboratory Standards Institute. [ Links ]
Cornejo-Juárez, P., Velásquez-Acosta, C., Sandoval, S., Gordillo, P., Volkow-Fernández, P. (2007). Patrones de resistencia bacteriana en urocultivos en un hospital oncológico. Salud Pública de México, 49 (5): 330-336. [ Links ]
Erazo, S., Delporte, C., Negrete, R., García, R., Zaldívar, M., Iturra, G., Caballero, E., López, J.L., Backhouse, N. (2006). Constituents and biological activities of Schinus polygamus. J Ethnopharmacol, 107 (3): 395-400. [ Links ]
Gil, R., Mejías, R., Carmona, J., Mejías, R., Rodríguez, M. (2003). Estudio etnobotánico de algunas plantas medicinales expendidas en los herbolarios de Mérida, Ejido y Tabay (Estado Mérida - Venezuela). Revista de la Facultad de Farmacia, 45 (1): 69-76. [ Links ]
González-Salvatierra, R., Guzmán-Blanco, M. (1999). La resistencia a antimicrobianos en las Américas. Rev Panam Salud Publica, 6 (6): 437-439. [ Links ]
Guzmán, M., Lozada, R. (2007). Detección de Staphylococcus aureus meticilinoresistentes de pacientes con Infecciones nosocomiales y adquiridas en la comunidad. Revista de la Sociedad Venezolana de Microbiología, 27: 45-49. [ Links ]
Mora, F., Ríos, N., Rojas, L.B., Díaz, T., Velasco, J., Carmona, J., Silva, B. (2011). Chemical composition and in vitro antibacterial activity of the essential oil of Phthirusa adunca from Venezuelan Andes. Nat Prod Commun, 6 (7): 1051-1053. [ Links ]
Nieves, E., Fernández, J., Lias, J., Rondón, M., Briceño, B. (2010). Actividad repelente de aceites esenciales contra las picaduras de Lutzomyia migonei (Diptera: Psychodidae). Rev. Biol.Trop, 58 (4): 1549-1560. [ Links ]
Peña, A., Rojas, L., Aparicio, R., Alarcón, L., Baptista, J.G., Velasco, J., Carmona, J., Usubillaga, A. (2012). Chemical composition and antibacterial activity of the essential oil of Espeletia nana. Nat Prod Commun, 7(5): 661-662. [ Links ]
Peña, C., Suárez, C., Tubau, F., Gutierrez, O., Domínguez, A., Oliver, A., Pujol, M., Gudiol, F., Ariza, J. (2007). Nosocomial spread of Pseudomonas aeruginosa producing the metallo-beta-lactamase VIM-2 in a Spanish hospital: clinical and epidemiological implications. Clin Microbiol Infect, 13 (10): 1026-1029. [ Links ]
Sonboli, A., Babakhani, B., Mehrabian, A.R. (2006). Antimicrobial activity of six constituents of essential oil from Salvia. Z Naturforsch C, 61 (3-4): 160-164. [ Links ]
Trombetta, D., Castelli, F., Sarpietro, M., Venuti, V., Cristani, M., Daniele, C., Saija, A., Mazzanti, G., Bisignano, G. (2005). Mechanisms of antibacterial action of three monoterpenes. Antimicrobial Agents and Chemotherapy, 49 (6): 2474-2478. [ Links ]
Velasco, J., Rojas, J., Salazar, P., Rodríguez, M., Díaz, T., Morales, A, Rondón, M. (2007). Antibacterial activity of the essential oil of Lippia oreganoides against multiresistant bacterial strains of nosocomial origin. Nat Prod Commun, 2 (1): 85-88. [ Links ]
Yan-qiu, Cui., Xin-hong, Cui., Yi, Zhu., Qun, Zhang., Peng, Nan. (2008). Chemical Composition and Antimicrobial Activity of Volatile Oil of Six Gymnosperm Species Leaves from Shanghai. Bioinformatics and Biomedical Engineering, 2nd International Conference: 4573 - 4577. [ Links ]














